Abstract
Nervous necrosis virus (NNV) is a member of the Betanodavirus genus that causes fatal diseases in over 40 species of fish worldwide. Mortality among NNV-infected fish larvae is almost 100%. In order to elucidate the mechanisms responsible for the susceptibility of fish larvae to NNV, we exposed zebrafish larvae to NNV by bath immersion at 2, 4, 6, and 8 days postfertilization (dpf). Here, we demonstrate that developing zebrafish embryos are resistant to NNV at 2 dpf due to the protection afforded by the egg chorion and, to a lesser extent, by the perivitelline fluid. The zebrafish larvae succumbed to NNV infection during a narrow time window around the 4th dpf, while 6- and 8-day-old larvae were much less sensitive, with mortalities of 24% and 28%, respectively.
INTRODUCTION
Aquaculture is the fastest increasing type of food production system in the world, with an annual growth rate of 9% since 1985 (1). The fish produced by aquaculture account for about 50% of the total amount of fish produced for human consumption (1). Nevertheless, the rapid development of aquaculture is associated with environmental costs, such as habitat degradation, diseases, and pollution (2). Viral nervous necrosis disease (VNND) is one of the most devastating threats to cultured marine fish worldwide and results in great economic loss. The disease is caused by the nervous necrosis virus (NNV), a member of the Betanodavirus genus. The virus is highly contagious and virulent to at least 40 marine and brackish water fish species, including groupers, sea bass, temperate basses, barramundi, mullet, sea bream, and flounder (3).
NNV is a spherical, nonenveloped virus with a bipartite, positive, single-stranded RNA genome. The virus genome is composed of RNA1 (3,107 nucleotides [nt]) and RNA2 (1,421 nt), which encode the RNA-dependent RNA polymerase (RdRp) (4) and the coat protein (CP), respectively (5). A subgenomic transcript of the RNA1 segment (RNA3) encodes the nonstructural proteins B2 and B1 (6, 7). The B2 protein prevents host RNA interference-mediated cleavage (8). The B1 protein is expressed at the early stage of infection and exhibits an anti-necrotic cell death function (7).
Mortality among NNV-infected larvae and juveniles is almost 100%, resulting in serious economic losses to producers of high-value fish species. Mature fish are more resistant to VNND but are still capable of horizontally transferring the virus to mature fish and vertically to their offspring. The high level of susceptibility to NNV of fish larvae and juveniles, including zebrafish (9), and the recent report describing an NNV outbreak in zebrafish (10) led us to establish an NNV-zebrafish larva infection model to study host-pathogen interactions in the early stages of development. Zebrafish characteristics make them an attractive model for studying host-microbe interactions and immune system development. The optical transparency of zebrafish larvae, the availability of transgenic lines, their small size, and the rapid development of zebrafish embryos are some of their many advantages. Zebrafish genomic sequencing enables the use of functional genomic and reverse genetic techniques. This experimental platform therefore provides significant opportunities for understanding host-microbe interactions in the context of a rapidly developing vertebrate host (11). This study describes the fluctuations in zebrafish larva sensitivity to NNV during the first 11 days postfertilization (dpf), providing a suitable system to study the host factors involved in protecting larvae against pathogens.
MATERIALS AND METHODS
Zebrafish, larvae, and eggs.
Adult wild-type AB zebrafish (Danio rerio) were maintained under conditions previously described (12). The eggs used for the experiments and the mother fish that spawned the eggs for this study were NNV free, as validated by reverse transcriptase (RT) real-time PCR (see below). Naturally spawned zebrafish eggs were collected as described before (13). Following collection, the eggs were kept in plastic containers at a density of 100 eggs in 100 ml egg water, which contained 60 mg/liter of Instant Ocean in deionized water and 0.25 mg/liter methylene blue. The eggs were kept at a temperature of 28.5°C for 1 to 7 days, until they were used for the experiments. Larvae were fed twice a day with NobilFluid Artemia (JBL, Neuhofen, Germany) from 5 dpf until the end of the experiment (11 days postinfection).
Virus.
The NNV strain used in this study was originally isolated from a sick white grouper (Epinephelus aeneus) in Israel (new GenBank accession number KP748520). Its viral genome is 99% similar to that of the sevenband grouper nervous necrosis virus (SGNNV). The virus was propagated on goldfish (Carassius auratus) cultured skin cells (GFSk-S1 cells). The GFSk-S1 cells were grown in Leibovitz medium (L15; Biological Industries, Beth Haemek, Israel) supplemented with 10% fetal bovine serum, 2 mM glutamine (Biological Industries, Beth Haemek, Israel), and 1% penicillin-streptomycin (Biological Industries, Beth Haemek, Israel) at 25°C, as previously described (14). Virus titration was evaluated by plaque assay in 96-well plates. Virus was inactivated by UV irradiation for 4 min at room temperature at 25 nm and 214 mJ/cm2.
Larva infection.
