Abstract
Mesophilic and psychrotrophic organism viable counts, as well as high-throughput 16S rRNA gene-based pyrosequencing, were performed with the aim of elucidating the origin of psychrotrophic lactic acid bacteria (LAB) in a ready-to-eat (RTE) meal manufacturing plant. The microbial counts of the products at the end of the shelf life were greatly underestimated when mesophilic incubation was implemented due to overlooked, psychrotrophic members of the LAB. Pseudomonas spp., Enterobacteriaceae, Streptococcaceae, and Lactobacillus spp. constituted the most widespread operational taxonomic units (OTUs), whereas Leuconostoc gelidum was detected as a minor member of the indigenous microbiota of the food ingredients and microbial community of the processing environment, albeit it colonized samples at almost every sampling point on the premises. However, L. gelidum became the most predominant microbe at the end of the shelf life. The ability of L. gelidum to outgrow notorious, spoilage-related taxa like Pseudomonas, Brochothrix, and Lactobacillus underpins its high growth dynamics and severe spoilage character under refrigeration temperatures. The use of predicted metagenomes was useful for observation of putative gene repertoires in the samples analyzed in this study. The end products grouped in clusters characterized by gene profiles related to carbohydrate depletion presumably associated with a fast energy yield, a finding which is consistent with the fastidious nature of highly competitive LAB that dominated at the end of the shelf life. The present study showcases the detrimental impact of contamination with psychrotrophic LAB on the shelf life of packaged and cold-stored foodstuffs and the long-term quality implications for production batches once resident microbiota are established in the processing environment.
INTRODUCTION
Theoretically, a food commodity harbors a wide range of different microorganisms (1) associated with the ecological niches wherefrom foods originate. This initial diverse community can be enriched by other biota after transportation to industrial production facilities (2). Due to handling during processing and storage, microbes are eventually subjected to a selection pressure (3), which defines a dominant microbial consortium. In this way, certain members of this association proliferate greatly and cause alterations constituting the specific spoilage organisms (SSOs) (4). In the past few years in Belgium, the persisting problem of psychrotrophic lactic acid bacteria (LAB) prevailing at the end of the shelf life of packaged and refrigerated foodstuffs has been substantiated (5–7). These cold-adapted species of LAB contaminate foods from numerous industrial plants, but little is known about their environmental occurrence and the possible contamination routes. Their introduction in production lines can be mediated by crude material, given the fact that LAB are ubiquitous in plant ecosystems and also constitute the commensal biota of livestock (8–10). Moreover, there is emerging evidence that psychrotrophic LAB can adapt to premises and establish a resident community (11, 12).
Currently, the focus is mainly on the systematic study of well-known spoilage-related species (13–18) in various food matrices and the development of methods to suppress their growth and offensive metabolism through the application of innovative technologies. The spoilage-related populations can be studied as a consortium with the help of culture-independent approaches in order to look at changes to the composition of the microbial communities in response to environmental factors related to food storage, such as temperature and packaging technology (19, 20), as well as technological parameters (21). However, the isolation and characterization of spoilage microorganisms from food are also fundamental in order to differentiate the biotype-specific traits that delineate the intraspecies diversity (22–25). The spoilage of food is a complex phenomenon; in ready-to-eat (RTE) foods, it usually occurs with off odors, acidification, and appearance defects (26–28). Few documented studies have dealt with the investigation of ecological niches that serve as reservoirs or commodities harboring spoilage microbiota like Pseudomonas (29–32), LAB (21, 33–38), and Brochothrix thermosphacta (39) in actual processing environments.
In the present study, a food company manufacturing RTE meals was surveyed in order to investigate the origin of contamination with psychrotrophic LAB. The processing plant in question was periodically challenged by unexpected spoilage characterized by very high counts of psychrotrophic LAB, acute acidification of the products, and a sour off odor usually occurring before the end of the shelf life. Previously, a consistent underestimation of the counts of members of psychrotrophic LAB allocated to the genera Leuconostoc, Lactobacillus, and Lactococcus was observed for several samples deriving from this facility (5, 7). More recently, the problem progressed from sporadic events into a recurring spoilage outburst (6). To evaluate this problem, comparative enumeration and a standard enrichment method were used to detect the presence of populations of psychrotrophic LAB, whereas high-throughput 16S rRNA gene pyrosequencing was implemented to assess the composition of the microbiota of environmental samples, surfaces, food ingredients, and end products. The study aimed at investigating the psychrotrophic microorganisms principally involved in the RTE meal spoilage and identifying the most probable sources of contamination within the processing plant.
MATERIALS AND METHODS
Processing plant and RTE meals.
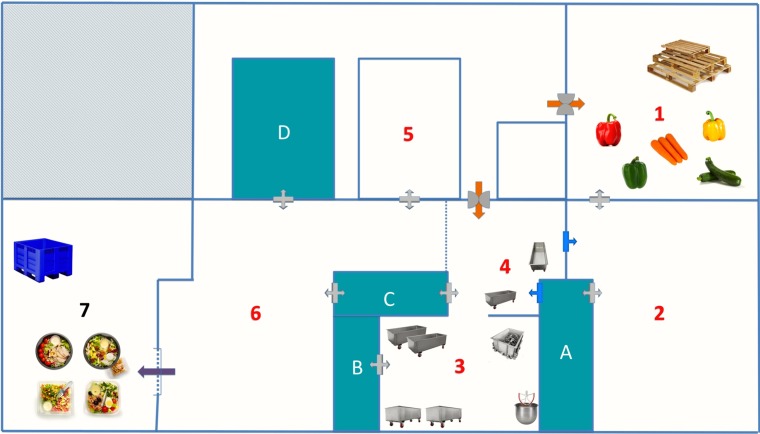
The industrial plant manufactured RTE meals containing the following ingredients: raw vegetables (i.e., broccoli, cabbage, carrot, celery, corn, cucumber, leek, lettuce, onion, parsley, peas, sweet bell pepper, tomato, zucchini, etc.), boiled starchy constituents (i.e., cereals, noodles, pasta, potatoes, etc.), cooked meat products (i.e., bacon, chicken pieces, ham), fishery products (i.e., sardine, shrimp, tuna), dairy products (i.e., feta cheese, mozzarella cheese, parmesan cheese), sauces (i.e., fish, mayonnaise, mustard, tomato, soy, teriyaki), and boiled eggs. The packaged end products had a shelf life of 7 to 8 days at 4°C, according to the specifications of the manufacturer. For the purposes of the study, two visits to the company were performed over a period of 2 months. Figure 1 presents a schematic representation of the spatial arrangement of the different domains of the plant, and Table 1 describes the areas of the processing environment from which all samples were recovered.
FIG 1.
Layout of the processing plant, which is divided into domains (domains 1 to 7) allocated to a specific production process (Table 1). Domain 1, cold room for storage of whole, raw, and unprocessed vegetables; domain 2, high-care area for handling of vegetables (i.e., rinsing, washing, cutting, and chopping); domain 3, preparation room where vegetables are assembled and mixed together with pasta and meat or fish ingredients; domain 4, second preparation room where mixtures are completed with the addition of sauces and spices; domain 5, area exclusively allocated to boiling and cooling of starchy constituents (i.e., pasta, potatoes, etc.); domain 6, production lines where the intermediate products are combined, portioned into plastic containers, styled with food toppings (i.e., eggs, cheese, sardines, etc.), and packaged; domain 7, storage of end products; A to D, refrigerators.
TABLE 1.
Food material and environmental samples recovered from each domain of the production planta
| Domain no. | Domain description | Food sample(s) |
Environmental sample(s) | |
|---|---|---|---|---|
| 1st company visit | 2nd company visit | |||
| 1 | Raw vegetable storage | I.1, I.2, I.3, I.7 | II.19, II.20, II.21 | |
| 2 | Vegetable preparation | I.4, I.5, I.6, I.8, I.9 | W.1, W.4, W.5, W.6, S.7, S.8, S.9, S.10 | |
| 3 | Premixing | S.6, S.11 | ||
| 4 | Mixing | II.1 | W.2, W.3, S.1, S.2, S.5, S.12, S.13 | |
| 5 | Boiling/cooling | S.3 | ||
| 6 | Production lines | I.16, I.17, I.18, I.19, I.20 | II.2, II.3, II.4, II.8, II.9, II.12, II.16 | S.4, S.14, S.20, S.21 |
| 7 | End products | EP1, EP2, EP3, EP4, EP5, EP6 | ||
| Refrigerators | ||||
| A | Processed vegetables | I.10, I.11, I.13, I.14, I.15 | II.10, II.11 | |
| B | Cooked, starchy ingredients | S.19 | ||
| C | Toppings | I.12 | II.18 | S.17, S.18 |
| D | Assembled intermediate products | II.5, II.6, II.7, II.13, II.14, II.15, II.17 | S.15, S.16 | |
See Fig. 1 for the layout of the domains and refrigerators. Samples I.1 to I.20 (Table 2 and 3) were collected in October 2013 (1st visit). Samples II.1 to II.21 (Tables 2 and 3), water samples W.1 to W.6 and swab samples S.1 to S.21 (described below), and end product samples EP.1 to EP.6 (Table 4) were collected in November 2013 (2nd visit). W.1, chlorine bath; W.2, mixing vessel; W.3, mixing basket; W.4 to W.6, washing bath (cucumber, lettuce, and endive, respectively); S.1, mixing vessel (sanitized); S.2, scoop (sanitized); S.3, pasta-cooling vessel; S.4, bench; S.5, carrier vessel; S.6, mixing vessel (sanitized); S.7 and S.8, washing bath tank; S.9, rotating belt of dicer; S.10, box; S.11, big mixing vessel (sanitized); S.12, wings of mixing machine; S.13, wing of mixing basket; S.14, balance; S.15 to S.19, wall; S.20 and S.21, gloves.
Environmental sampling.
