Abstract
Serratia spp. are opportunistic human pathogens responsible for an increasing number of nosocomial infections. However, little is known about the virulence factors and regulatory circuits that may enhance the establishment and long-term survival of Serratia liquefaciens in the hospital environment. In this study, two reporter strains, Chromobacterium violaceum CV026 and VIR24, and high-resolution triple-quadrupole liquid chromatography–mass spectrometry (LC-MS) were used to detect and to quantify N-acyl-homoserine lactone (AHL) quorum-sensing signals in 20 S. liquefaciens strains isolated from clinical samples. Only four of the strains produced sufficient amounts of AHLs to activate the sensors. Investigation of two of the positive strains by high-performance liquid chromatography (HPLC)-MS confirmed the presence of significant amounts of short-acyl-chain AHLs (N-butyryl-l-homoserine lactone [C4-HSL] and N-hexanoyl-l-homoserine lactone [C6-HSL]) in both strains, which exhibited a complex and strain-specific signal profile that included minor amounts of other short-acyl-chain AHLs (N-octanoyl-l-homoserine lactone [C8-HSL] and N-3-oxohexanoyl-l-homoserine lactone [OC6-HSL]) and long-acyl-chain (C10, C12, and C14) AHLs. No correlation between biofilm formation and the production of large amounts of AHLs could be established. Fimbria-like structures were observed by transmission electron microscopy, and the presence of the type 1 fimbrial adhesin gene fimH in all strains was confirmed by PCR. The ability of S. liquefaciens to adhere to abiotic surfaces and to form biofilms likely contributes to its persistence in the hospital environment, increasing the probability of causing nosocomial infections. Therefore, a better understanding of the adherence properties of this species will provide greater insights into the diseases it causes.
INTRODUCTION
Serratia spp. are opportunistic Gram-negative bacteria that belong to the family Enterobacteriaceae. Serratia marcescens and Serratia liquefaciens are frequently encountered in nosocomial infections (1–4). S. liquefaciens is an increasingly recognized cause of transfusion-related sepsis and has been reported as a cause of meningitis, thrombophlebitis, corneal ulcers, and other infections (5–10). Moreover, Serratia species are inherently resistant to several antibiotics and are capable of readily acquiring resistance (11, 12).
N-Acylhomoserine lactone (AHL)-mediated quorum-sensing (QS) systems are cell density-dependent intercellular signaling mechanisms that regulate many physiological processes in Gram-negative bacteria. When the concentration of the molecule exceeds a threshold, signaling pathways are modulated, and the bacteria respond by modifying gene expression in a concerted manner throughout the population. This AHL-dependent QS system has been investigated extensively in S. marcescens strain MG1 (previously misidentified as S. liquefaciens) (13). Moreover, quorum sensing may play a role in biofilm formation in S. marcescens (14, 15). A biofilm can be defined as a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface. Growth in biofilms enhances the survival of bacterial populations in hospital environments and during host infections (i.e., in the presence of antibiotics), increasing the probability of causing nosocomial infections (16–18). In addition, some biocides used in hospitals are ineffective against nosocomial pathogens growing as biofilms attached to surfaces (19). Biofilm formation has been connected to infections associated with indwelling medical devices, such as central venous catheters, urinary catheters, and contact lenses (20). Also, in hospitals, some medicinal products need to be stored at lower than ambient temperatures to ensure their quality and efficacy (vaccines, insulin, biotechnology products, drugs, etc.). Moreover, red blood cells or whole blood must always be stored at a temperature between 2 and 6°C to maintain the oxygen-carrying ability of blood and to minimize bacterial contamination. However, some psychrophilic, primarily Gram-negative pathogenic bacteria can proliferate from very low to clinically significant concentrations under storage conditions.
The goal of the present study was to analyze the abilities of 20 S. liquefaciens strains to produce quorum-sensing molecules, to express surface appendages, and to form biofilms during growth at different temperatures and under different medium conditions. Identifying these parameters should contribute to a better understanding of the correlation between the adherence capabilities and the pathogenicity of this bacterium.
MATERIALS AND METHODS
Bacterial strains.
Twenty S. liquefaciens strains were isolated from different clinical samples at the Hospital Universitario Marqués de Valdecilla in Santander, Spain (Table 1). All the strains have caused moderate or severe illness in monomicrobial infections and were considered not merely colonizers. All isolates were primarily identified as S. liquefaciens complex by the Vitek 2 automated system (bioMérieux, Marcy l'Etoile, France). Species identification by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) was performed in a Bruker Daltonics MALDI-TOF mass spectrometry device (Microflex; Bruker Daltonics) with flexControl software, which is able to differentiate between the three species of the S. liquefaciens complex (S. liquefaciens, Serratia grimesii, and Serratia proteamaculans). This system uses Serratia liquefaciens (ATCC 27592), S. grimesii (ATCC 14460), Serratia proteamaculans subsp. proteamaculans (ATCC 19323), and Serratia proteamaculans subsp. quinovora (ATCC 33765) as reference strains.
TABLE 1.