To optimize the conditions for NNV infection, groups of 10 or 20 zebrafish larvae were immersed in a bath at 4 days postfertilization under the environmental conditions described in Table 1. Larvae were exposed to the virus for 3, 12, or 24 h in water at a temperature of 25°C or 28°C containing 5 × 104 PFU/ml NNV. Groups of larvae were maintained in flat-bottomed, 12-well plates containing 1 ml of egg water and 1 ml of NNV suspension, while the control groups were maintained in the same fluid lacking the virus. Following infection, larvae were kept in 2 ml egg water, which was replaced daily throughout the experimental period. The larvae were monitored daily for a period of 11 days postinfection for clinical signs of disease and mortality. Condition evaluation trials were repeated three times for each treatment. Following these trials, all experiments were performed with 60 larvae, divided equally in three wells of flat-bottomed, 12-well plates. All experiments were repeated three times.
TABLE 1.
Environmental conditions required for enhancing the sensitivity of larvae to NNV at 4 dpfa
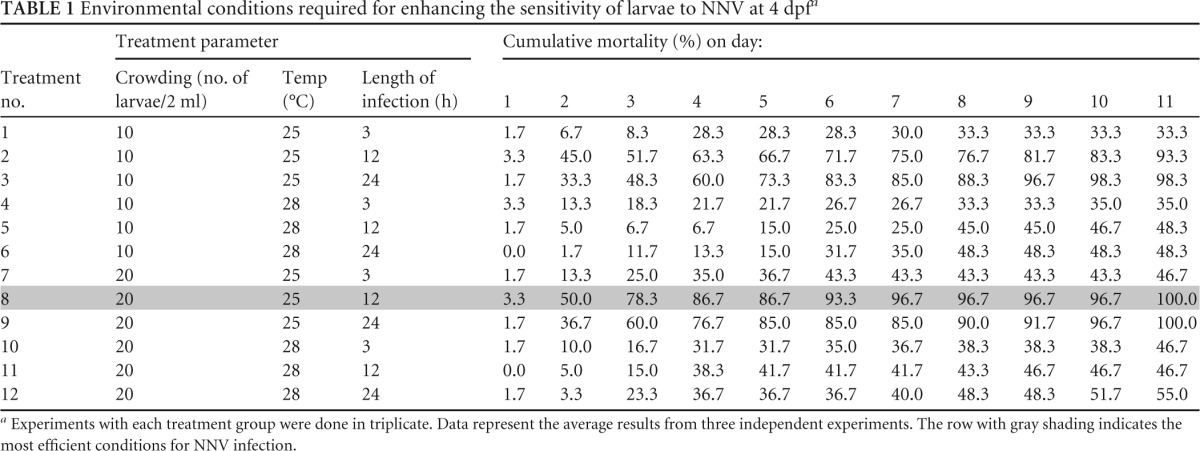
Experiments with each treatment group were done in triplicate. Data represent the average results from three independent experiments. The row with gray shading indicates the most efficient conditions for NNV infection.
Egg piercing.
Eggs were held by tweezers, and the chorion was pierced by an insulin needle (29 gauge by 1/2 in.; BD, NJ, USA) while the larvae were kept submersed in the perivitelline fluid (see Fig. 5AII).
FIG 5.
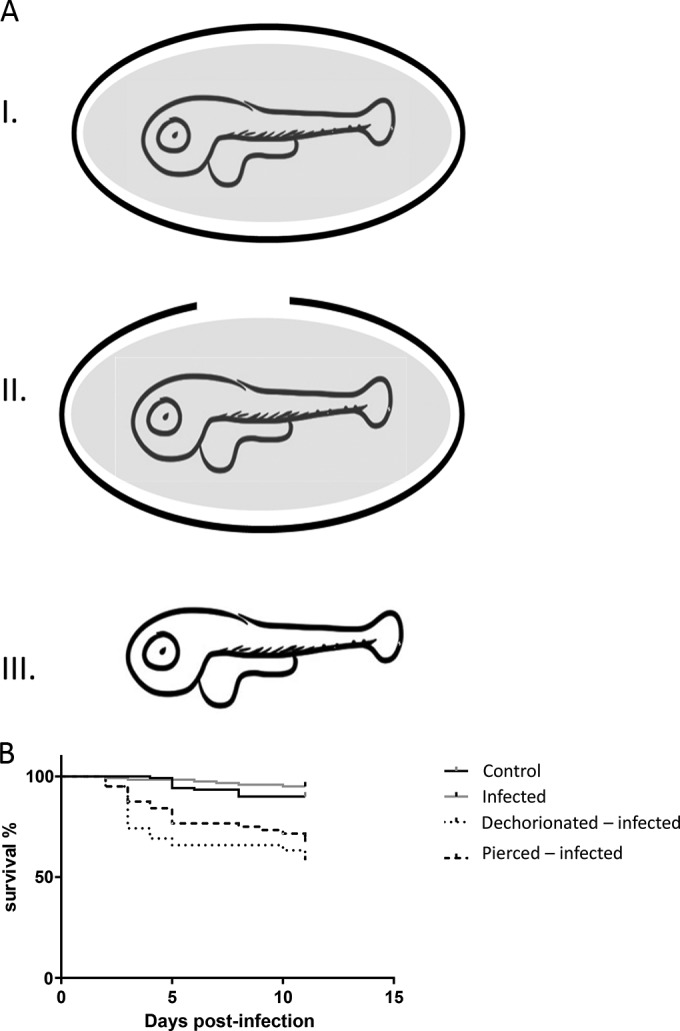
Zebrafish larvae infected with NNV on day 2 postfertilization. (A) Schematic presentation of dechorionated larvae and pierced eggs. The schematics show eggs containing larvae (I), pierced larvae (II), and dechorionated larvae (III). The gray background represents perivitelline fluid. (B) Survival curves. The groups of eggs described in the legend to panel A were infected with NNV by bath immersion, and their survival rate was recorded. The data are representative of those from three independent experiments (n = 60 for each age group).