Surface swab and water samples were recovered from various sites of the processing installation before and after sanitation in order to assess the occurrence of bacterial contaminants and, consequently, evaluate the hygienic plan (Table 1). Sterile rayon swabs with a plastic shaft in individual tubes (Cultiplast, product code 111598; LP Italiana SPA) were used for swabbing of food contact surfaces and processing equipment. The tubes were filled with 10 ml of physiological solution (0.85% [wt/vol] NaCl in distilled water) and then closed aseptically. After the heads of the swabs were moistened in physiological solution, they were applied to a surface of approximately 25 cm2. Subsequently, the tubes were vortexed vigorously at maximum capacity for 1 min in order to dislodge the cells from the swab. Aliquots of 1 ml of the suspension were centrifuged at 120,006 × g for 1 min, and the cells were pelleted. The supernatant was discarded, and the swabs were vortexed following the same procedure until all of the cell suspension was pelleted. Finally, the cells were resuspended in 300 μl of sterile distilled water and were used for total genomic DNA extraction. Water samples (50 ml) were collected from various areas of the processing environment and placed in sterile containers. The water samples were passed through a 0.2-μm-pore-size filter, and the compacted cells were washed off the filter with sterile distilled water and resuspended in 300 μl of distilled water. The cell suspensions were used for genomic DNA extraction.
Microbiological analysis of food material and end products.
Samples of raw materials, RTE meal ingredients, and intermediate products were recovered during the two company visits. All food materials were immediately subjected to comparative microbiological analysis, with mesophilic (30°C) and psychrotrophic (22°C) incubation (see Table 2) being implemented as previously described (5), while some of the samples underwent a standard enrichment-incubation procedure (10 days of anaerobic storage at 4°C; see Table 3) favoring the growth of psychrotrophic LAB (38). For a selection of samples (identified in Table 2), a portion was homogenized in physiological solution and the first decimal dilution was used for culture-independent analysis of the microbiota. End products were collected only during the second sampling; stored until the end of the shelf life, when they were eventually analyzed microbiologically (see Table 4); and used for pyrosequencing analysis as well. Statistical analysis by paired t test with a 95% level of confidence was performed as well, comparing the counts determined at 22 and 30°C.
TABLE 2.
Microbial counts in all samples of raw materials, processed constituents, and intermediate products determined during preenrichment enumerationa
| Visit and sample | Sample description | Count (avg log no. of CFU/g ± SD) |
Underestimation (log no. of CFU/g) at 30°C (medium) | |||||
|---|---|---|---|---|---|---|---|---|
| PCA |
RCA |
MRS |
||||||
| 22°C | 30°C | 22°C | 30°C | 22°C | 30°C | |||
| 1st company visit | ||||||||
| I.1b | Whole green sweet bell pepper | 6.20 ± 0.11 | 6.08 ± 0.05 | 5.31 ± 0.03 | 5.25 ± 0.02 | <3 | <3 | |
| I.2 | Whole yellow sweet bell pepper | 5.51 ± 0.07 | 5.46 ± 0.03 | <3 | <3 | <3 | <3 | |
| I.3 | Whole red sweet bell pepper | 5.57 ± 0.05 | 5.55 ± 0.09 | 4.05 ± 0.10 | 3.92 ± 0.08 | <3 | <3 | |
| I.4 | Cut green sweet bell pepper | 6.31 ± 0.07 | 5.98 ± 0.12 | 4.87 ± 0.15 | 4.75 ± 0.18 | <3 | <3 | |
| I.5b | Cut yellow sweet bell pepper | <3 | <3 | 3.77 ± 0.07 | 3.33 ± 0.35 | 3.30 ± 0.00 | 3.00 ± 0.00 | |
| I.6 | Cut red sweet bell pepper | 5.19 ± 0.13 | 5.12 ± 0.11 | <3 | <3 | <3 | <3 | |
| I.7 | Whole peeled carrots | 7.31 ± 0.01 | 7.26 ± 0.05 | 6.52 ± 0.06 | 6.34 ± 0.05 | 3.95 ± 0.05 | 3.69 ± 0.09 | |
| I.8b | Sliced red sweet bell pepper | 3.74 ± 0.13 | 3.69 ± 0.09 | <3 | <3 | <3 | <3 | |
| I.9 | Sliced yellow sweet bell pepper | 4.23 ± 0.03 | 4.21 ± 0.04 | 3.91 ± 0.12 | <3 | 3.69 ± 0.09 | <3 | >0.91 (RCA) |
| I.10b | Sliced red sweet bell pepper | 6.59 ± 0.02 | 6.58 ± 0.02 | 6.55 ± 0.12 | 6.52 ± 0.10 | 3.77 ± 0.00 | <3 | >0.77 (MRS) |
| I.11b | Sliced yellow sweet bell pepper | 7.11 ± 0.03 | 7.07 ± 0.01 | 6.50 ± 0.06 | 6.41 ± 0.05 | 4.57 ± 0.04 | 3.92 ± 0.20 | 0.65 (MRS) |
| I.12 | Halved eggs | 5.29 ± 0.19 | 5.03 ± 0.11 | 3.94 ± 0.14 | <3 | 4.01 ± 0.06 | 3.52 ± 0.07 | >0.94 (RCA) |
| I.13b | Carrot sticks | 7.35 ± 0.02 | 7.31 ± 0.02 | 6.83 ± 0.03 | 6.79 ± 0.07 | 5.52 ± 0.07 | 5.19 ± 0.14 | |
| I.14 | Curly carrot sticks | 7.35 ± 0.02 | 7.25 ± 0.02 | 7.02 ± 0.03 | 6.96 ± 0.04 | 4.67 ± 0.02 | 4.31 ± 0.12 | |
| I.15 | Onion rings | 7.15 ± 0.01 | 7.04 ± 0.05 | 7.01 ± 0.03 | 6.98 ± 0.04 | <3 | <3 | |
| I.16 | Mix (peppers, broccoli, corn, peas) | 7.31 ± 0.03 | 7.30 ± 0.02 | 6.82 ± 0.07 | 6.75 ± 0.13 | 5.15 ± 0.07 | 4.39 ± 0.09 | 0.76 (MRS) |
| I.17b | Wok mix (peppers, carrot, leek) | 7.26 ± 0.02 | 7.23 ± 0.03 | 6.91 ± 0.05 | 6.83 ± 0.03 | 5.53 ± 0.01 | 4.99 ± 0.13 | 0.55 (MRS) |
| I.18b | Tuna | 4.11 ± 0.23 | 4.01 ± 0.37 | <3 | <3 | <3 | <3 | |
| I.19 | Shrimp | 3.96 ± 0.24 | 3.67 ± 0.35 | <3 | <3 | <3 | <3 | |
| I.20b | Chicken cubes | 6.86 ± 0.04 | 6.81 ± 0.07 | 6.59 ± 0.08 | 6.56 ± 0.15 | 5.39 ± 0.11 | 5.22 ± 0.11 | |
| 2nd company visit | ||||||||
| II.1b | Mix (carrot, cabbage, peppers, lettuce, beans) | 5.92 ± 0.01 | 5.89 ± 0.03 | 5.10 ± 0.03 | 5.03 ± 0.02 | 4.45 ± 0.03 | 4.40 ± 0.02 | |
| II.2b | Quinoa with peas | 4.89 ± 0.06 | 4.85 ± 0.06 | 4.67 ± 0.06 | 4.57 ± 0.02 | 4.33 ± 0.04 | 4.28 ± 0.07 | |
| II.3 | Lettuce | 6.70 ± 0.05 | 6.62 ± 0.06 | 5.62 ± 0.02 | 5.58 ± 0.04 | <3 | <3 | |
| II.4 | Tuna | 6.56 ± 0.04 | 6.40 ± 0.05 | 5.01 ± 0.06 | 5.05 ± 0.08 | 6.51 ± 0.03 | 6.45 ± 0.02 | |
| II.5b | Bulgur tandoori | 5.28 ± 0.07 | 5.23 ± 0.03 | 4.40 ± 0.04 | 4.26 ± 0.10 | 3.58 ± 0.17 | 3.42 ± 0.10 | |
| II.6 | Mix (carrot, cabbage, peppers, lettuce, beans) | 5.46 ± 0.01 | 5.44 ± 0.02 | 5.05 ± 0.13 | 5.02 ± 0.08 | 4.44 ± 0.07 | 4.40 ± 0.04 | |
| II.7 | Mix (carrot, cabbage, sauce) | 4.88 ± 0.09 | 4.86 ± 0.09 | 4.40 ± 0.02 | 4.31 ± 0.08 | 3.90 ± 0.05 | 3.84 ± 0.06 | |
| II.8 | Sardines | <3 | <3 | <3 | <3 | <3 | <3 | |
| II.9b | Halved eggs | <3 | <3 | <3 | <3 | <3 | <3 | |
| II.10b | Sliced green sweet bell peppers | 7.23 ± 0.02 | 7.18 ± 0.03 | 7.16 ± 0.05 | 7.09 ± 0.02 | 4.57 ± 0.07 | 3.58 ± 0.27 | 0.99 (MRS) |
| II.11b | Sliced red sweet bell peppers | 7.47 ± 0.04 | 7.42 ± 0.04 | 7.42 ± 0.02 | 7.35 ± 0.01 | 4.98 ± 0.04 | 4.16 ± 0.15 | 0.82 (MRS) |
| II.12 | Farfalle (carrot, peppers, chicken, rocket, tomato) | 6.00 ± 0.04 | 5.75 ± 0.18 | 4.35 ± 0.08 | 4.05 ± 0.08 | 3.46 ± 0.15 | <3 | |
| II.13 | Spirelli (carrot, zucchini) | 5.38 ± 0.00 | 5.25 ± 0.05 | 4.37 ± 0.07 | 4.28 ± 0.02 | 3.98 ± 0.07 | 3.69 ± 0.08 | |
| II.14b | Mix (sweet bell peppers, broccoli, corn) | 6.99 ± 0.01 | 6.86 ± 0.02 | 5.52 ± 0.09 | 5.40 ± 0.04 | 5.04 ± 0.04 | 5.01 ± 0.02 | |
| II.15 | Mix (cucumber, tomato, carrot) | 7.53 ± 0.02 | 7.48 ± 0.02 | 6.88 ± 0.04 | 6.81 ± 0.04 | 4.72 ± 0.05 | 4.34 ± 0.04 | |
| II.16 | Chicken pieces | 4.95 ± 0.05 | 4.36 ± 0.07 | 4.66 ± 0.02 | 4.60 ± 0.04 | 3.75 ± 0.05 | 3.10 ± 0.17 | 0.65 (MRS) |
| II.17 | Spirelli (ham, tomato, egg, parsley) | 4.40 ± 0.02 | 4.26 ± 0.00 | 3.10 ± 0.17 | 3.10 ± 0.17 | 3.42 ± 0.10 | <3 | |
| II.18b | Chicken cubes | 5.33 ± 0.10 | 5.12 ± 0.05 | 5.36 ± 0.07 | 5.23 ± 0.07 | 4.74 ± 0.13 | 3.86 ± 0.09 | 0.88 (MRS) |
| II.19 | Whole green sweet bell pepper | <3 | <3 | <3 | <3 | <3 | <3 | |
| II.20 | Whole yellow sweet bell pepper | 6.97 ± 0.01 | 6.95 ± 0.01 | 5.65 ± 0.06 | 5.58 ± 0.08 | <3 | <3 | |
| II.21 | Whole red sweet bell pepper | 7.45 ± 0.02 | 7.42 ± 0.04 | 5.77 ± 0.03 | 5.70 ± 0.01 | 4.00 ± 0.04 | 3.92 ± 0.08 | |
Preenrichment enumeration was performed immediately after recovery of the samples. The counts were generated on three media (i.e., plate count agar [PCA], MRS, and reinforced clostridial agar [RCA]) and at two different temperatures (i.e., 30 and 22°C). Plating was performed in triplicate.