S. liquefaciens strains used in this study and their phenotypic characteristics
| Strain no.a | Clinical source | QSb detected with: |
Biofilm formationc at 4°C in: |
HAd | Fimbriaee | AAf | Diam (mm) of swimming motility zoneg at: |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| CV026 | VIR24 | LB | LB1/4 | 37°C | 24°C | |||||
| 1 | Bone | − | − | ++ | +/− | − | ++++ | + | 0 | 7 |
| 2 | Blood | +++ | + | ++ | ++ | MRHA | ++++ | + | 0 | 58 |
| 3 | Wound exudate | − | − | − | − | − | +++ | + | 0 | 14 |
| 4 | Abdominal drainage | +++ | + | +++ | ++ | − | +++ | + | 11 | 41 |
| 5 | Surgical wound exudate | − | − | +/− | +/− | − | +++ | − | 11 | 43 |
| 6 | Diabetic foot exudate | − | − | +++ | ++ | − | ++ | − | 9 | 31 |
| 7 | Buccal smear | ++ | + | +/− | +/− | MSHA | +++ | + | 0 | 0 |
| 8 | Bile | − | − | +++ | ++ | MSHA | +++ | − | 0 | 41 |
| 9 | Urine | − | − | − | − | − | +++ | − | 12 | 40 |
| 10 | Bone | − | − | +++ | ++ | − | + | − | 11 | 46 |
| 11 | Ulcer | − | − | +++ | ++ | MSHA | +++ | − | 7 | 54 |
| 12 | Tracheal aspirate | − | − | +++ | ++ | − | +++ | − | 7 | 36 |
| 13 | Urine | − | − | +/− | +/− | − | ++++ | + | 15 | 35 |
| 14 | Skin vesicle | − | − | ++ | ++ | MRHA | +++ | − | 8 | 40 |
| 15 | Blood | − | − | ++ | ++ | − | +++ | − | 9 | 32 |
| 16 | Ulcer | +++ | + | +++ | ++ | MSHA | +++ | + | 20 | 27 |
| 17 | Skin smear | − | − | +++ | +/− | MSHA | +++ | − | 8 | 25 |
| 18 | Urine | − | − | +/− | +/− | − | +++ | − | 10 | 25 |
| 19 | Urine | − | − | +++ | ++ | MSHA | +++ | − | 26 | 51 |
| 20 | Thigh bone graft | − | − | +++ | ++ | − | +++ | − | 20 | 42 |
Strains (from the Hospital Universitario Marqués de Valdecilla) are designated as follows: 1, HUMV 526; 2, HUMV 6339; 3, HUMV 6313; 4, HUMV 4524; 5, HUMV 4205; 6, HUMV 3632; 7, HUMV 3365; 8, HUMV 701; 9, HUMV 412; 10, HUMV 6817; 11, HUMV 5610; 12, HUMV 4222; 13, HUMV 2617; 14, HUMV 335; 15; HUMV 2935; 16, HUMV 21; 17, HUMV 185; 18, HUMV 405; 19, HUMV 779; 20, HUMV 3250.
Induction of violacein biosynthesis by AHLs in the C. violaceum reporter strains CV026 and VIR24 using the biosensor plate bioassay at 37°C, 25°C, and 4°C. Symbols indicate no induction − or weak (+), moderate (++), or strong (+++) induction.
Biofilm formation in LB or LB1/4 at 4°C after 5 days. Symbols indicate no biofilm formation − or weak (+/−), moderate (++), or strong (+++) biofilm formation.
MSHA, mannose-sensitive hemagglutination; MRHA, mannose-resistant hemagglutination; −, no agglutination of human red blood cells.
Data obtained by TEM were expressed as the percentage of fimbriated bacteria at 37°C, and strains were scored as follows: ++++, >95% fimbriated; +++, >75% fimbriated; ++, ∼40 to 60% fimbriated; +, <5% fimbriated.
AA, autoagglutination at 4°C in 96-U-bottom microplates. +, autoagglutination; −, no autoagglutination.
Results are averages from three independent experiments showing standard deviations of <10%.
The strains were routinely cultured on blood agar (BA) plates and in Luria-Bertani (LB) broth at 37°C and were frozen at −80°C with 20% glycerol. Chromobacterium violaceum strain CV026, used in bioassays for the production of quorum-sensing molecules, has been described elsewhere (21). C. violaceum strain VIR24 is a new reporter strain for long-N-acyl-chain homoserine lactones, which cannot be detected by CV026 (22). Both C. violaceum strains were grown at 25°C on LB agar plates.
Enterobacter sp. strains HUMV 2104, HUMV 4605, and HUMV 5198 were used as non-AHL-producing strains.
AHL bioassays.
Acylhomoserine lactone production by S. liquefaciens strains was detected by streaking against the biosensor strains CV026 and VIR24. Assay results were judged positive by induction of the purple pigment violacein in the C. violaceum reporters, as described previously (23). The plates were incubated for 48 h at 25°C, the optimal growth temperature for the C. violaceum biosensors. After incubation, the boundary of each reporter strain with each S. liquefaciens isolate tested was examined for violacein biosynthesis.
The production of AHLs in the extracellular products (ECPs) of S. liquefaciens was also tested after growth at 37°C, 25°C, and 4°C. S. liquefaciens strains were grown either for 24 h at 37°C or 25°C in tryptic soy broth (TSB), LB broth, or brain heart infusion broth (BHIB) or for 5 days at 4°C in LB broth or LB broth diluted 1:4 (LB1/4). Strains were then collected by centrifugation at 7,000 rpm for 5 min at room temperature (RT) using a microcentrifuge. Spent culture media (cell-free ECPs) were sterilized via membrane filtration (0.22 μm; Millipore), and 100 μl was used to stimulate violacein production in the reporter strains.
To prepare C. violaceum suspensions, bacteria were grown on LB agar plates at 25°C for 48 h, resuspended in phosphate-buffered saline (PBS), and adjusted spectrophotometrically to approximately 5.5 × 109 CFU ml−1 (optical density at 620 nm [OD620], 0.15). One hundred microliters of each culture suspension was added to the plates and was air dried for 30 min. Control plates with CV026 or VIR24 were incubated with the same volumes (100 μl) of fresh bacterial culture medium. Plates were incubated for 48 h at 25°C.
Mixing experiments using ECPs from AHL-producing strains.
Biofilm formation was also analyzed qualitatively in different strains after growth in the presence of spent culture media from the four AHL-producing strains, in order to determine if the presence of AHLs will favor or prevent biofilm formation in heterologous strains. In each well (24-well plate format), spent culture media from AHL-producing strains (750 μl) were mixed with 750 μl of LB broth and were inoculated with the test strain. Plates were incubated at 37°C or 25°C for 24 h, and biofilms were stained with crystal violet (CV) and were photographed. LB broth alone was inoculated with the test strain as a control.
AHL extraction and liquid chromatography (LC)-MS identification.
Samples (100 ml) from spent culture media of S. liquefaciens strains grown in liquid LB medium were obtained 24 h after inoculation at 37°C, acidified to pH 2 with 1 M HCl in a shaker at 200 rpm for 24 h at 20°C to ensure the absence of any AHL lactonolysis products, and extracted with dichloromethane as described previously (24). Dried extracts were reconstituted in 1 ml acetonitrile and were stored at −20°C until further analysis.