Egg dechorionation.
Eggs were manually dechorionated under a binocular microscope using two tweezers. Each naked larva was washed twice in 10 ml distilled water to remove the perivitelline fluid (see Fig. 5AIII).
Viral nucleic acid and immune gene expression analyses.
Larvae were sacrificed by fast freezing at −70°C. Each larva was washed twice in 10 ml distilled water. Total RNA was extracted from a pool of five larvae that were ground manually using a sterile plastic pestle in a 1.5-ml Eppendorf tube. Further RNA extraction was performed using an EZ RNA purification kit (Biological Industries, Beth Haemek, Israel). cDNA was synthesized using a Master Script RT-PCR system (5 PRIME, Hamburg, Germany), according to the manufacturer's instructions, and a random hexamer primer. Real-time RT-PCR was performed with an AB 7300 instrument (Applied Biosystems, CA, USA). Each reaction mixture contained 5 μl of primers (300 nM each), 5 μl of cDNA (diluted 1/10), and 10 μl of SYBR green PCR core reagent (Applied Biosystems, CA, USA). Samples were first incubated for 2 min at 50°C and for 10 min at 95°C and then subjected to 40 amplification cycles (95°C for 15 and 60°C for 1 min). The expression data for the genes were normalized to those for a 150-bp fragment of the β-actin gene using the following primers: β-actin 1f (ATGGATGAGGAAATCGCTG) and β-actin 2r (ATGCCAACCATCACTCCCTG) (9), where f and r represent forward and reverse, respectively. Original data were analyzed using the comparative threshold cycle (CT) method (2−ΔΔCT). All PCRs were performed with triplicate samples and were repeated at least twice. NNV cDNA was identified and quantitated by real-time PCR targeting two capsid gene fragments: an 81-bp fragment, using primers NTR f (GCCCCTGATGGAGCAGTCT) and NTR r (AGCACGGTCAACATCTCCAGTT) (primer set 1 [9]), and a 203-bp fragment, using primers 203f (GACGCGCTTCAAGCAACTC) and 203r (CGAACACTCCAGCGACACAGCA) (primer set 2 [15]). For immune gene analyses, the following primers were used: for the Mx(a) gene, Mx f (AGTACCGGGGAAGAGAGCTA) and Mx r (AAGGTGGCATGATTGTCTGT) (9); for the interleukin 1β (IL-1β) gene, IL-1β f (GGCTGTGTGTTTGGGAATCT) and IL-1β r (TGATAAACCAACCGGGACA) (16), and for the tumor necrosis factor alpha (TNF-α) gene, TNF-α f (GCGCTTTTCTGAATCCTACG) and TNF-α r (TGCCCAGTCTGTCTCCTTCT) (16).
Eggs used for the experiments were NNV free, as validated by RT-PCR of total RNA extracted from five randomly collected pools of 10 eggs (data not shown). RNA extracted from brain and liver tissues of the 10 randomly collected egg-producing mother fish used for this study were found to be NNV free using RT-PCR, as described above (data not shown).
Histology.
Five larvae from each group of NNV-infected or mock-infected larvae were collected at 24 hours postinfection (hpi) and fixed in a 10% solution of phosphate-buffered formalin. The samples were dehydrated using graded ethanol concentrations, embedded in 2-hydroxyethyl methacrylate, and sectioned at a thickness of 3 μm. The sections were stained with toluidine blue.
Statistical analysis.
Data were analyzed using the statistical analysis software SPSS (version 18.0) and GraphPad Prism (version 6.05). Statistical significance was set at a P value of <0.05. Larva survival was calculated by Kaplan-Meier analysis, and statistical significance was determined by the log rank (Mantel-Cox) test.
Ethics statement.
All animal studies were carried out according to the European Union Regulations for animal experimentation and approved by the Hebrew University Animal Care Committee (approval number MD-13-13789-3).
Nucleotide sequence accession number.
Newly determined sequence data for NNV have been deposited in GenBank under accession number KP748520.
RESULTS
Optimal conditions for NNV-zebrafish larva infection.
The environmental condition evaluation trials (Table 1) indicated that the ultimate conditions for NNV-zebrafish larva infection are an infection time of 12 h at a temperature of 25°C with a density of 20 larvae per 2 ml. These conditions were used for all further infection assays.
Identification of NNV RNA in infected zebrafish larvae.