Samples used for the pyrosequencing analysis.
TABLE 3.
Microbial counts in all samples of all raw materials, processed constituents, and intermediate products determined during postenrichment enumerationa
| Sample | Sample description | Count (avg log no. of CFU/g ± SD) |
Underestimation (log no. of CFU/g) at 30°C (medium) | |||||
|---|---|---|---|---|---|---|---|---|
| PCA |
RCA |
MRS |
||||||
| 22°C | 30°C | 22°C | 30°C | 22°C | 30°C | |||
| 1st company visit | ||||||||
| I.1 | Whole green sweet bell pepper | 7.67 ± 0.04 | 7.66 ± 0.04 | 7.66 ± 0.13 | 7.57 ± 0.37 | 7.64 ± 0.12 | 6.84 ± 0.06 | 0.80 (MRS) |
| I.2 | Whole yellow sweet bell pepper | 8.01 ± 0.02 | 7.90 ± 0.04 | 7.73 ± 0.07 | 7.69 ± 0.08 | 7.70 ± 0.01 | 7.36 ± 0.10 | |
| I.3 | Whole red sweet bell pepper | 7.98 ± 0.04 | 7.96 ± 0.05 | 7.80 ± 0.04 | 7.76 ± 0.03 | 6.52 ± 0.02 | 6.50 ± 0.02 | |
| I.4 | Cut green sweet bell pepper | 7.20 ± 0.03 | 7.01 ± 0.06 | 7.23 ± 0.05 | 7.11 ± 0.03 | <5 | <5 | |
| I.5 | Cut yellow sweet bell pepper | 7.84 ± 0.06 | 7.48 ± 0.02 | 7.38 ± 0.02 | 6.35 ± 0.08 | 7.55 ± 0.07 | 6.24 ± 0.07 | 1.31 (MRS) |
| I.6 | Cut red sweet bell pepper | 5.98 ± 0.19 | 5.79 ± 0.10 | <5 | <5 | <5 | <5 | |
| I.7 | Whole peeled carrots | 7.58 ± 0.02 | 7.48 ± 0.05 | 7.16 ± 0.08 | 7.12 ± 0.04 | 5.59 ± 0.11 | 5.36 ± 0.10 | |
| I.8 | Sliced red sweet bell pepper | 7.59 ± 0.04 | 7.58 ± 0.02 | 7.46 ± 0.03 | 7.32 ± 0.04 | 5.85 ± 0.15 | <5 | >0.85 (MRS) |
| I.9 | Sliced yellow sweet bell pepper | 8.86 ± 0.01 | 8.83 ± 0.01 | 8.79 ± 0.03 | 8.73 ± 0.05 | 8.86 ± 0.02 | 8.85 ± 0.01 | |
| I.10 | Sliced red sweet bell pepper | 8.78 ± 0.06 | 8.69 ± 0.08 | 8.70 ± 0.03 | 8.68 ± 0.01 | 8.49 ± 0.09 | 7.96 ± 0.05 | 0.53 (MRS) |
| I.11 | Sliced yellow sweet bell pepper | 8.98 ± 0.04 | 8.89 ± 0.04 | 8.32 ± 0.10 | 8.30 ± 0.02 | 8.56 ± 0.02 | 8.03 ± 0.11 | 0.52 (MRS) |
| I.12 | Halved eggs | 9.43 ± 0.02 | 9.37 ± 0.02 | 8.77 ± 0.04 | 8.72 ± 0.01 | 8.92 ± 0.05 | 8.78 ± 0.02 | |
| I.13 | Carrot sticks | 8.93 ± 0.07 | 8.90 ± 0.04 | 8.59 ± 0.03 | 8.38 ± 0.05 | 6.71 ± 0.04 | 6.12 ± 0.11 | 0.59 (MRS) |
| I.14 | Curly carrot sticks | 8.61 ± 0.04 | 8.56 ± 0.02 | 7.95 ± 0.02 | 7.78 ± 0.03 | <5 | <5 | |
| I.15 | Onion rings | 9.30 ± 0.02 | 9.25 ± 0.01 | 9.22 ± 0.01 | 9.19 ± 0.02 | 5.65 ± 0.00 | <5 | >0.65 (MRS) |
| I.16 | Mix (peppers, broccoli, corn, peas) | 9.38 ± 0.03 | 9.29 ± 0.02 | 9.10 ± 0.03 | 9.07 ± 0.02 | 8.54 ± 0.08 | 8.37 ± 0.05 | |
| I.17 | Wok mix (peppers, carrot, leek) | 9.09 ± 0.01 | 9.06 ± 0.02 | 8.94 ± 0.04 | 8.89 ± 0.02 | 8.16 ± 0.14 | 7.64 ± 0.02 | 0.53 (MRS) |
| I.18 | Tuna | 8.13 ± 0.11 | 7.92 ± 0.08 | 7.88 ± 0.04 | 7.84 ± 0.01 | 7.40 ± 0.04 | 6.67 ± 0.02 | 0.73 (MRS) |
| I.19 | Shrimp | 6.01 ± 0.15 | 6.08 ± 0.04 | <5 | <5 | <5 | <5 | |
| I.20 | Chicken cubes | 9.24 ± 0.03 | 9.20 ± 0.02 | 8.93 ± 0.02 | 8.88 ± 0.07 | 8.27 ± 0.08 | 7.61 ± 0.02 | 0.66 (MRS) |
| 2nd company visit | ||||||||
| II.1 | Mix (carrot, cabbage, peppers, lettuce, beans) | 9.20 ± 0.02 | 9.10 ± 0.03 | 8.47 ± 0.05 | 8.04 ± 0.04 | 8.60 ± 0.03 | 7.10 ± 0.10 | 1.50 (MRS) |
| II.2 | Quinoa with peas | 9.44 ± 0.04 | 9.37 ± 0.01 | 8.94 ± 0.06 | 7.48 ± 0.08 | 9.02 ± 0.04 | 8.33 ± 0.07 | 1.46 (RCA) |
| II.3 | Lettuce | 9.44 ± 0.01 | 9.43 ± 0.03 | 9.12 ± 0.03 | 9.09 ± 0.02 | 7.44 ± 0.04 | 7.3 ± 0.03 | |
| II.4 | Tuna | 9.29 ± 0.03 | 9.22 ± 0.03 | 8.68 ± 0.09 | 8.68 ± 0.10 | <5 | <5 | |
| II.5 | Bulgur tandoori | 6.91 ± 0.06 | 6.75 ± 0.03 | 6.06 ± 0.06 | 5.88 ± 0.09 | 6.04 ± 0.04 | <5 | >1.04 (MRS) |
| II.6 | Mix (carrot, cabbage, peppers, lettuce, beans) | 9.28 ± 0.02 | 9.21 ± 0.02 | 8.66 ± 0.04 | 8.63 ± 0.05 | 8.58 ± 0.02 | 8.48 ± 0.07 | |
| II.7 | Mix (carrot, cabbage, sauce) | <5 | <5 | <5 | <5 | <5 | <5 | |
| II.8 | Sardines | <5 | <5 | <5 | <5 | <5 | <5 | |
| II.9 | Halved eggs | 6.26 ± 0.04 | <5 | 6.14 ± 0.06 | 5.88 ± 0.10 | 5.84 ± 0.02 | <5 | >1.26 (PCA) |
| II.10 | Sliced green sweet bell peppers | 8.80 ± 0.05 | 8.62 ± 0.03 | 6.16 ± 0.08 | 5.95 ± 0.05 | 5.84 ± 0.07 | <5 | >0.84 (MRS) |
| II.11 | Sliced red sweet bell peppers | 9.38 ± 0.02 | 9.35 ± 0.02 | 8.96 ± 0.01 | 8.25 ± 0.06 | 9.05 ± 0.06 | 8.34 ± 0.05 | >0.71 (MRS) |
| II.12 | Farfalle (carrot, peppers, chicken, rocket, tomato) | 6.09 ± 0.09 | 5.93 ± 0.08 | <5 | <5 | 5.46 ± 0.15 | 5.36 ± 0.32 | |
| II.13 | Spirelli (carrot, zucchini) | 5.46 ± 0.15 | 5.42 ± 0.10 | 5.46 ± 0.15 | 5.10 ± 0.17 | 5.10 ± 0.17 | 5.10 ± 0.17 | |
| II.14 | Mix (sweet bell peppers, broccoli, corn) | 9.16 ± 0.03 | 7.44 ± 0.07 | 8.79 ± 0.01 | 8.74 ± 0.08 | 9.11 ± 0.03 | 6.77 ± 0.03 | 2.34 (MRS) |
| II.15 | Mix (cucumber, tomato, carrot) | 8.73 ± 0.06 | 8.27 ± 0.05 | 8.42 ± 0.05 | 8.00 ± 0.04 | 8.65 ± 0.07 | 7.64 ± 0.04 | 1.01 (MRS) |
| II.16 | Chicken pieces | 7.90 ± 0.02 | 7.76 ± 0.04 | 8.65 ± 0.08 | 8.44 ± 0.06 | 7.80 ± 0.04 | 7.71 ± 0.04 | |
| II.17 | Spirelli (ham, tomato, egg, parsley) | <5 | <5 | <5 | <5 | <5 | <5 | |
| II.18 | Chicken cubes | 7.89 ± 0.08 | 7.74 ± 0.08 | 8.93 ± 0.04 | 8.79 ± 0.01 | 7.76 ± 0.02 | 7.22 ± 0.18 | 0.54 (MRS) |
| II.19 | Whole green sweet bell pepper | <5 | <5 | <5 | <5 | <5 | <5 | |
| II.20 | Whole yellow sweet bell pepper | 6.29 ± 0.07 | 6.09 ± 0.05 | <5 | <5 | <5 | <5 | |
| II.21 | Whole red sweet bell pepper | <5 | <5 | <5 | <5 | <5 | <5 | |
Postenrichment enumeration was performed after 10 days of anaerobic incubation at 4°C. This enrichment procedure was used to select for psychrotrophic LAB. Plating was performed in triplicate. PCA, plate count agar; RCA, reinforced clostridial agar.