Analyses were carried out using an Agilent 1100 series high-performance liquid chromatograph (HPLC) (Agilent, Santa Clara, CA). The column was a Zorbax Eclipse XDB-C18 column (length, 150 mm; inside diameter, 4.6 mm; particle size, 5 μm). The mobile phase was built by 0.1% formic acid in water (A) and methanol (B), and the flow rate was 0.4 ml min−1. The gradient profile was as follows: first 50% B from 0 to 10 min, then a linear gradient from 50 to 90% B over 15 min, and finally 90% B for 25 min. The column was reequilibrated for a total of 4 min. Samples (2 μl) were diluted in 0.1% formic acid in acetonitrile and were injected onto the column.
MS experiments were conducted on an API 4000 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a Turbo ion source using positive-ion electrospray, multiple-reaction-monitoring (MRM) mode. The MRM signals were used to generate relative quantification information and to trigger subsequent quality product ion spectra (product ion, MS2). The conditions for the generation of the MRM-triggered spectra were as follows: declustering potential (DP) ramped from 35 to 57; collision energy (CE), 14 to 28; collision cell exit potential (CXP), 8.
AHLs with acyl chains of 4 (C4-HSL, 3-hydroxy-C4-HSL), 6 (C6-HSL, 3-hydroxy-C6-HSL, OC6-HSL, 3-oxo-C6-HSL), 8 (C8-HSL, 3-hydroxy-C8-HSL), 10 (C10-HSL, 3-hydroxy-C10-HSL), 12 (C12-HSL, 3-hydroxy-C12-HSL), or 14 (C14-HSL, 3-hydroxy-C14-HSL) carbon atoms were used as standards (Sigma). AHLs were identified and confirmed by comparing both the elution time and the spectra from any peaks obtained with those of the standards. The AHLs were quantified by comparison with a calibration curve constructed for molecular ion abundance, using each of the appropriate AHL synthetic standards (25).
Biofilm formation.
Biofilm formation was estimated in 96-well U-bottom polystyrene microtiter plates (Nunc, Thermo Fisher Scientific) by the method of O'Toole and Kolter (26) with some modifications. S. liquefaciens strains were grown in LB medium for 24 h at 37°C with shaking, and a 1:1,000 dilution was prepared in PBS (OD620, ∼0.01). Five microliters was placed in each well containing 145 μl of culture medium. The microplates were incubated for 24 h at 24°C or 37°C. Planktonic cells were removed, and the number of CFU was determined. Wells containing biofilms were rinsed three times with distilled water (200 μl/well), and the remaining adherent bacteria were stained with 190 μl/well of crystal violet (0.7% [wt/vol] solution; Sigma-Aldrich) for 12 min. Excess stain was removed by three washes with distilled water. Crystal violet was extracted by an ethanol-acetone solution (80:20, vol/vol), and the plates were incubated at RT in an orbital shaker for 1 min at 400 rpm (Thermomixer comfort; Eppendorf) to release the dye into the solution. Then a sample of 100 μl was transferred to another 96-well flat-bottom plate, and the amount of dye (proportional to the density of adherent cells) was determined at 620 nm using a microplate reader (Multiskan FC; Thermo Fisher). In each experiment, results were corrected for background staining by subtracting the value for crystal violet bound to uninoculated controls. The biofilm assay was performed three times, with octuplicates in each assay. TSB, LB broth, and BHIB were employed in experiments carried out at 24°C and 37°C. LB broth and LB broth diluted 1:4 in PBS were employed in experiments carried out at 4°C in 96- and 24-well plates. Biofilms were formed under static conditions. The number of CFU was converted to a logarithmic scale, and normalized biofilms were calculated by dividing the total biofilm value (expressed as the OD620) by the bacterial growth for each strain (expressed in CFU).
SEM.
Biofilm formation was also analyzed qualitatively using scanning electron microscopy (SEM) in 24-well plates (Nunc, Thermo Scientific). Biofilms were processed directly inside the plates after the removal of culture media and washing. The entire wells were fixed with ice-cold 3% glutaraldehyde for 20 min at 4°C. Wells were then dehydrated in a graded ethanol series, cut into small pieces with a hot lancet, dried by the critical point method, coated with gold in a Fine Coat ion sputter (JFC-1100; JEOL), and observed with an Inspect S microscope (FEI Company) working at 15 or 20 kV (see Fig. S1 in the supplemental material).
CLSM.
Bacteria were grown in uncoated 4-well μ-slides (Ibidi, Martinsried, Germany) without shaking. The slides were placed inclined (∼45°) into an incubator to form a liquid-air interface (see Fig. S2A in the supplemental material). After 24 h, unfixed planktonic cells were removed by rinsing with saline (0.85% NaCl), and bacterial viability within biofilms was determined by using the BacLight LIVE/DEAD bacterial viability kit (Molecular Probes Inc.). A series of optical sections was obtained with a Nikon A1R confocal scanning laser microscope (CLSM); the excitation wavelengths were 488 nm (green) and 561 nm (red), and 500- to 550-nm and 570- to 620-nm emission filters were used, respectively. Images at the liquid-air interface were captured at random with a 20× Plan Apo (numerical aperture [NA], 0.75) objective. Reconstructions of confocal sections were assembled using NIS-Elements software, version 3.2.
HA and HA inhibition tests.
To identify other factors associated with biofilm formation, we studied the hemagglutinating activities of the strains with human group A erythrocytes and the mannose sensitivities of these agglutinations. Human erythrocytes were obtained from healthy volunteers after informed consent. Hemagglutination (HA) tests were performed on microscope slides using 10% suspensions of human group A erythrocytes. Bacteria were cultured at 37°C for 24 h in LB medium, washed, and suspended in PBS to a concentration of ∼5 × 109 per ml. Twenty-five microliters of cultures was mixed with 25 μl of erythrocytes with or without 1% d-mannose (Sigma). Agglutination of erythrocytes was examined visually after a short period (up to 1 min) of rocking at RT. Hemagglutination was considered resistant to mannose (MRHA) when it occurred despite the presence of mannose and sensitive to mannose (MSHA) when it was inhibited by the presence of this carbohydrate.
Detection of genes encoding type I fimbriae by PCR.