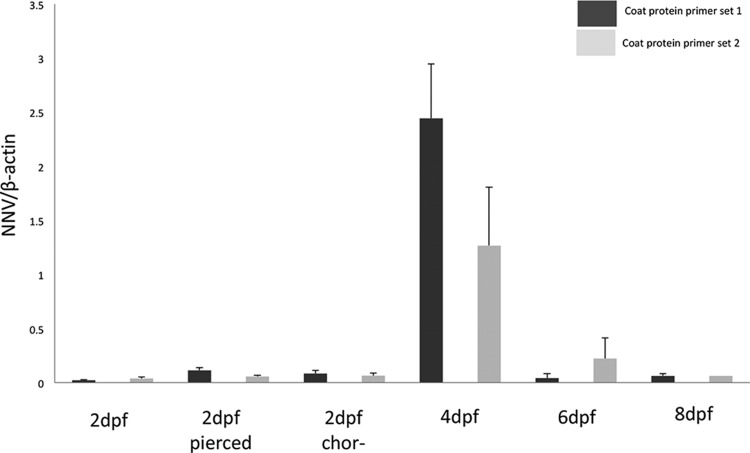
To confirm NNV infection in zebrafish larvae exposed to the virus on the 2nd, 4th, 6th, and 8th days postfertilization, pools of five larvae were collected from each group at 24 hpi. The control groups included noninfected larvae of the same age. Using real-time RT-PCR, we detected NNV genomic RNA in extracts of all larvae exposed to the virus (Fig. 1). We demonstrated NNV genomic RNA by analyzing two independent capsid fragments. Those two fragments showed similar results (Fig. 1). These results indicate that zebrafish larvae are infected by the virus on the 2nd, 4th, 6th, and 8th dpf. To rule out the possibility that the RT-PCR-positive larvae were not externally contaminated, dead larvae, sacrificed by fast freezing at −70°C at the age of 2 dpf and 4 dpf (n = 20, each group), were immersed in a water bath containing 5 × 104 PFU/ml NNV under the same conditions as the experimental larvae. The larvae were washed, and RNA extraction was performed as described above. All the immersed dead larvae were found to be RT-PCR negative for NNV (data not shown).
FIG 1.
Quantification analysis of NNV genomic RNA in zebrafish larvae exposed to the virus. RNA was extracted from a pool of five larvae taken from each group at 24 h postinfection. NNV cDNA was identified and quantitated by real-time RT-PCR targeting two capsid gene fragments, an 81-bp fragment (primer set 1) and a 203-bp fragment (primer set 2). Each bar represents the mean ± SE of triplicate readings from pooled larvae, and the data are representative of those from three independent experiments. The NNV expression levels were normalized against the β-actin expression level. All control groups and the group infected with inactivated virus were found to be negative by the two primer sets. dpf, days postfertilization; 2dpf pierced, eggs were pierced with a needle before infection; 2dpf chor−, eggs were manually dechorionated before infection.
Zebrafish larvae are susceptible to NNV on the 4th day postfertilization.
Zebrafish larvae (n = 60, each age group) at the ages of 2, 4, 6, and 8 dpf were exposed to NNV infection for 12 h at a temperature of 25°C in 2 ml water. Figure 2 shows that larvae exposed to the virus at 2 dpf were mostly resistant to NNV infection (5% mortality), while all larvae exposed to the virus on the 4th dpf died (100% mortality) at 48 to 72 hpi. The susceptibility to VNND was drastically reduced for larvae exposed to the virus at days 6 and 8 postfertilization (24% and 28% mortality, respectively).
FIG 2.
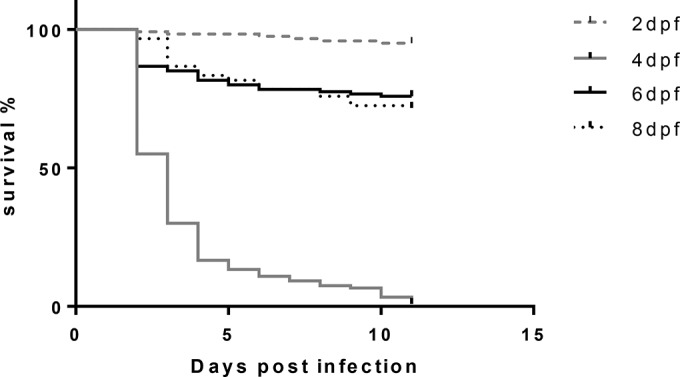
Survival curves for zebrafish larvae infected with NNV by bath immersion on days 2, 4, 6, and 8 postfertilization. The data are representative of those from three independent experiments (n = 60 for each age group). Mock-infected larvae of all age groups had survival rates of 95 to 100%. dpf, days postfertilization.
As a control, at 4 dpf larvae were infected with UV-inactivated NNV. Bath immersion with inactivated virus did not affect the fish, with similar mortality rates being found for the mock-infected control and the UV-inactivated virus-infected larvae (P = 0.35, Mantel-Cox test). The survival rate of larvae in both groups was significantly higher than that of NNV-infected larvae (P < 0.0001, Mantel-Cox test; Fig. 3), indicating that propagated virus is exclusively the cause of VNND in zebrafish larvae.
FIG 3.
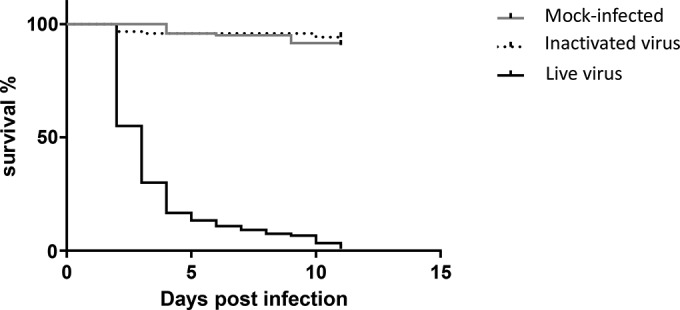
Survival curves for zebrafish larvae infected with NNV by bath immersion on day 4 postfertilization with live virus versus or inactivated virus. The data are representative of those from three independent experiments (n = 60 for each group).