TABLE 4.
Results of microbiological analysis of end products at end of shelf life based on comparative enumeration by mesophilic (30°C) and psychrotrophic (22°C) incubationa
| Sample | End product | Count (avg log no. of CFU/g ± SD) |
Underestimation (log no. of CFU/g) at 30°Cb | |||||
|---|---|---|---|---|---|---|---|---|
| PCA |
RCA |
MRS |
||||||
| 22°C | 30°C | 22°C | 30°C | 22°C | 30°C | |||
| EP.1 | Farfalle (carrot, peppers, chicken, rocket, tomato) | 8.45 ± 0.00 | 7.46 ± 0.03 | 8.32 ± 0.02 | 7.46 ± 0.08 | 8.39 ± 0.08 | 7.09 ± 0.05 | 1.30 |
| EP.2 | Niçoise salad | 8.77 ± 0.02 | 8.25 ± 0.06 | 8.67 ± 0.04 | 7.69 ± 0.06 | 8.75 ± 0.06 | 6.97 ± 0.02 | 1.78 |
| EP.3 | Noodle pasta with shrimp and peppers | 9.29 ± 0.02 | 8.94 ± 0.03 | 9.02 ± 0.02 | 7.83 ± 0.03 | 9.16 ± 0.04 | 7.70 ± 0.02 | 1.46 |
| EP.4 | Potato salad with bacon | 7.57 ± 0.05 | 7.53 ± 0.08 | 7.59 ± 0.02 | 7.20 ± 0.10 | 7.39 ± 0.09 | 6.10 ± 0.04 | 1.29 |
| EP.5 | Bulgur tandoori | 7.34 ± 0.09 | 6.17 ± 0.13 | 7.11 ± 0.03 | 5.73 ± 0.05 | 7.16 ± 0.16 | 5.69 ± 0.09 | 1.47 |
| EP.6 | Quinoa with carrot and beans | 8.86 ± 0.05 | 8.43 ± 0.06 | 8.95 ± 0.13 | 8.20 ± 0.03 | 9.16 ± 0.04 | 8.30 ± 0.02 | 0.85 |
All samples were used for the pyrosequencing analysis. Plating was performed in triplicate. PCA, plate count agar; RCA, reinforced clostridial agar.
Underestimation on MRS medium.
Culture-independent DNA extraction, 16S rRNA gene amplicon library preparation, and sequencing.
Extraction of DNA from selected foods (see Tables 2 and 4) and all the environmental samples was performed using a Biostic bacteremia DNA isolation kit (Mo Bio Laboratories, Inc., Carlsbad, CA). The aforementioned cell suspensions obtained from surface swabs, water samples, and food material were pelleted at 120,006 × g for 1 min, and the extraction protocol was applied following the instructions of the manufacturer.
The microbial diversity was evaluated by pyrosequencing of the V1 to V3 hypervariable region of the 16S rRNA gene. The primers and PCR conditions for the amplification of 520-bp fragments have been described previously (40). 454 adaptors were added to the amplicons obtained with the forward primer, followed by addition of a 10-bp sample-specific multiplex identifier (MID). The PCR products were subjected to agarose gel electrophoresis and were subsequently purified two times using an Agencourt AMPure kit (Beckman Coulter, Milan, Italy) and quantified using a QuantiFluor system (Promega, Milan, Italy). An equimolar pool was then generated. The amplicon pool was subjected to pyrosequencing on a GS Junior platform (454 Life Sciences, Roche, Italy) using titanium chemistry according to the specifications of the manufacturer.
Bioinformatics.
Raw reads were first filtered according to the 454 processing pipeline. The sequences were then analyzed and further filtered using QIIME (quantitative insights into microbial ecology), version 1.8.0, software (41). In order to guarantee a higher level of accuracy in terms of operational taxonomic unit (OTU) detection, after the split library script was performed by QIIME, reads were excluded from the analysis if they had an average quality score of less than 25, if they were shorter than 300 bp, and if there were ambiguous base calls. OTUs defined at 99% similarity were picked using the uclust method (42), and representative sequences were submitted to analysis with the RDPII classifier (43) to obtain a taxonomy assignment using the Greengenes 16S rRNA gene database (44). OTUs identified to be chloroplasts were filtered out before further analyses. Alpha and beta diversities were evaluated by QIIME, as previously reported (39). The correlation analysis was carried out using the psych package in the R environment to identify patterns of co-occurrence/coexclusion between OTUs. Co-occurrence/coexclusion matrices were plotted using the corrplot package in R. Only significant correlations (false discovery rate [FDR] < 0.05) were considered.
Metagenome prediction.
PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states; http://picrust.github.io/picrust/), a bioinformatics tool that predicts the abundance of gene families on the basis of the 16S rRNA-based structure of the microbiota (45), was used to predict the functional profiles of the microbial communities in the subsets of ingredients and end products. For the analysis with PICRUSt, using the closed reference method, OTUs with 97% identity to sequences in the Greengenes database (May 2013 version) were picked using QIIME, version 1.8. Data were normalized for 16S rRNA copy numbers, and the metagenomes were predicted. From the inferred metagenomes, orthologs in the Kyoto Encyclopedia of Genes and Genomes (KEGG) were identified, and the table obtained was rarefied at the lowest number of sequences per sample. KEGG orthologs were then collapsed at level 3 of the hierarchy, and the resulting table was imported into R (www.r-project.org). The made4 package was used to produce a heat plot by using hierarchical Ward-linkage clustering based on the Spearman correlation coefficients of the proportion of activities belonging to carbohydrates and amino acid metabolism pathways. Nearest sequenced taxon indexes (NSTIs) were calculated in order to evaluate the accuracy of the metagenome predictions, which depends on how closely related the microbes in a given sample are to microbes with sequenced genome representatives; NSTIs with lower values indicate a closer mean relationship (45). In order to compare the samples in this study on the basis of the predicted metagenomes, a nonphylogenetic Bray-Curtis-based principal coordinate analysis (PCoA) was implemented in QIIME using the whole set of data on gene abundances produced by PICRUSt.
Nucleotide sequence accession number.
The 16S rRNA gene sequences are available at the Sequence Read Archive of NCBI (accession number SRP049628).
RESULTS
Microbiological analysis.
The initial contamination levels (Table 2), based on total psychrotrophic organism counts, were very high (5.3 to 7.5 log CFU/g) for numerous raw materials (samples I.1 to I.3, I.7, II.20, and II.21) and intermediate products (samples I.4, I.10 to I.17, I.20, II.1, II.3 to II.6, II.10 to II.15, and II.18), while the counts for the populations of LAB were 2 to 3 log units lower and were even below 3 log CFU/g in several cases (samples I.1 to I.4, I.15, II.3, and II.20). Vegetable material (e.g., sweet bell peppers, carrots, lettuce, onions) especially exhibited high total psychrotrophic organism counts but had very low levels of occurrence of LAB (Table 2). Comparison of the two incubation temperatures showed that the counts at 22°C were significantly higher (P ≤ 0.05) than the counts at 30°C for 10 samples (Table 2). The same was observed in 20 samples during the second enumeration (postenrichment), suggesting the presence of very small populations of psychrotrophic microbiota in the initial samples that grew competently during the anaerobic cold storage (Table 3). Sweet bell peppers, eggs, chicken pieces, tuna, and intermediate products containing peppers were mainly the samples where overlooked psychrotrophic microbial populations unable to grow at 30°C were found. It is also interesting that the populations of LAB increased considerably during anaerobic storage, and from very low densities they became dominant and equaled the total psychrotrophic organism count (e.g., for samples I.1, I.5, I.9 to I.12, II.2, II.11, II.14, II.15, and II.18). Eventually, for the small number of sampled end products, a sour off odor as well as a high underestimation on MRS medium was observed (0.85 to 1.78 log CFU/g) for all RTE meals (Table 4) at the end of the shelf life.
16S rRNA gene sequencing-based structure of the microbiota.
A total of 307,732 reads passed the filters applied through the QIIME split_library.py script, with an average value of 3,489 reads per sample after the chloroplast filtering and an average length of 462 bp being obtained. The number of OTUs, Good's estimated sample coverage (ESC), and the Chao1 and Shannon indices were obtained for all the samples (see Table S1 in the supplemental material). In general, the alpha diversity showed that environmental samples and some of the ingredients showed higher values of diversity indices than the other samples (see Table S1 in the supplemental material). The estimated sample coverage was satisfactory for 90% of the samples.