All S. liquefaciens strains were analyzed genotypically by PCR using primers specific for the type 1 fimbrial adhesin FimH (fim-f, 5′-AACAGCGATCATTTCCAGTTTGTGTG-3′; fim-r, 5′-ATTGCGTACCAGCATTAGCAATGTCC-3′) as described previously (27). Briefly, bacterial DNA was extracted using the QIAamp DNA minikit (Qiagen) according to the manufacturer's instructions, and PCR amplification was carried out in a Mastercycler pro S thermal cycler (Eppendorf, Germany) in a 50-μl reaction mixture containing 1× PCR buffer, 1.5 mM MgCl2, 200 nM each 2′-deoxynucleoside 5′-triphosphate (dNTP), 1 μM each forward and reverse primer, 1.25 U of DreamTaq DNA polymerase (Thermo Scientific), and 25 ng of genomic DNA. PCR conditions were typically as follows: one initial denaturation at 94°C for 3 min; 40 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min; and a final extension at 72°C for 10 min. PCR products were resolved by electrophoresis on 1.5% agarose gels in Tris-acetate-EDTA (TAE) buffer and were stained with Sybr Safe stain (Invitrogen). Escherichia coli strain MG1655 was used as a positive control.
TEM.
All S. liquefaciens strains were examined by transmission electron microscopy (TEM) after growth at 37°C to confirm the presence of pili and flagella. Some strains were arbitrarily selected after growth at 4°C to compare the effects of temperature on the production of pili and flagella. Bacteria were applied to Formvar-coated grids and were air dried. The cells were then negatively stained with 1% phosphotungstic acid in distilled water for 20 s and were examined with a JEM-1011 transmission electron microscope (JEOL) operating at 80 kV and equipped with an Orius SC1000 charge-coupled device (CCD) camera (Gatan). At least 200 bacterial cells from each strain were examined for the presence of pili, and the percentage of piliated cells was determined.
Motility assays.
S. liquefaciens strains were grown in LB medium for 24 h at 37°C with shaking, and a 1:1,000 dilution was prepared in PBS (OD620, ∼0.01). Motility assays were performed as described previously (27). Briefly, tryptone swim plates (1% tryptone, 0.5% NaCl, and 0.3% agar) dried overnight at RT were point inoculated with bacteria using sterile toothpicks and were incubated for 24 h at 24°C or 37°C, and motility was assessed by the size of the circular zone. For swarming, tryptone swarm plates (1% tryptone, 0.5% NaCl, and 0.6% agar) dried overnight at RT were inoculated with bacteria using sterile toothpicks and were incubated for 24 h at 24°C or 37°C; the sizes of the swarming zones were observed macroscopically; and motility was assessed by the size of the circular zone. The diameters of the swimming motility zones were measured and are expressed as means for two independent experiments. Appropriate positive- and negative-control strains for swarming were included in the study (Pseudomonas aeruginosa strain PAO1 and Aeromonas hydrophila HUMV 1439, respectively).
Statistics.
The effects of temperature and the culture medium on biofilm formation were analyzed statistically using one-way analysis of variance (ANOVA) and Tukey's test using Excel (Microsoft Corporation, USA). Statistical significance was set at a two-tailed P value of <0.05. Data are presented as means ± standard deviations (SD) for three independent experiments. Each experiment was carried out in octuplicate wells.
RESULTS
Induction of violacein biosynthesis in AHL reporters by S. liquefaciens clinical isolates.
The AHL reporter strains C. violaceum CV026 and VIR24 differed in sensitivity to the AHL molecules in the simple biosensor plate assay. Only four S. liquefaciens isolates (20%) were found to induce violacein production in the reporter strains (Table 1; Fig. 1). The production of AHLs by S. liquefaciens was also tested after growth at 37°C or 25°C using cell-free ECPs (see Fig. S3 in the supplemental material) and after growth at 4°C using cell-free ECPs from wells containing biofilms (see Fig. S4a in the supplemental material). Only ECPs from the same four strains induced the production of pigment in C. violaceum. The presence of AHLs was also investigated by HPLC-MS in ethyl acetate extracts of acidified culture media from two positive S. liquefaciens strains (strains no. 7 and 16) and one negative isolate (strain no. 15). The low C4-HSL concentration retrieved in the culture medium of S. liquefaciens strain no. 15 (Table 2) is consistent with the negative response of this strain in the bioassay, where it failed to induce violacein production in either biosensor (Table 1). In contrast, LC-MS analysis revealed complex and strain-specific AHL profiles for the two strains capable of activating the sensors. Large amounts of N-butyryl-l-homoserine lactone (C4-HSL) and N-hexanoyl-l-homoserine lactone (C6-HSL) were found in the two positive strains studied, results consistent with the strong activation of the short-chain biosensor in these strains. N-Octanoyl-l-homoserine lactone (C8-HSL) was also present in small amounts in the two strains producing positive results in the bioassay. Small amounts of N-dodecanoyl-l-homoserine lactone (C12-HSL) and N-tetradecanoyl-l-homoserine lactone (C14-HSL) were also detected in the two strains (see Fig. S5 in the supplemental material). However, the production of N-decanoyl-l-homoserine lactone (C10-HSL) and N-3-oxohexanoyl-l-homoserine lactone (OC6-HSL) was strain dependent (Table 2).
FIG 1.
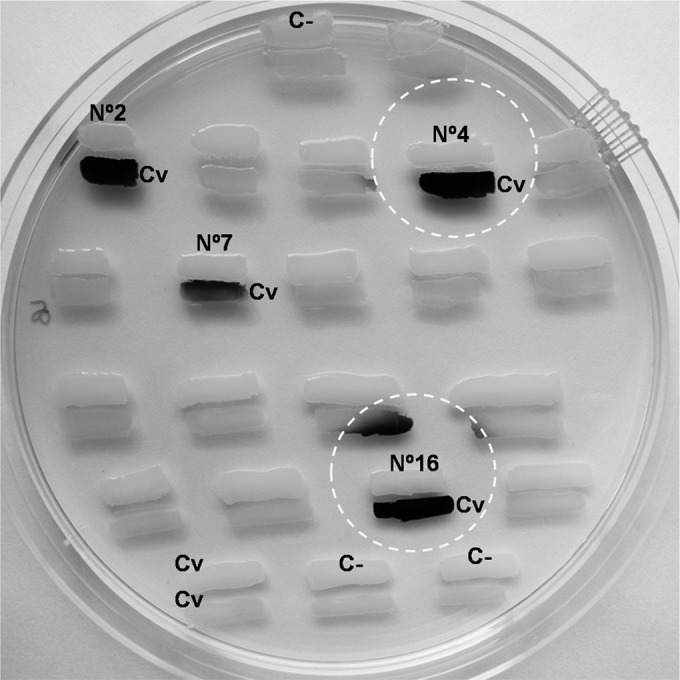
Bioreporter-based detection of AHL production by S. liquefaciens strains. Shown are examples of agar plate assays for AHL production at 25°C by S. liquefaciens isolates from clinical samples. The reporter strain (CV026) was streaked parallel to the test strains. The development of violet pigment in the reporter strain indicated the production of violacein. Strains no. 2, 4, 7, and 16 were positive. Negative controls (Enterobacter spp.) (top and bottom) were also included. Dashed circles indicate apparent diffusion halos. C−, negative control; Cv, C. violaceum.