The mortality rate of larvae infected on the 2nd dpf did not differ significantly from that of the larvae in the control group (P = 0.145, Mantel-Cox test), while the survival rates of the control groups of larvae were significantly higher than those of larvae infected at days 4, 6, and 8 postfertilization (P < 0.0001, P = 0.001, and P < 0.0001, respectively, Mantel-Cox test). Although the mortality rates for larvae infected at the ages of 6 and 8 dpf were higher than those for larvae in their control groups, the mortality rate for larvae infected at the age of 4 dpf was significantly higher than that for larvae infected on the 2nd, 6th, and 8th dpf (P < 0.0001, P < 0.0001, and P < 0.0001, respectively, Mantel-Cox test). These results clearly demonstrate that the susceptibility of zebrafish larvae to NNV is limited to a narrow window of time around the 4th dpf.
Histopathology.
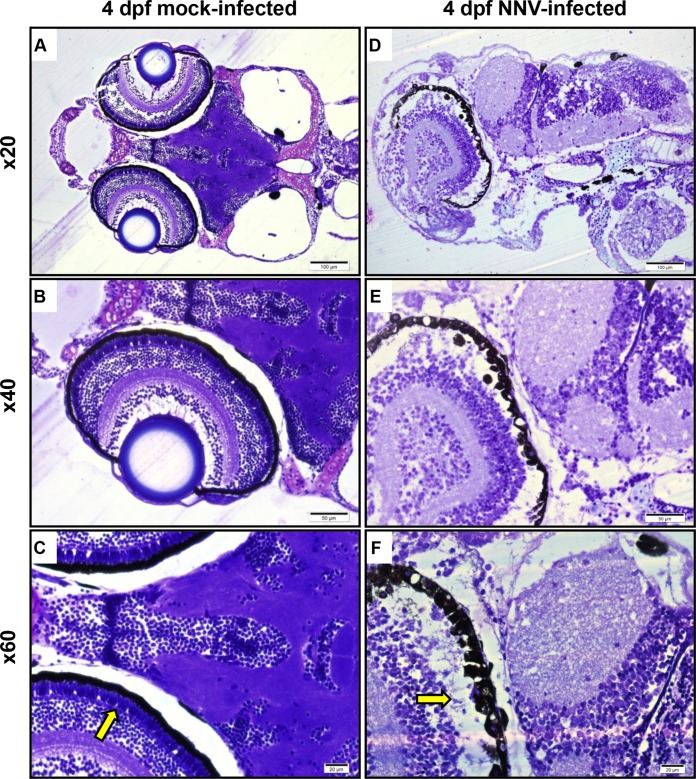
To further characterize the disease process, pools of five larvae were collected at 24 hpi from each group infected or mock infected at 2, 4, 6, and 8 dpf and analyzed for the histopathological changes induced by viral infection. Zebrafish larvae infected with NNV at the age of 4 dpf showed marked neuropil vacuolation, as well as a relative paleness of neurons involving both the brain and retina. The most prominent injury was in the photoreceptor layer of the retina, where almost all of the cells appeared to be lost (Fig. 4). The brain and retina of larvae infected with NNV at the ages of 6 and 8 dpf were relatively preserved and almost identical to those of the control group (data not shown). Undeveloped brain and retina without obvious injury were observed in 2-day-old infected larvae, but they could not be used for diagnosis at this early stage of development (data not shown).
FIG 4.
Methacrylate-embedded sections of zebrafish larvae at 4 dpf stained with toluidine blue. (A to C) Control mock-infected larvae; (D to F) NNV-infected larvae showing marked neuropil vacuolation, as well as a relative paleness of neurons involving both the brain and retina. The most prominent injury appears to be in the photoreceptor layer (yellow arrows) of the retina, with apparent nearly total lysis of the photoreceptors. dpf, days postfertilization.
The egg chorion and perivitelline fluid protect the larvae from NNV infection at day 2 postfertilization.
To determine whether the egg chorion and/or the perivitelline fluid protects the embryo from NNV infection, at 2 dpf, dechorionated larvae and larvae with a pierced chorion were mock or NNV infected. NNV infection of embryos with a pierced chorion and dechorionated larvae reduced the survival rate by 24% and 37%, respectively, relative to that for the mock-infected groups (P < 0.0001, Mantel-Cox test for both groups). There was no significant difference in the survival rates between the pierced chorion and dechorionated groups infected on the 2nd dpf (P = 0.112, Mantel-Cox test) (Fig. 5B).
Innate immunity gene expression.