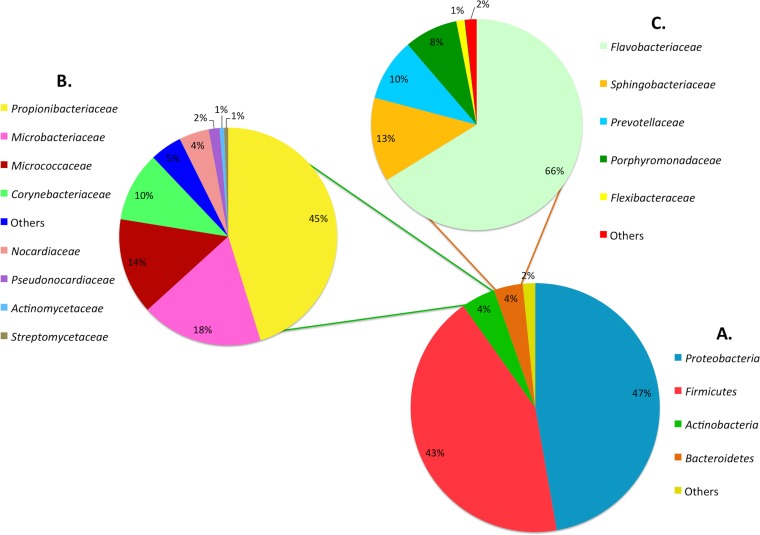
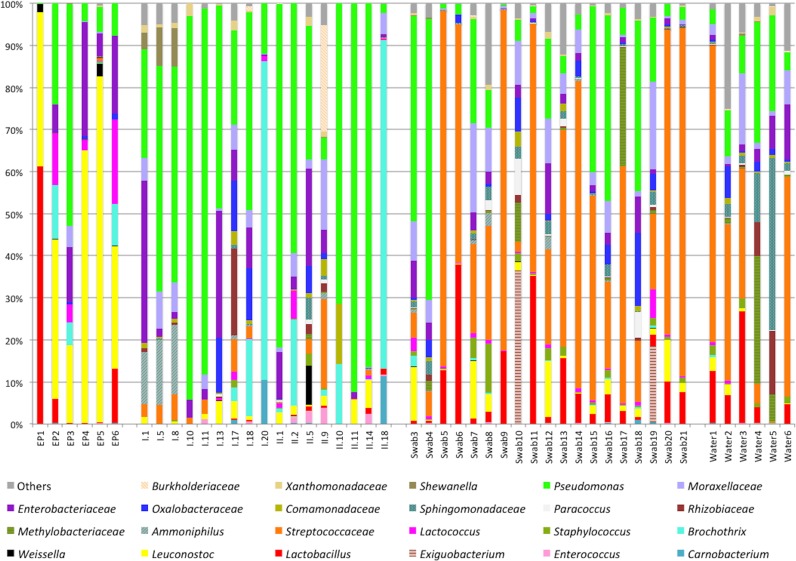
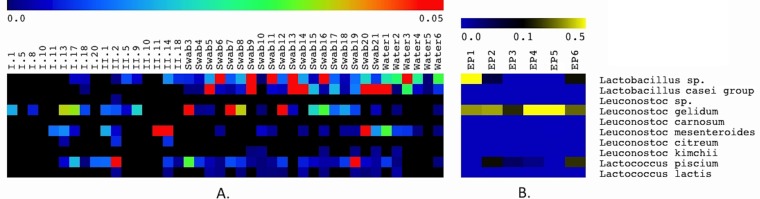
For the entire study, the majority of OTUs were attributed to the phyla Proteobacteria (47%) and Firmicutes (43%), which were more abundant in food (Fig. 2). On the other hand, the phyla Actinobacteria and Bacteriodetes were more sporadically encountered, being mainly encountered on equipment and premises, and represented about 8% of the identified OTUs; major families belonging to these two phyla are reported in Fig. 2. For the food ingredients, Pseudomonas and Enterobacteriaceae were the most abundant OTUs, shared by almost all samples (Fig. 3), while Brochothrix and Carnobacterium were also high in chicken cubes and tuna (i.e., samples I.18, I.20, and II.18). In surface swab and water samples, a core microbiota of Streptococcaceae, Moraxellaceae, Pseudomonas, and Lactobacillus was found. Leuconostocs were present in 12 food samples at low abundances ranging from 0.4 to 6.5% as well as in 24 environmental samples with incidences of between 0.05 and 12.3% (Fig. 3). Leuconostoc gelidum and Leuconostoc mesenteroides were the most prevalent among the Leuconostoc species (Fig. 4). Despite the low initial populations of LAB (Table 2) and the very low abundances of leuconostocs (Fig. 3), psychrotrophic L. gelidum was the most abundant taxon at the end of the shelf life, representing 18.6 to 81% of the spoilage-related microbial consortium in all spoiled end products (Fig. 4). L. gelidum was the only Leuconostoc species found to be predominant at the end of storage. Additionally, a Lactobacillus sp., a Pseudomonas sp., Lactococcus piscium, a Brochothrix sp., Enterobacteriaceae, and a Weissella sp. were also found to be presumptive spoilage microbiota.
FIG 2.
Distribution of the identified OTUs of bacterial phyla. (A) The proportion of each phylum was calculated on the basis of its relative abundance in each sample (n = 48). (B and C) Bacterial families identified for the minor members assigned to the Actinobacteria (B) and Bacteriodetes (C) are summarized.
FIG 3.
Abundance (in percent) of bacterial families and genera belonging to the two major phyla (i.e., Firmicutes and Proteobacteria) obtained by 16S rRNA gene pyrosequencing analysis. Only OTUs with abundance values above 0.1% in at least five samples are shown.
FIG 4.
Pseudo-heat maps showing species diversity and relative abundance of species of LAB belonging to the genera Lactobacillus, Leuconostoc, and Lactococcus. Assignment of the sequences to the species level was performed by manually aligning representative sequences from all the amplicon read clusters attributed to each genus using the BLAST search tool (http://www.ncbi.nlm.nih.gov/blast). The similarity score in all cases was greater than 97% when the sequences were compared to well-described 16S rRNA sequences. The color scale at the top indicates the percentages of the species in ingredients and environmental samples (i.e., 0 to 5%) (A) and in spoiled end products (i.e., 0 to 50%) (B).
With respect to the different areas of the plant, L. gelidum was more abundant in the vegetable preparation area (i.e., domain 2), whereas Lactobacillus spp. were detected on the empty, sanitized equipment of domain 3, where processed vegetables were assembled. In domain 4 (mixing area), surface swab samples (i.e., swab samples 1 and 2) from the clean utensils yielded no DNA, but L. gelidum was found in the equipment in use (Fig. 4). Moreover, L. gelidum was also present on the surface of the pasta-cooling vessel (i.e., domain 5), along with Lactococcus piscium. Lactobacillus and Leuconostoc mesenteroides were found primarily in water samples, in the surface swabs of the production lines (i.e., domain 6), and on the gloves of the personnel. Interestingly, L. gelidum, L. piscium, and lactobacilli were also found on the walls of the refrigerators (i.e., refrigerators B, C, and D) (Table 1).
Beta diversity and co-occurrence/coexclusion patterns.
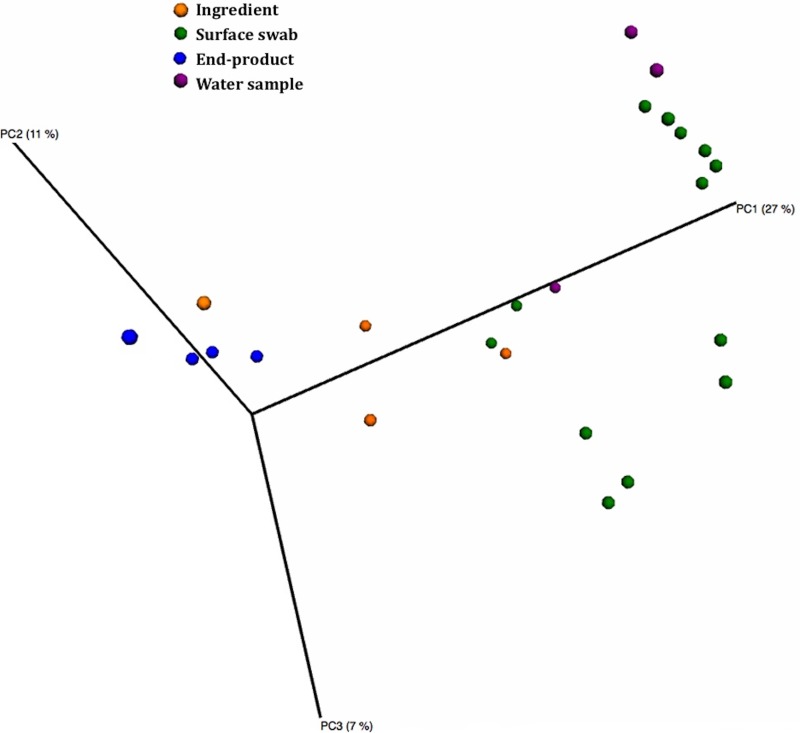
The results of analysis of the beta diversity, based on unweighted UniFrac analysis, indicated that the four different types of samples (i.e., water, surface swabs, ingredients, and end products) could be clearly distinguished on the basis of their microbiota (Fig. 5). The spoiled food samples, characterized by a lower microbial diversity and an abundance of only selected species, formed a discrete group that was close to the ingredients and distinguished from the environmental samples.
FIG 5.
Principal coordinate analysis of jackknifed unweighted UniFrac distances for the 16S rRNA gene sequence data.