TABLE 2.
Quantification of AHLs in ECPs from different S. liquefaciens strains by HPLC-MS
| S. liquefaciens strain no. | Activation of biosensors | Concn (nM)a |
||||||
|---|---|---|---|---|---|---|---|---|
| C4-HSL | C6-HSL | C8-HSL | C10-HSL | C12-HSL | C14-HSL | OC6-HSL | ||
| 7 | + | 669.85 | 7.41 | 0.19 | <0.1b | <0.1b | <0.1b | ND |
| 16 | + | 4.3 | 25.79 | 0.72 | ND | <0.1b | 0.66 | 13.79 |
| 15 | − | 0.8 | ND | ND | ND | ND | ND | ND |
S. liquefaciens cultures were grown in liquid LB medium for 24 h at 37°C. ND, not found/not determined.
The signal was detected and was correctly identified by MS, but the concentration did not allow reliable quantification.
Biofilm formation by S. liquefaciens.
The ability of S. liquefaciens to form biofilms was assessed by culturing the clinical isolates in three different culture media at 24°C and 37°C. Biofilms formed were quantified by crystal violet (CV) staining after 24 h. Biofilm analysis revealed a wide range of phenotypes. To quantify specific biofilm-forming ability, the normalized biofilm value was calculated by dividing the total biofilm value (expressed as the OD620) by the bacterial growth (expressed in CFU) for each strain (Fig. 2). Temperature had a significant impact on the biofilm-forming ability of S. liquefaciens (see Fig. S6 in the supplemental material). S. liquefaciens strains grew faster at 24°C than at 37°C, but bacterial growth was, in general, not strongly influenced by the culture medium, as estimated by the CFU of planktonic cells at both temperatures (see Fig. S7 in the supplemental material).
FIG 2.
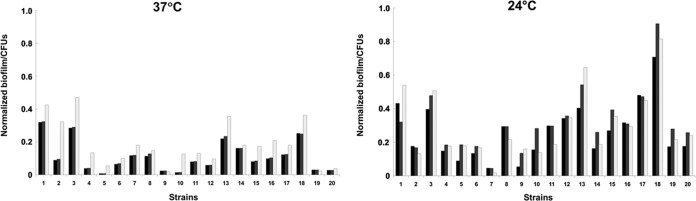
Normalized biofilm formation, calculated as total biofilm (expressed as the OD620) divided by growth (expressed in CFU). Incubation temperatures were 37°C (left) and 24°C (right). Black bars, LB medium; gray bars, TSB; white bars, BHIB. Strain designations corresponding to the numbers are given in Table 1, footnote a.
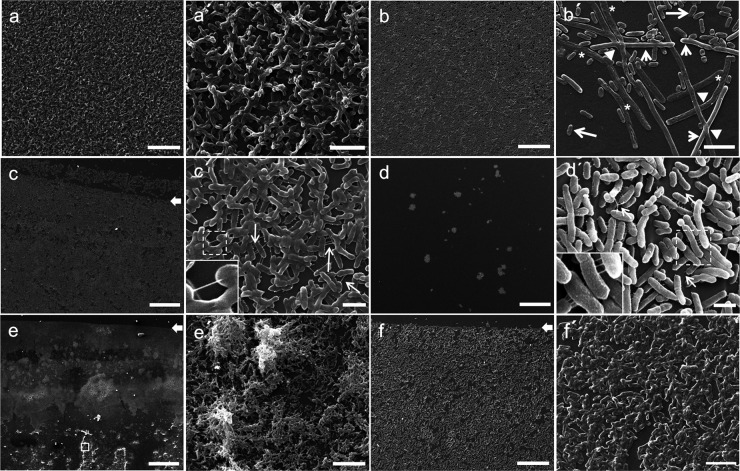
For the investigation of biofilm morphology in S. liquefaciens, three strains (strains no. 1, 7, and 13) were arbitrarily selected. The biofilms were found at the liquid-air interface, and this was imaged by SEM (Fig. 3). Similar results were observed using different plastic brands (Costar, Fisher Scientific, and Eppendorf) (data not shown).
FIG 3.
Scanning electron microscopy of S. liquefaciens biofilms. Shown are representative scanning electron micrographs of bacterial biofilms formed on plastic surfaces at the liquid-air interface in LB medium. (a and a′) Strain no. 1 at 24°C; (b and b′) strain no. 1 at 37°C; (c and c′) strain no. 13 at 24°C; (d and d′) strain no. 13 at 37°C; (e and e′) strain no. 7 at 24°C; (f and f′) strain no. 7 at 37°C. Arrows in panels c, e, and f indicate the air-liquid interface. Arrows in panels c′ and d′ indicate putative pili. Note that pili were not limited to the polar region (as a polar flagellum would be). Panel b′ is a detail of strain no. 1 showing three distinct morphotypes in a biofilm: normal cells (long arrows), elongated cells (short arrows), and ghost cells (asterisks). Arrowheads indicate elongated cells that appear to intersect. Panel e′ is a detail of the boxed area in panel e. Magnifications: ×1,800 (a and b), ×10,000 (a′, b′, and f′), ×1,000 (c), ×15,000 (c′), ×200 (d and e), ×20,000 (d′), ×3,500 (e′), and ×2,000 (f). Bars: 25 μm (a, b, and f), 5 μm (a′, b′, and f′), 50 μm (c), 2.5 μm (c′ and d′), 250 μm (d and e), and 15 μm (e′).