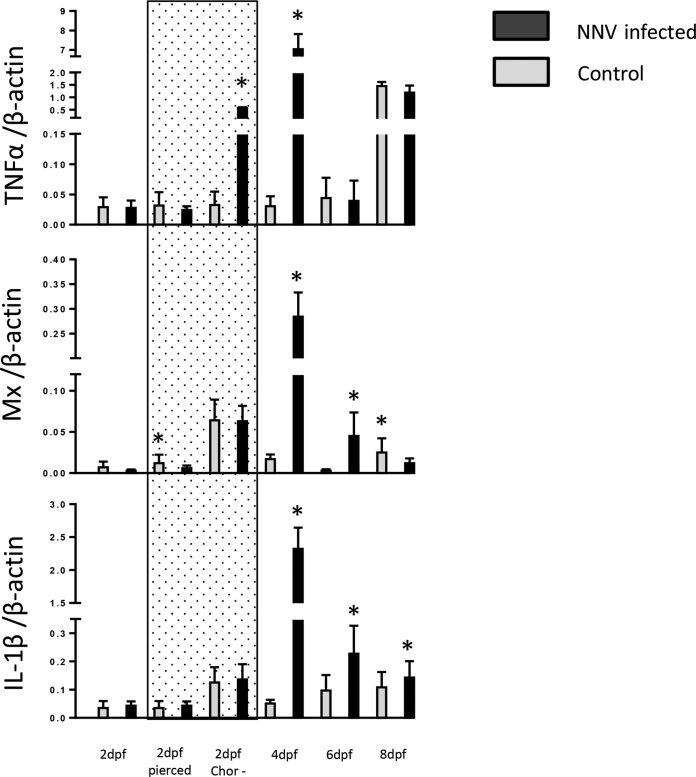
To determine why larvae are vulnerable to NNV at 4 dpf, we determined the expression levels of three genes for proteins representative of the major cytokine groups, TNF-α, Mx, and IL-1β, at 24 hpi. Figure 6 shows that the expression level of the TNF-α gene in naive larvae was low and stable during the first 6 days but rapidly increased on day 8 postfertilization. Unlike TNF-α, the expression of Mx and IL-1β remained at about the same levels throughout the experiment until 8 dpf. However, NNV infection on day 4 induced a remarkable increase in the levels of TNF-α, Mx, and IL-1β expression, while infection on the other days caused only a moderate boost in the expression of these genes (Fig. 6).
FIG 6.
Temporal expression levels of genes for TNF-α, Mx, and IL-1β following NNV infection at different days postfertilization. RNA was extracted from a pool of five larvae taken from each group at 24 h postinfection. cDNA was used to quantify innate immune gene expression by real-time RT-PCR. Each bar represents the mean ± SE of triplicate readings from three independent experiments. Gene expression levels were normalized against the β-actin expression level. dpf, days postfertilization; 2dpf pierced, eggs were pierced with a needle before infection; 2dpf Chor−, eggs were manually dechorionated before infection. The area marked with shading represents results for larvae that underwent piercing and dechorionation at 2 dpf. Statistically significant differences are marked with asterisks.
DISCUSSION
Zebrafish larvae are susceptible to NNV infection during the first 8 dpf, but the lethal effect exerted by the virus is mostly restricted to 4 dpf. Infection of 4-day-old larvae resulted in 100% mortality, while infection of larvae on the 2nd, 6th, and 8th dpf caused death for 5%, 24%, and 28% of the larvae, respectively. The standard water temperature for raising zebrafish larvae is 28.5°C, while the optimal temperature for NNV propagation is 25°C. It is feasible that a water temperature of 25°C, used in our study, could suppress the development of some immune factors (17), leading to the high rate of mortality on the 4th dpf. Previously, it was reported that 98% of zebrafish larvae challenged with NNV by microinjection died within 24 hpi, whereas the mortality for the mock-injected group was 24% (9). Infection of zebrafish larvae by bath immersion is a natural route of infection requiring the minimal manipulation of the larvae (18).
The ontogenesis of zebrafish embryos is rapid, and on the 5th dpf most organ systems are fully developed (19). However, the adaptive immune system is morphologically and functionally mature only at 4 to 6 weeks postfertilization (20). Hence, the adaptive immune system does not play a role during the first 11 dpf surveyed in this study. Interestingly, we showed that larvae are susceptible to the virus during the early period of development, but extensive mortality is mostly restricted to the 4th day postfertilization. The reasons for the partial resistance of larvae during the early stage of their lives and the hypersensitivity to the virus at 4 dpf are yet unknown.
First, we evaluated the anti-NNV protection provided by the egg chorion and perivitelline fluid. Dechorionation comprises removal of the egg chorion and the perivitelline fluid from the developing embryo, while piercing of the egg chorion preserves the perivitelline fluid attached to the embryos but eliminates the physical protection offered by the chorion (Fig. 5A). The chorion, comprising highly insoluble proteins and glycoproteins (21), provides a mostly physical barrier (22). The perivitelline fluid is a protein storage component that contains lectins, protease inhibitors (23), growth factors, and antimicrobial agents (24, 25). The fish mothers used by us to produce larvae were NNV free, and they had never been exposed to NNV, ruling out the possibility of specific anti-NNV antibodies being transferred to the larvae. Exposure of pierced or dechorionated eggs to NNV resulted in similar mortality rates. These findings emphasize the cardinal role of the egg chorion and the limited function of the perivitelline fluid in protecting zebrafish embryos against NNV.
To determine whether factors involved in the innate immune response provide the larvae with an additional layer of defense, we compared cytokine expression levels in naive and infected larvae. TNF-α is an important proinflammatory cytokine in fish and mammals (26). The Mx genes are used as indicators of interferon production in a number of fish species (27). IL-1β is a proinflammatory cytokine that is expressed in the early stage of microbial infection (28) and regulates the expression of other cytokines (29).