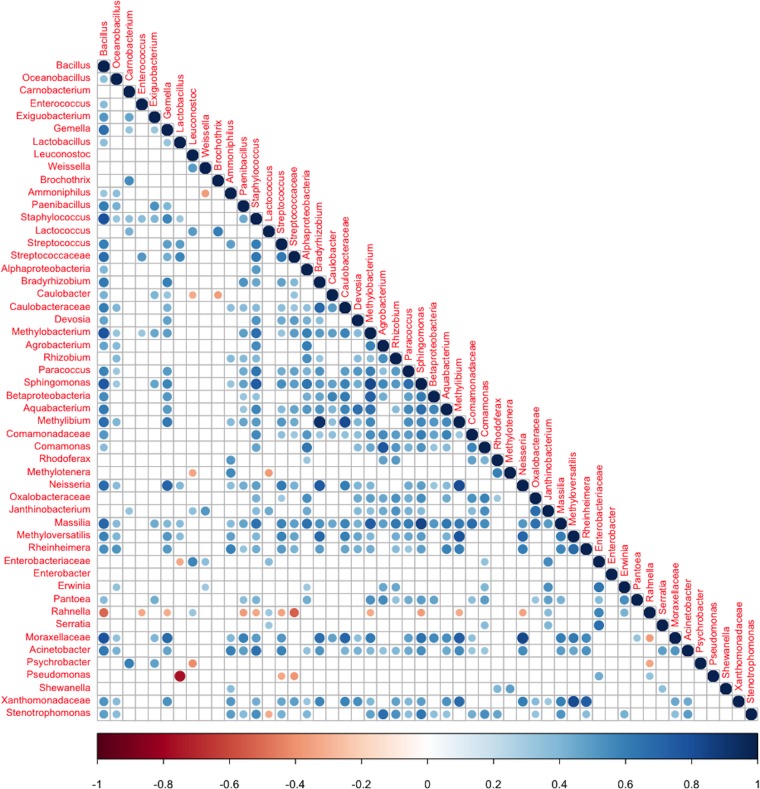
Analysis of the significant (FDR < 0.05) co-occurrence/coexclusion relationships of the identified OTUs demonstrated a strong coexclusion between Pseudomonas and Lactobacillus and another significant coexclusion between Pseudomonas and Streptococcaceae (Fig. 6). Remarkably, Rahnella was present to the exclusion of Streptococcaceae and several other OTUs, whereas Bacillus and Staphylococcus co-occurred with several other OTUs (Fig. 6).
FIG 6.
Significant co-occurrence and coexclusion relationships between bacterial OTUs. Spearman's rank correlation matrix of OTUs with an abundance of ≥0.1% in at least 5 samples. Only phylotypes assigned to the phyla Proteobacteria and Firmicutes were considered. Strong correlations are indicated by large circles, whereas weak correlations are indicated by small circles. The colors of the scale bar denote the nature of the correlation, with 1 indicating a perfectly positive correlation (dark blue) and −1 indicating a perfectly negative correlation (dark red) between two phylotypes. Only significant correlations (FDR < 0.05) are shown.
Diversity of predicted metagenomes.
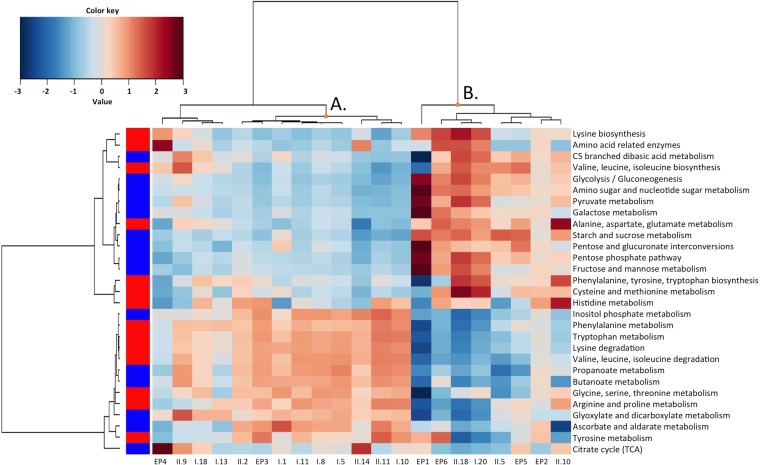
The PICRUSt tool was used to predict the metagenomes by comparison of the sequences recovered with the sequences in the 16S rRNA gene sequence database (45, 46). The weighted nearest sequenced taxon index (NSTI) for the samples of the present study was 0.092 ± 0.064. The developers of the PICRUSt tool (45) found that human-associated samples had the lowest (best) NSTI values (0.03 ± 0.2), thanks to the large number of sequenced genomes available, whereas other mammalian gut or soil samples had mean NSTI values above 0.1. Considering the whole set of samples (including water, swabs, ingredients, and end products), differentiation between the different groups of samples obtained from the Bray-Curtis distance on the basis of the predicted gene abundance showed a certain extent of distinction between ingredients and environmental samples, while the end products did not cluster together (see Fig. S1 in the supplemental material). Regarding raw materials and end products, they could be clearly differentiated on the basis of the tentative gene repertoires associated with their microbiota when genes related to amino acid and carbohydrate metabolism were taken into account (Fig. 7). A cluster with samples mainly derived from the first domains of the plant, basically encompassing raw materials (cluster A), and a cluster with end products and processed ingredients originating from the final production steps (cluster B) were identified. It could be inferred that the dominant spoilage-related biota at the end of the shelf life could be regarded as a microbial consortium presumably associated with a fast energy yield due to carbohydrate depletion. The other cluster showed a higher presumptive abundance of genes related to amino acid metabolism and metabolism of short-chain organic acids, such as propanoate and butanoate.
FIG 7.
Heat plot of the abundances of genes presumptively belonging to carbohydrate (blue squares) and amino acid (red squares) metabolism pathways in the ingredients and end products. A hierarchical Ward linkage based on the Spearman correlation coefficients was used for the clustering. TCA, tricarboxylic acid.
DISCUSSION
Apart from the ecological aspects characterizing a food matrix, the interaction of foodstuffs with the environment is equally crucial concerning the establishment of a microbial community. Therefore, high-quality raw materials, good hygiene practices, thorough sanitation of food contact surfaces, and effective preservation technologies need to be used in combination (47, 48) in order to ensure acceptable organoleptic properties from the moment that food products are retailed in the market until the end of their shelf life (49, 50). Unprocessed or minimally processed foodstuffs, like vegetable-based RTE meals, are appreciated by consumers because they contain fewer additives, are convenient, and are not subjected to invasive preservation methods that have a detrimental impact on nutrients (49, 51). However, they are a priori inhabited by large numbers of ubiquitous microorganisms and are thus considered highly perishable (52), unstable, and more prone to spoilage manifestations from LAB (26, 50, 53). In this study, widely diverse microbes were described in the ingredients and environmental samples from the RTE meal processing plant. Pseudomonas, Lactobacillus, and Streptococcaceae were the most abundant OTUs, and interesting findings came from the analysis of the significant coexclusion patterns, where it was shown that Pseudomonas and Lactobacillus/Streptococcaceae are coexcluded in these commodities. Additionally, the structure of the microbiota allowed the differentiation of the analyzed end products on the basis of the UniFrac distance, confirming a strict selection of taxa among the initial biota during storage. Members of the LAB allocated to the family Streptococcaceae (with Streptococcus thermophilus being the most abundant OUT; data not shown) were found only on surfaces and in water samples, for which microbiological counts (i.e., contamination levels and viability) were not determined, but were absent in the intermediate samples and the end products. Their occurrence could be attributed to the dairy products handled in-house, which could have facilitated this environmental spreading. On the other hand, the thermophilic character of dairy starter cultures could explain their absence during cold storage. Pseudomonas was encountered in the majority of raw materials and environmental sites and constituted the most largely represented OTU, as reported in a previous study, in which it was a typical contaminant of fresh produce and meat carcasses (27, 54). Additionally, among the species of LAB, Pseudomonas was highly abundant in two spoiled end products (Fig. 3). However, it should be emphasized that all the raw materials were highly contaminated with species of non-LAB (total psychrotrophic organism count range, 5 to 7 log CFU/g) from the start. On the contrary, all end products were dominated by psychrotrophic LAB that emerged from very low initial contamination levels and dictated the spoilage pattern (i.e., acidification and a sour off odor). This finding underpins the significance of the community of LAB in relation to the wide microbial diversity observed. Predicted metagenomes were useful for observation of putative gene repertoires for the samples analyzed in this study (46) but did not support a clear explanation of the mechanisms of adaptation of the microbiota to the specific environment. Considering the ingredients and end products and the presumptive genes involved in carbohydrate and amino acid metabolism, the spoiled RTE meals appeared to be contaminated by microorganisms presumably associated with a fast energy yield due to carbohydrate depletion. This is in accordance with the prototrophic and fastidious nature of highly competitive LAB (8, 9) that dominated at the end of the shelf life. Among these species of psychrotrophic LAB, Leuconostoc gelidum prevailed.
Leuconostoc gelidum subsp. gasicomitatum has been extensively studied for its involvement in extreme cases of spoilage in Finland (12, 55–60). Recently, L. gelidum subsp. gasicomitatum and L. gelidum subsp. gelidum were also identified to be the most predominant spoilage-associated microbes for packaged and refrigerated foodstuffs in Belgium (6, 7). To our knowledge, all references to L. gelidum subsp. gasicomitatum describe a potent spoilage microbe, and in the few studies in which it represented a fermentation-specific microorganism, its dominance among other members of the LAB was highlighted (61–63).
In the present study, the levels of LAB in food ingredients was low and in several cases was <3 log CFU/g, while the total psychrotrophic organism count ranged from 6 to 7 log CFU/g. Despite the low initial level of contamination, psychrotrophic members of Leuconostoc, Lactobacillus, and Lactococcus prevailed at the end of the shelf life. This corroborates the competitiveness of psychrotrophic LAB (64, 65) and especially leuconostocs (66), which occur at very low numbers but proliferate rapidly during storage. L. gelidum was detected at a low abundance in 25 samples subjected to 16S rRNA pyrosequencing analysis (Fig. 4), however, suggesting its wide occurrence in food materials as well as the processing environment and the premises. In swab and water samples, the relative abundance of leuconostocs was <10% in the majority of cases. These findings are in agreement with those of our previous study on RTE vegetable salads (38). L. gelidum subsp. gasicomitatum and L. gelidum subsp. gelidum were isolated from surfaces, air, and water (before and after disinfection) as well as from raw materials, especially sweet bell peppers. Similar to the findings of the present case study, all the manufactured RTE salads had been cross contaminated and exhibited acute acidification at the end of the shelf life, and counts were significantly underestimated when mesophilic incubation was performed. Apparently, these taxa of LAB occur as environmental contaminants belonging to the house microbiota and also as a microbial group introduced into the food-processing environment by crude material (12, 38, 55). The ability of certain L. gelidum subsp. gasicomitatum biotypes to adhere strongly to food contact surfaces, forming dense cell aggregations that could presumably facilitate habitation in production lines, has also been investigated (67).