Morphology was found to be variable, even for biofilms formed by the same strain assayed at different temperatures. At 24°C, strains no. 1 and 13 formed thick biofilms with small, hilly structures spatially distributed (Fig. 3a and c). In contrast, the same strains formed smooth, flat biofilms at 37°C (Fig. 3b and d). Interestingly, many cells of strain no. 1 showed a wide range of abnormalities; some appeared to have lost the cytoplasmic material and had collapsed. These cells seemed transparent, with a ghost-like appearance. Moreover, the elongated cells crossed over/under each other or appeared to intersect (Fig. 3b′). This phenomenon was clearly observed only in this strain. CLSM revealed that these cells were likely to undergo filamentous growth before dying, measuring as much as 60 μm in length, or even more, as shown in Fig. S2B in the supplemental material.
SEM analysis of strain no. 7 cultured at 24°C showed the presence of strong biofilms and visible microcolonies (Fig. 3e and e′). However, this strain formed a few small groups of bacteria sparsely distributed at 37°C (Fig. 3f and f′). SEM also showed that cells of each of the three strains were linked to each other through extracellular appendages, possibly pilus-like structures (arrows in Fig. 3c′ and d′). Additionally, bacteria were almost entirely encased in an exopolysaccharide matrix (Fig. 3a′, c′, and d′). Figure 3e′ shows a more sparsely populated region on the plastic surface where the individual bacteria can be visualized.
The biofilm formation assay was also performed at 4°C in 96-well plates. At that temperature, 7 strains showed autoagglutination (data not shown), while the other 13 strains did not (Table 1). Consequently, we decided to perform a qualitative biofilm assay at 4°C in 24-well plates using LB medium and LB medium diluted 1:4 in order to observe biofilm formation under reduced-nutrient conditions. Representative examples of biofilm formation in 24-well plates by the 20 S. liquefaciens strains after 5 days of growth at 4°C are shown in Fig. 4 and Fig. S4b in the supplemental material. At 4°C, the colonization of plastic surfaces was very slow. However, after 5 days of incubation, many strains formed biofilms that could be classified as strong, moderate, or weak. Fourteen strains were found to form moderate to strong biofilms, and six strains exhibited weak biofilm formation or none. At this temperature, bacterial populations increased progressively over time for all strains, reaching counts from ∼6 × 108 CFU ml−1 for strain no. 3 to ∼2.8 × 109 CFU ml−1 for strain no. 8. Similar counts were obtained in cultures growing on LB1/4 medium (data not shown). Curiously, nutrient levels in the system did not greatly affect biofilm formation (Table 1).
FIG 4.
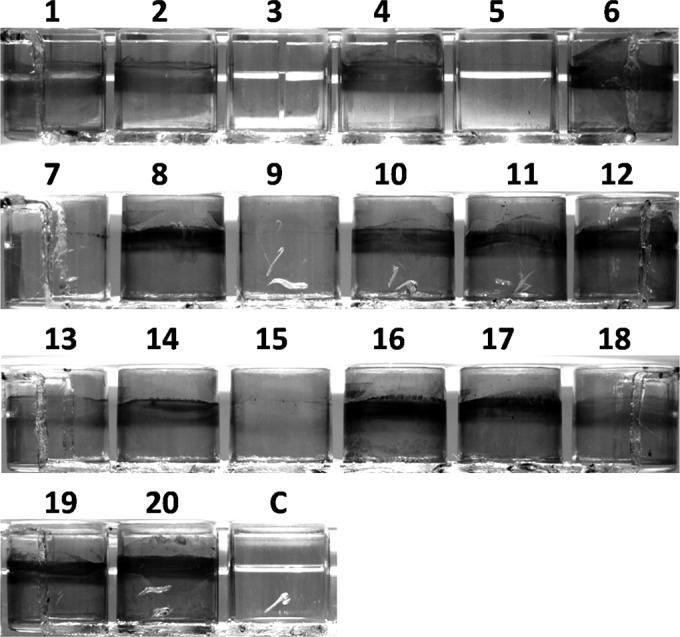
Biofilm formation at 4°C by the 20 S. liquefaciens strains. Shown are representative examples of biofilm formation in 24-well plates by the 20 S. liquefaciens strains after 5 days of growth at 4°C in LB medium. Wells were stained with CV. Strain designations corresponding to the numbers are given in Table 1, footnote a. C, uninoculated control.
In mixing experiments using S. liquefaciens ECPs from the four AHL-producing strains, we observed that spent media from these strains did not promote or affect biofilm formation in the same strain or in heterologous strains (producing or nonproducing strains). The same results were observed at 37°C and at 25°C. Some examples are shown in Fig. S8 in the supplemental material.
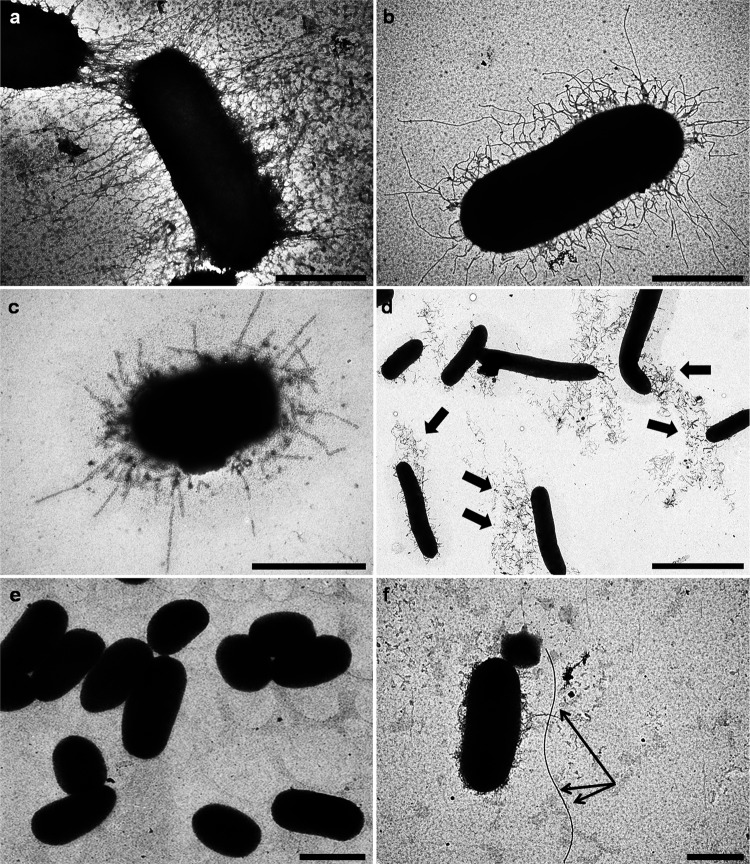
Surface appendages in S. liquefaciens.