Infection of control and pierced larvae at the 2nd dpf with NNV did not significantly alter the mRNA levels of these genes; however, increased expression of the three genes is evident in the dechorionated larvae following NNV infection. The expression of the Mx and IL-1β genes was also upregulated in the naive dechorionated larvae, probably due to the stress from the operation and/or environmental antigenic stimulation. These and previously published results (30) indicate that the innate immune system is active in larvae at the 2nd dpf. On the other hand, untreated eggs were not stimulated, suggesting that the larva shell reduces the interaction with external stimulators, including NNV. Upon NNV infection of larvae on the 4th dpf, when larvae are not protected by the eggshells, the expression levels of TNF-α, Mx, and IL-1β genes were dramatically upregulated. However, this response is not sufficient to protect the larvae, and the larvae succumbed to the virus.
Are there additional factors that make the larvae sensitive to NNV at the 4th dpf? Maternal fish components, such as the complement component C3, factor B (Bf), lectins, lysozymes, and vitellogenin (Vg)-derived yolk proteins, are transferred from mother to offspring, protecting the larvae from the assault of pathogens, including NNV (31). In addition, maternal IgM molecules protect some teleost offspring, including zebrafish larvae, from pathogen infection (30). The level of maternal Ig molecules gradually decreases in zebrafish and tilapia from the 1st day postfertilization to the self-feeding period at 5 to 6 dpf, when yolk absorption is completed (32). The larva mouth slit is open by 72 h postfertilization (hpf) (13), and at 96 hpf, the digestive organs allow the uptake and processing of external food (33).
The increased survival rates of zebrafish larvae on the 6th dpf correlate with the upregulation of T-cell receptor alpha chain (TCRAC), immunoglobulin lambda light chain (IgLC) 1, IgLC 2, IgLC 3 (34), Ikaros, and recombination activating gene 1 (RAG1) (34). We detected a significant increase in IL-1β gene expression, suggesting that it plays a protective role in the larvae at 6 and 8 dpf.
We speculate that maternal components, together with the egg chorion and the perivitelline fluid, are responsible for protection of zebrafish larvae while they are still in the egg. However, after emerging from the chorion, the larvae are exposed to external stimuli and environmental agents, including NNV. Even though there is increased expression of genes involved in innate immunity, the larvae are extremely sensitive to NNVD. Soon after this critical period, the innate factors, together with the other immune factors developing in the larvae, are capable of protecting the larvae from NNV.
ACKNOWLEDGMENTS
This research was supported by the Israeli Ministry of Agriculture and Rural Development, Chief Scientist Office (grant no. 894-0183-10).
We thank A. Dishon, KoVax Co. (Jerusalem, Israel), for providing the goldfish (Carassius auratus) skin (GFSk-S1) cell cultures.
REFERENCES
- 1.Khan M, Khan S, Miyan K. 2011. Aquaculture as a food production system: a review. Biol Med 3:291–302. [Google Scholar]
- 2.Bostock J, McAndrew B, Richards R, Jauncey K, Telfer T, Lorenzen K, Little D, Ross L, Handisyde N, Gatward I, Corner R. 2010. Aquaculture: global status and trends. Philos Trans R Soc London B Biol Sci 365:2897–2912. doi: 10.1098/rstb.2010.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munday BL, Kwang J, Moody N. 2002. Betanodavirus infections of teleost fish: a review. J Fish Dis 25:127–142. doi: 10.1046/j.1365-2761.2002.00350.x. [DOI] [Google Scholar]
- 4.Tan C, Huang B, Chang SF, Ngoh GH, Munday B, Chen SC, Kwang J. 2001. Determination of the complete nucleotide sequences of RNA1 and RNA2 from greasy grouper (Epinephelus tauvina) nervous necrosis virus, Singapore strain. J Gen Virol 82:647–653. [DOI] [PubMed] [Google Scholar]
- 5.Delsert C, Morin N, Comps M. 1997. A fish encephalitis virus that differs from other nodaviruses by its capsid protein processing. Arch Virol 142:2359–2371. doi: 10.1007/s007050050248. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto T, Mise K, Takeda A, Okinaka Y, Mori K-I, Arimoto M, Okuno T, Nakai T. 2005. Characterization of striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J Gen Virol 86:2807–2816. doi: 10.1099/vir.0.80902-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen L-J, Su Y-C, Hong J-R. 2009. Betanodavirus non-structural protein B1: a novel anti-necrotic death factor that modulates cell death in early replication cycle in fish cells. Virology 385:444–454. doi: 10.1016/j.virol.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Fenner BJ, Thiagarajan R, Chua HK, Kwang J. 2006. Betanodavirus B2 is an RNA interference antagonist that facilitates intracellular viral RNA accumulation. J Virol 80:85–94. doi: 10.1128/JVI.80.1.85-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu MW, Chao YM, Guo TC, Santi N, Evensen Ø, Kasani SK, Hong JR, Wu JL. 2008. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish infection model. Mol Immunol 45:1146–1152. doi: 10.1016/j.molimm.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Binesh CP. 2013. Mortality due to viral nervous necrosis in zebrafish Danio rerio and goldfish Carassius auratus. Dis Aquat Organ 104:257–260. doi: 10.3354/dao02605. [DOI] [PubMed] [Google Scholar]