Monitoring of microbial contamination in industrial plants facilitates acquisition of an in-depth understanding of the patterns through which spoilage-related microorganisms thrive, contaminate, survive, prevail, circulate, or succeed each other under realistic conditions. Source-tracking studies are based on the detection of distinct microbial groups, species, or strains within a heterogeneous context, providing valuable information about how and when contamination occurs (68, 69). Tracing of the origin of microbial contamination is usually implemented in epidemiological investigations determining the primary sources of pathogens that inflict foodborne illnesses. Therefore, the output of such applications is crucial for food safety and the prevention of outbreaks that could potentially jeopardize public health (70). The occurrence of virulent microbes is often indicative of poor standards of hygiene (69), but the presence of microorganisms naturally present in food commodities, like LAB, is expected. Apparently, raw, whole untreated vegetable material and processed food ingredients of all types harbor Leuconostoc gelidum. Moreover, Leuconostoc gelidum strains possess the physiological mechanisms needed to colonize different sites of a food-processing environment (67), the ability to adapt to low temperatures in practically any food matrix (7, 71), and fast outgrowth from very small populations. All these characteristics classify Leuconostoc gelidum as a unique specimen of spoilage potency, and methods for its rapid detection would be valuable for food industries.
In 2011, the first inquiry into the products of the RTE meal manufacturing plant described here documented a sporadic spoilage problem (5) related to the presence of psychrotrophic LAB unable to grow at 30°C (7). The incidence of quality implications increased during 2012, when entire production batches were not reaching the anticipated shelf life (6). Currently, the situation has improved after a very thorough and systematic disinfection of the facility on the basis of the results of the present study.
Supplementary Material
ACKNOWLEDGMENTS
The Ghent University Geconcerteerde Onderzoeks Actie (GOA project BOF10/GOA/010), funded by the Flemish government, is acknowledged for the financial support provided for the present research. Vasileios Pothakos was financially supported by the International Committee on Food Microbiology and Hygiene (ICFMH) under Mobility Grant Program 2014.
We are grateful to Francesca De Filippis for helping with the preparation of the heat plot for the PICRUSt data.
The Laboratory of Food Microbiology and Food Preservation, Department of Food Safety and Food Quality, Faculty of Bioscience Engineering, Ghent University, is a member of Food2Know.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03941-14.
REFERENCES
- 1.Gram L, Ravn L, Rasch M, Bruhn JB, Christensen AB, Givskov M. 2002. Food spoilage—interactions between food spoilage bacteria. Int J Food Microbiol 78:79–97. doi: 10.1016/S0168-1605(02)00233-7. [DOI] [PubMed] [Google Scholar]
- 2.Nychas G, Skandamis P, Tassou C, Koutsoumanis K. 2008. Meat spoilage during distribution. Meat Sci 78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Borch E, Kant-Muermans ML, Blixt Y. 1996. Bacterial spoilage of meat and cured meat products. Int J Food Microbiol 33:103–120. doi: 10.1016/0168-1605(96)01135-X. [DOI] [PubMed] [Google Scholar]
- 4.Huis in't Veld J. 1996. Microbial and biochemical spoilage of foods: an overview. Int J Food Microbiol 33:1–18. doi: 10.1016/0168-1605(96)01139-7. [DOI] [PubMed] [Google Scholar]
- 5.Pothakos V, Samapundo S, Devlieghere F. 2012. Total mesophilic counts underestimate in many cases the contamination levels of psychrotrophic lactic acid bacteria (LAB) in chilled-stored food products at the end of their shelf life. Food Microbiol 32:437–443. doi: 10.1016/j.fm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Pothakos V, Taminiau B, Huys G, Nezer C, Daube G, Devlieghere F. 2014. Psychrotrophic lactic acid bacteria associated with production batch recalls and sporadic cases of early spoilage in Belgium between 2010 and 2014. Int J Food Microbiol 191:157–163. doi: 10.1016/j.ijfoodmicro.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Pothakos V, Snauwaert C, De Vos P, Huys G, Devlieghere F. 2014. Psychrotrophic members of Leuconostoc gasicomitatum, Leuconostoc gelidum and Lactococcus piscium dominate at the end of shelf life in packaged and chilled-stored food products in Belgium. Food Microbiol 39:61–67. doi: 10.1016/j.fm.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Björkroth J, Holzapfel W. 2006. Genera Leuconostoc, Oenococcus and Weissella, p 267–319. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes. Springer, New York, NY. [Google Scholar]
- 9.Axelsson L. 2004. Lactic acid bacteria: classification and physiology. In Salminen S, von Wright A, Ouwehand A (ed), Lactic acid bacteria. Microbiological and functional aspects, 3rd ed Marcel Dekker, New York, NY. [Google Scholar]
- 10.Makarova K, O'Sullivan O, O'Callaghan J, Sangrador-Vegas A, McAuliffe O, Slattery L, Kaleta P, Callanan M, Fitzgerald GF, Ross RP, Beresford T. 2006. Comparative genomics of lactic acid bacteria. Proc Natl Acad Sci U S A 103:15611–15616. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Björkroth J. 2005. Microbiological ecology of marinated meat products. Meat Sci 70:477–480. doi: 10.1016/j.meatsci.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Vihavainen EJ, Björkroth KJ. 2009. Diversity of Leuconostoc gasicomitatum associated with meat spoilage. Int J Food Microbiol 136:32–36. doi: 10.1016/j.ijfoodmicro.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Martín A, Benito MJ, Hernández A, Pérez-Nevado F, Córdoba JJ, Córdoba MG. 2008. Characterisation of microbial deep spoilage in Iberian dry-cured ham. Meat Sci 78:475–484. doi: 10.1016/j.meatsci.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Laursen BG, Leisner JJ, Dalgaard P. 2006. Carnobacterium species: effect of metabolic activity and interaction with Brochothrix thermosphacta on sensory characteristics of modified atmosphere packed shrimp. J Agric Food Chem 54:3604–3611. doi: 10.1021/jf053017f. [DOI] [PubMed] [Google Scholar]
- 15.Ercolini D, Russo F, Torrieri E, Masi P, Villani F. 2006. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol 72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korkeala HJ, Björkroth JK. 1997. Microbiological spoilage and contamination of vacuum-packaged cooked sausages. J Food Prot 60:724–731. [DOI] [PubMed] [Google Scholar]
- 17.Doulgeraki AI, Paramithiotis S, Kagkli DM, Nychas G-JE. 2010. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol 27:1028–1034. doi: 10.1016/j.fm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Pin C, García De Fernando GD, Ordonez JA. 2002. Effect of modified atmosphere composition on the metabolism of glucose by Brochothrix thermosphacta. Appl Environ Microbiol 68:4441–4447. doi: 10.1128/AEM.68.9.4441-4447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doulgeraki AI, Ercolini D, Villani F, Nychas G-JE. 2012. Spoilage microbiota associated to the storage of raw meat in different conditions. Int J Food Microbiol 157:130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Ercolini D. 2013. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecology. Appl Environ Microbiol 79:3148–3155. doi: 10.1128/AEM.00256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieminen TT, Koskinen K, Laine P, Hultman J, Säde E, Paulin L, Paloranta A, Johansson P, Björkroth J, Auvinen P. 2012. Comparison of microbial communities in marinated and unmarinated broiler meat by metagenomics. Int J Food Microbiol 157:142–149. doi: 10.1016/j.ijfoodmicro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Ercolini D, Casaburi A, Nasi A, Ferrocino I, Di Monaco R, Ferranti P, Mauriello G, Villani F. 2010. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int J Food Microbiol 142:120–131. doi: 10.1016/j.ijfoodmicro.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Casaburi A, Nasi A, Ferrocino I, Di Monaco R, Mauriello G, Villani F, Ercolini D. 2011. Spoilage-related activity of Carnobacterium maltaromaticum strains in air-stored and vacuum-packed meat. Appl Environ Microbiol 77:7382–7393. doi: 10.1128/AEM.05304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casaburi A, De Filippis F, Villani F, Ercolini D. 2014. Activities of strains of Brochothrix thermosphacta in vitro and in meat. Food Res Int 62:366–374. doi: 10.1016/j.foodres.2014.03.019. [DOI] [Google Scholar]
- 25.Pothakos V, Nyambi C, Zhang B-Y, Papastergiadis A, De Meulenaer B, Devlieghere F. 2014. Spoilage potential of psychrotrophic lactic acid bacteria (LAB) species: Leuconostoc gelidum subsp. gasicomitatum and Lactococcus piscium, on sweet bell pepper (SBP) simulation medium under different gas compositions. Int J Food Microbiol 178:120–129. doi: 10.1016/j.ijfoodmicro.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 26.García-Gimeno RM, Zurera-Cosano G. 1997. Determination of ready-to-eat vegetable salad shelf life. Int J Food Microbiol 36:31–38. doi: 10.1016/S0168-1605(96)01238-X. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez J, Rodriguez G, Barry-Ryan C, Bourke P. 2008. Efficacy of plant essential oils against foodborne pathogens and spoilage bacteria associated with ready-to-eat vegetables: antimicrobial and sensory screening. J Food Prot 71:1846–1854. [DOI] [PubMed] [Google Scholar]