Hemagglutination was observed in eight strains (40%). HA was mannose sensitive in six cases and mannose resistant (MR) in the other two (Table 1). All but one of the isolates, including all the HA-positive isolates, were piliated at 37°C (Table 1). Representative TEM images of selected strains are shown in Fig. 5. Growth temperature affects pilus production. For example, strain no. 10 showed a smooth surface in the majority of the population (Fig. 5e) at 37°C. In contrast, more piliated cells appeared in this strain at 4°C (Fig. 6b).
FIG 5.
Examples of transmission electron micrographs of negatively stained bacteria grown for 24 h in LB medium at 37°C. (a) Strain no. 14; (b) strain no. 19; (c) strain no. 20; (d) strain no. 1; (e) strain no. 10; (f) strain no. 16. Note the detachment of pili from the bacterial surface of strain no. 1 (indicated by arrows in panel d). Arrows in panel f indicate a single flagellum. Magnifications: ×40,000 (a and b), ×50,000 (c), ×8,000 (d), ×10,000 (e), ×25,000 (f). Bars: 0.5 μm (a through c), 2.5 μm (d), 1.25 μm (e), and 1 μm (f).
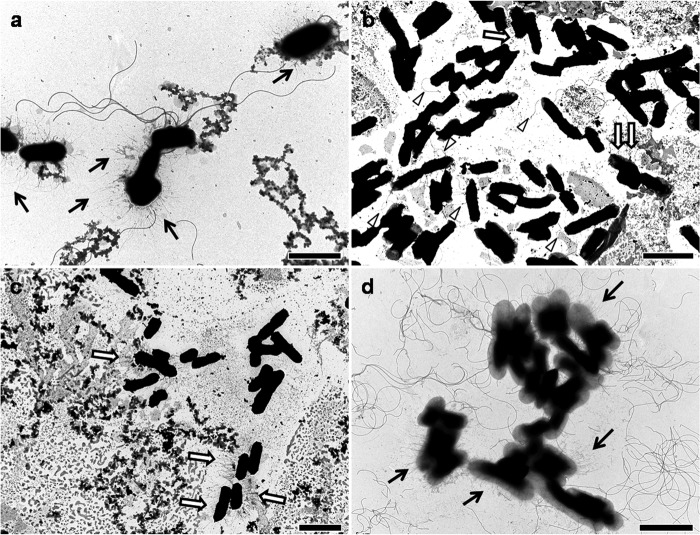
FIG 6.
Examples of transmission electron micrographs of negatively stained bacteria after growth at a low temperature (4°C). (a) Strain no. 1; (b) strain no. 10; (c) strain no. 7; (d) strain no. 11. Arrows indicate pili, and arrowheads in panel b indicate flagella. Magnifications: ×12,000 (a and d), ×10,000 (b), ×8,000 (c). Bars: 2.5 μm (a and d) and 5 μm (b and c).
There was no correlation between biofilm formation and MR agglutination of human erythrocytes. Of note, strains exhibiting strong biofilm formation at 37°C (strains no. 1, 3, 13, and 18) gave negative results in all the hemagglutination tests. Furthermore, the strains were examined genotypically by PCR for the presence of type I fimbriae, and all strains were positive (data not shown).
Interestingly, in several strains, including strain no. 1 (Fig. 6a), strong biofilm production at 4°C was accompanied by the presence of multiple flagella. In this strain, pili were long at 4°C, becoming shorter at 24°C and scarce at 37°C. Interestingly, strain no. 7 was unable to form biofilms at 4°C (Fig. 4; see also Fig. S4b in the supplemental material), and no flagella could be detected (Fig. 6c).
Motility in S. liquefaciens.
To investigate whether motility was correlated with biofilm formation, swimming motility and swarming motility were analyzed at 24°C and 37°C for all S. liquefaciens strains. Most strains showed swimming ability at 24°C, but this ability was reduced or suppressed at 37°C (Table 1). On swarming plates, most of the isolates were unable to spread minimally and showed only pinpoint colonies at both temperatures. The positive-control strain (P. aeruginosa PAO1) swarmed, producing tendrils that covered >80% of the plates within 24 h, while the negative-control A. hydrophila strain did not swarm (not shown).
DISCUSSION
Many bacterial behaviors, including biofilm formation, are regulated by quorum sensing. We aimed to gain knowledge about this system in S. liquefaciens clinical isolates. C. violaceum CV026 is a well-known AHL reporter strain, but this strain could not recognize long-acyl-chain homoserine lactones such as C10- and C14-HSLs. Strain VIR24 could detect long-acyl-chain HSL molecules not detected by CV026 (22). It is well known that Serratia spp. may utilize quorum sensing for motility, extracellular and cell-associated enzyme production, and pigment synthesis (15, 28). However, the role of an AHL-mediated QS system in S. liquefaciens is less well understood, because most studies were performed with an S. liquefaciens strain, MG1, that has recently been reclassified as Serratia marcescens. In the present work, there is no evidence of any correlation between AHL production and motility for the different S. liquefaciens strains. To complicate this matter, at least four different QS systems have been described in Serratia spp. (29). Our results showed that AHL-mediated QS in S. liquefaciens is not dominant (only four strains showed pigment induction in C. violaceum), and other molecules may play important signaling roles in this species.
The ability of nosocomial pathogens to form biofilms is of significant clinical interest, since biofilm formation influences the efficacy of antimicrobial therapy and the outcome of an infection. Moreover, biofilm formation may contribute to the establishment and long-term survival of bacterial pathogens in the hospital environment.