- 11.Kanther M, Rawls JF. 2010. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol 22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerfield M. 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio Rerio), 2nd ed University of Oregon Press, Corvallis, OR. [Google Scholar]
- 13.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 14.Ronen A, Perelberg A, Abramowitz J, Hutoran M, Tinman S, Bejerano I, Steinitz M, Kotler M. 2003. Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine 21:4677–4684. doi: 10.1016/S0264-410X(03)00523-1. [DOI] [PubMed] [Google Scholar]
- 15.Kuo HC, Wang TY, Chen PP, Chen YM, Chuang HC, Chen TY. 2011. Real-time quantitative PCR assay for monitoring of nervous necrosis virus infection in grouper aquaculture. J Clin Microbiol 49:1090–1096. doi: 10.1128/JCM.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galindo-Villegas J, Garcia-Moreno D, de Oliveira S, Meseguer J, Mulero V. 2012. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc Natl Acad Sci U S A 109:E2605–E2614. doi: 10.1073/pnas.1209920109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Morvan C, Troutaud D, Deschaux P. 1998. Differential effects of temperature on specific and nonspecific immune defences in fish. J Exp Biol 201:165–168. [DOI] [PubMed] [Google Scholar]
- 18.López-Muñoz A, Roca FJ, Sepulcre MP, Meseguer J, Mulero V. 2010. Zebrafish larvae are unable to mount a protective antiviral response against waterborne infection by spring viremia of carp virus. Dev Comp Immunol 34:546–552. doi: 10.1016/j.dci.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Meeker ND, Trede NS. 2008. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol 32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Novoa B, Figueras A. 2012. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv Exp Med Biol 946:253–275. doi: 10.1007/978-1-4614-0106-3_15. [DOI] [PubMed] [Google Scholar]
- 21.Bonsignorio D, Perego L, Del Giacco L, Cotelli F. 1996. Structure and macromolecular composition of the zebrafish egg chorion. Zygote 4:101–108. [DOI] [PubMed] [Google Scholar]
- 22.Hamodrakas SJ. 1992. Molecular architecture of helicoidal proteinaceous eggshells. Results Probl Cell Differ 19:115–186. [DOI] [PubMed] [Google Scholar]
- 23.Nagle GT, de Jong-Brink M, Painter SD, Li KW. 2001. Structure, localization and potential role of a novel molluscan trypsin inhibitor in Lymnaea. Eur J Biochem 268:1213–1221. doi: 10.1046/j.1432-1327.2001.01972.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez JF, Lescar J, Chazalet V, Audfray A, Gagnon J, Alvarez R, Breton C, Imberty A, Mitchell EP. 2006. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J Biol Chem 281:20171–20180. doi: 10.1074/jbc.M603452200. [DOI] [PubMed] [Google Scholar]
- 25.Nagle GT, Akalal DB, Painter SD. 1999. Maternal impact on egg development in Lymnaea stagnalis: a growth factor is produced by the albumen gland in the reproductive tract. Invertebr Reprod Dev 36:171–174. doi: 10.1080/07924259.1999.9652695. [DOI] [Google Scholar]
- 26.Wang WL, Liu W, Gong HY, Hong JR, Lin CC, Wu JL. 2011. Activation of cytokine expression occurs through the TNFα/NF-κB-mediated pathway in birnavirus-infected cells. Fish Shellfish Immunol 31:10–21. doi: 10.1016/j.fsi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Thanasaksiri K, Sakai N, Yamashita H, Hirono I, Kondo H. 2014. Influence of temperature on Mx gene expression profiles and the protection of sevenband grouper, Epinephelus septemfasciatus, against red-spotted grouper nervous necrosis virus (RGNNV) infection after poly (I:C) injection. Fish Shellfish Immunol 40:441–445. doi: 10.1016/j.fsi.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Huising MO, van der Meulen T, Flik G, Verburg-van Kemenade BML. 2004. Three novel carp CXC chemokines are expressed early in ontogeny and at nonimmune sites. Eur J Biochem 271:4094–4106. doi: 10.1111/j.1432-1033.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello CA. 1997. Interleukin-1. Cytokine Growth Factor Rev 8:253–265. doi: 10.1016/S1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Zhang S, Tong Z, Li L, Wang G. 2009. Maternal transfer and protective role of the alternative complement components in zebrafish Danio rerio. PLoS One 4:e4498. doi: 10.1371/journal.pone.0004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HM, Ji DR, Shao JZ, Zhang SC. 2012. Maternal transfer and protective role of antibodies in zebrafish Danio rerio. Mol Immunol 51:332–336. doi: 10.1016/j.molimm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Olsen YA, Press CML. 1997. Degradation kinetics of immunoglobulin in the egg, alevin and fry of Atlantic salmon, Salmo salar L, and the localisation of immunoglobulin in the egg. Fish Shellfish Immunol 7:81–91. doi: 10.1006/fsim.1996.0064. [DOI] [Google Scholar]
- 33.Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. 2012. Zebrafish embryos as an alternative to animal experiments. A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- 34.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. 2004. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol 28:9–28. doi: 10.1016/S0145-305X(03)00103-4. [DOI] [PubMed] [Google Scholar]