- 28.Jacxsens L, Devlieghere F, Van der Steen C, Debevere J. 2001. Effect of high oxygen modified atmosphere packaging on microbial growth and sensorial qualities of fresh-cut produce. Int J Food Microbiol 71:197–210. doi: 10.1016/S0168-1605(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 29.Koo OK, Mertz AW, Akins EL, Sirsat SA, Neal JA, Morawicki R, Crandall PG, Ricke SC. 2013. Analysis of microbial diversity on deli slicers using polymerase chain reaction and denaturing gradient gel electrophoresis technologies. Lett Appl Microbiol 56:111–119. doi: 10.1111/lam.12021. [DOI] [PubMed] [Google Scholar]
- 30.Ralyea RD, Wiedmann M, Boor KJ. 1998. Bacterial tracking in a dairy production system using phenotypic and ribotyping methods. J Food Prot 61:1336–1340. [DOI] [PubMed] [Google Scholar]
- 31.Andreani NA, Martino ME, Fasolato L, Carraro L, Montemurro F, Mioni R, Bordin P, Cardazzo B. 2014. Tracking the blue: a MLST approach to characterise the Pseudomonas fluorescens group. Food Microbiol 39:116–126. doi: 10.1016/j.fm.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Vangay P, Fugett EB, Sun Q, Wiedmann M. 2013. Food microbe tracker: a web-based tool for storage and comparison of food-associated microbes. J Food Prot 76:283–294. doi: 10.4315/0362-028X.JFP-12-276. [DOI] [PubMed] [Google Scholar]
- 33.Björkroth KJ, Korkeala HJ. 1996. Evaluation of Lactobacillus sake contamination in vacuum-packaged sliced cooked meat products by ribotyping. J Food Prot 59:398–401. [DOI] [PubMed] [Google Scholar]
- 34.Björkroth KJ, Korkeala HJ. 1997. Use of rRNA gene restriction patterns to evaluate lactic acid bacterium contamination of vacuum-packaged sliced cooked whole-meat product in a meat processing plant. Appl Environ Microbiol 63:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundström H-S, Björkroth KJ. 2011. Lactic acid bacteria associated with pig skin at pre- and post-scalding slaughter stages. Arch Lebensmittelhyg 62:26–31. doi: 10.2376/0003-925X-62-26. [DOI] [Google Scholar]
- 36.Samelis J, Kakouri A, Georgiadou KG, Metaxopoulos J. 1998. Evaluation of the extent and type of bacterial contamination at different stages of processing of cooked ham. J Appl Microbiol 84:649–660. doi: 10.1046/j.1365-2672.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 37.Vasilopoulos C, De Maere H, De Mey E, Paelinck H, De Vuyst L, Leroy F. 2010. Technology-induced selection towards the spoilage microbiota of artisan-type cooked ham packed under modified atmosphere. Food Microbiol 27:77–84. doi: 10.1016/j.fm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Pothakos V, Snauwaert C, De Vos P, Huys G, Devlieghere F. 2014. Monitoring psychrotrophic lactic acid bacteria contamination in a ready-to-eat vegetable salad production environment. Int J Food Microbiol 185:7–16. doi: 10.1016/j.ijfoodmicro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 39.De Filippis F, La Storia A, Villani F, Ercolini D. 2013. Exploring the sources of bacterial spoilers in beefsteaks by culture-independent high-throughput sequencing. PLoS One 8:e70222. doi: 10.1371/journal.pone.0070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ercolini D, De Filippis F, La Storia A, Iacono M. 2012. “Remake” by high-throughput sequencing of the microbiota involved in the production of water buffalo mozzarella cheese. Appl Environ Microbiol 78:8142–8145. doi: 10.1128/AEM.02218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z, Malmer D, Langille MGI, Way SF, Knight R. 2014. Which is more important for classifying microbial communities: who's there or what they can do? ISME J 8:2357–2359. doi: 10.1038/ismej.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh P, Wani AA, Saengerlaub S, Langowski H-C. 2011. Understanding critical factors for the quality and shelf life of MAP fresh meat: a review. Crit Rev Food Sci Nutr 51:146–177. doi: 10.1080/10408390903531384. [DOI] [PubMed] [Google Scholar]
- 48.Stellato G, La Storia A, Cirillo T, Ercolini D. 2014. Bacterial biogeographical patterns in a cooking centre for hospital foodservice. Int J Food Microbiol 193:99–108. doi: 10.1016/j.ijfoodmicro.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Gould GW. 1996. Methods for preservation and extension of shelf life. Int J Food Microbiol 33:51–64. doi: 10.1016/0168-1605(96)01133-6. [DOI] [PubMed] [Google Scholar]
- 50.Gould GW. 2000. Preservation: past, present and future. Br Med Bull 56:84–96. doi: 10.1258/0007142001902996. [DOI] [PubMed] [Google Scholar]
- 51.Abadias M, Usall J, Anguera M, Solsona C, Viñas I. 2008. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int J Food Microbiol 123:121–129. doi: 10.1016/j.ijfoodmicro.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Doyle ME. 2007. Microbial food spoilage—losses and control strategies. Food Research Institute Briefings. Food Research Institute, University of Wisconsin—Madison, Madison, WI. [Google Scholar]
- 53.Vasilopoulos C, De Vuyst L, Leroy F. 11 October 2013. Shelf-life reduction as an emerging problem in cooked hams underlines the need for improved preservation techniques. Crit Rev Food Sci Nutr. doi: 10.1080/10408398.2012.695413. [DOI] [PubMed] [Google Scholar]
- 54.Ercolini D, Russo F, Blaiotta G, Pepe O, Mauriello G, Villani F. 2007. Simultaneous detection of Pseudomonas fragi, P. lundensis, and P. putida from meat by use of a multiplex PCR assay targeting the carA gene. Appl Environ Microbiol 73:2354–2359. doi: 10.1128/AEM.02603-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vihavainen EJ, Björkroth KJ. 2007. Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by Leuconostoc gasicomitatum and Leuconostoc gelidum. Int J Food Microbiol 119:340–345. doi: 10.1016/j.ijfoodmicro.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Jääskeläinen E, Johansson P, Kostiainen O, Nieminen T, Schmidt G, Somervuo P, Mohsina M, Vanninen P, Auvinen P, Björkroth J. 2013. Significance of heme-based respiration in meat spoilage caused by Leuconostoc gasicomitatum. Appl Environ Microbiol 79:1078–1085. doi: 10.1128/AEM.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyhs U, Koort J, Lundström H-S, Björkroth KJ. 2004. Leuconostoc gelidum and Leuconostoc gasicomitatum strains dominated the lactic acid bacterium population associated with strong slime formation in an acetic-acid herring preserve. Int J Food Microbiol 90:207–218. doi: 10.1016/S0168-1605(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 58.Vihavainen EJ, Murros AE, Björkroth KJ. 2008. Leuconostoc spoilage of vacuum-packaged vegetable sausages. J Food Prot 71:2312–2315. [DOI] [PubMed] [Google Scholar]
- 59.Nieminen TT, Vihavainen E, Paloranta A, Lehto J, Paulin L, Auvinen P, Solismaa M, Björkroth KJ. 2011. Characterization of psychrotrophic bacterial communities in modified atmosphere-packed meat with terminal restriction fragment length polymorphism. Int J Food Microbiol 144:360–366. doi: 10.1016/j.ijfoodmicro.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Björkroth KJ, Geisen R, Schillinger U, Weiss N, De Vos P, Holzapfel WH, Korkeala HJ, Vandamme P. 2000. Characterization of Leuconostoc gasicomitatum sp. nov., associated with spoiled raw tomato-marinated broiler meat strips packaged under modified-atmosphere conditions. Appl Environ Microbiol 66:3764–3772. doi: 10.1128/AEM.66.9.3764-3772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong SH, Lee SH, Jung JY, Choi EJ, Jeon CO. 2013. Microbial succession and metabolite changes during long-term storage of kimchi. J Food Sci 78:M763–M769. doi: 10.1111/1750-3841.12095. [DOI] [PubMed] [Google Scholar]
- 62.Cho J, Lee D, Yang C, Jeon J, Kim J, Han H. 2006. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol Lett 257:262–267. doi: 10.1111/j.1574-6968.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 63.Matsui H, Tsuchiya R, Isobe Y, Narita M. 2013. Analysis of bacterial community structure in saba-narezushi (narezushi of mackerel) by 16S rRNA gene clone library. J Food Sci Technol 50:791–796. doi: 10.1007/s13197-011-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Björkroth J, Korkeala H. 1997. Ropy slime-producing Lactobacillus sake strains possess a strong competitive ability against a commercial biopreservative. Int J Food Microbiol 38:117–123. doi: 10.1016/S0168-1605(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 65.Nerbrink E, Borch E. 1993. Evaluation of bacterial contamination at separate processing stages in emulsion sausage production. Int J Food Microbiol 20:37–44. doi: 10.1016/0168-1605(93)90058-O. [DOI] [PubMed] [Google Scholar]
- 66.Rahkila R, Nieminen T, Johansson P, Säde E, Björkroth J. 2012. Characterization and evaluation of the spoilage potential of Lactococcus piscium isolates from modified atmosphere packaged meat. Int J Food Microbiol 156:50–59. doi: 10.1016/j.ijfoodmicro.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Pothakos V. 2014. Psychrotrophic lactic acid bacteria (LAB) as a source of fast spoilage occurring on packaged and cold-stored food products. Ph.D. dissertation. Ghent University, Ghent, Belgium. [Google Scholar]
- 68.Scott TM, Rose JB, Jenkins TM, Samuel R, Lukasik J, Farrah SR. 2002. Microbial source tracking: current methodology and future directions. Appl Environ Microbiol 68:5796–5803. doi: 10.1128/AEM.68.12.5796-5803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harwood VJ, Staley C, Badgley BD, Borges K, Korajkic A. 2014. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev 38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- 70.Foley SL, Lynne AM, Nayak R. 2009. Molecular typing methodologies for microbial source tracking and epidemiological investigations of Gram-negative bacterial foodborne pathogens. Infect Genet Evol 9:430–440. doi: 10.1016/j.meegid.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Säde E. 2011. Leuconostoc spoilage of refrigerated, packaged foods. Doctoral dissertation. Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.