A number of previous studies have shown that the nutrient content of the growth medium influences biofilm development in different organisms. More specifically, environmental factors such as glucose and temperature affect biofilm development in Enterobacteriaceae and other bacteria (30–32). Other environmental conditions, such as limited nutrient levels, may also serve as signals for the expression of biofilm-related genes. Given that biofilm formation is reported to enhance bacterial survival, colonization, and protection from antibiotics and host immune responses, the ability of S. liquefaciens to form biofilms may represent an important virulence mechanism in relation to its pathobiology. We determined the absence of correlation between the ability to form biofilms and the type of infection or body site from which isolates were collected. Furthermore, we demonstrated that the production of C4- or C6-HSL by S. liquefaciens is not essential for biofilm formation.
Representative isolates positive for biofilm formation, as determined by the microtiter method, were selected for SEM analysis. In agreement with the results from the CV assay, SEM studies demonstrated that the strains selected were capable of forming biofilms in a temperature-dependent fashion. In addition, the structure of the biofilms and the phenotypes observed were unique for each strain tested.
During biofilm formation, bacteria express a diverse arsenal of factors that might affect adhesion, including physical interactions between bacteria and the substratum, pili, flagella, and the presence of extracellular polymeric substances. MSHA results from the expression of type I pili, whereas MRHA results from the expression of P, S, and/or other adhesins. Labbate and coworkers (33) showed that type I fimbriae were necessary for the attachment of Serratia marcescens to epithelial cells. Also in S. marcescens, Shanks and coworkers showed that type I fimbriae were essential for biofilm formation (34). Moreover, several authors have found a positive correlation between biofilm formation, cell motility, and the presence of type I pili in Escherichia coli strains (35). In Klebsiella pneumoniae, the expression of type III fimbriae was found to strongly promote biofilm formation (36). Under TEM, a wide variety of different morphological types of pili have been observed in S. liquefaciens. Moreover, the expression of pili in individual cells of some strains is highly variable, as indicated by the presence of fimbriated and nonfimbriated bacterial cells in the same culture. This has also been reported for other species of the Enterobacteriaceae and could be indicative of phase variation (27, 37–40). However, not all fimbrial operons are regulated by phase variation; further work should be done, and genetic evidence should be provided, before any conclusion can be drawn on phase variation of specific fimbriae in S. liquefaciens.
Furthermore, SEM images showed pilus-like structures that seemed to promote bacterium-to-bacterium interactions in addition to adhesion to inert surfaces. However, we do not discard the possibility that these pilus-like structures could also be desiccated matrix material. Our study strongly indicates that strain background plays an important role in the impact of surface appendage expression on biofilm formation in S. liquefaciens. Hence, there is a great deal of interest in further identifying and characterizing these structures, including their exact adhesive capabilities.
Concerning flagellar expression, our observations reflect the fact that temperature is very important for the production of lateral flagella and motility in S. liquefaciens. Downregulation of swimming motility is observed in response to a temperature upshift from 24 to 37°C. At 24°C, swimming motility in S. liquefaciens (but not swarming) may play an important role in increasing biofilm biomass during growth. This phenomenon has also been observed in S. marcescens (41). We have found an exception, strain no. 7, which was able to form biofilms at 37°C despite its lack of swimming and swarming motility, indicating that flagella are not a prerequisite for biofilm formation, or that motility does not make a large contribution to biofilm formation in this strain. Once again, bacteria growing at 4°C were also able to express multiple flagella, which may contribute to biofilm formation at low temperatures. At this point, neither flagellated cells nor biofilm formation was observed in strain no. 7 at 4°C.
Also, nutrient limitation can produce diverse responses in bacteria, i.e., reductions in cell size, modulation of gene expression, and changes in bacterial morphology. We have shown that under reduced-nutrient conditions, the level of biofilm formation is maintained. Hence, the combination of temperature and nutrient availability can affect the attachment and survival of S. liquefaciens strains under storage conditions. Importantly, Greco and coworkers have shown recently that S. marcescens and S. liquefaciens form biofilms under platelet storage conditions in hospitals, a phenomenon associated with reduced detection by colony counting (42, 43). Further work is necessary to define the signaling mechanisms underlying this response.
We conclude that the ability of S. liquefaciens to survive at room temperature or under unfavorable environmental conditions (low temperatures and reduced nutrient levels) and the variability in biofilm formation among S. liquefaciens isolates may contribute to the risk for human infection posed by certain strains. Therefore, a better understanding of the adherence properties of this species will provide greater insights into the diseases it causes.
Supplementary Material
ACKNOWLEDGMENTS
S.R.-M. and M.L.-D. hold a contract from the Instituto de Investigación Marqués de Valdecilla (IDIVAL). J.R.-V. holds a Miguel Servet contract for Young Researchers from the Instituto de Salud Carlos III, Spain (grants CP08/00100 and PS09/00466). J.M.I. was supported by the Spanish Ministerio de Economía y Competitividad (grant CGL2008-04559/BOS). A.O. is supported by the Fundación Ramón Areces (grant CIVP16A1814).
We thank Tomohiro Morohoshi for kindly providing the VIR24 strain and Fidel Madrazo (Electron Microscopy Unit, Technology Support Services, IDIVAL) for help with TEM and CLSM.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00088-15.
REFERENCES
- 1.Arslan U, Erayman I, Kirdar S, Yuksekkaya S, Cimen O, Tuncer I, Bozdogan B. 2010. Serratia marcescens sepsis outbreak in a neonatal intensive care unit. Pediatr Int 52:208–212. doi: 10.1111/j.1442-200X.2009.02934.x. [DOI] [PubMed] [Google Scholar]
- 2.Chemaly RF, Rathod DB, Raad II. 2009. A tertiary care cancer center experience of the 2007 outbreak of Serratia marcescens bloodstream infection due to prefilled syringes. Infect Control Hosp Epidemiol 30:1237–1238. doi: 10.1086/648661. [DOI] [PubMed] [Google Scholar]
- 3.Ramírez-Arcos S, Chin-Yee I, Hume H, Fearon M, Goldman M, Eckert K, Martincic I, Peters G, Kovach D, Richardson SE. 2006. Fatal septic shock associated with transfusion-transmitted Serratia marcescens. Transfusion 46:679–681. doi: 10.1111/j.1537-2995.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Voelz A, Müller A, Gillen J, Le C, Dresbach T, Engelhart S, Exner M, Bates CJ, Simon A. 2010. Outbreaks of Serratia marcescens in neonatal and pediatric intensive care units: clinical aspects, risk factors and management. Int J Hyg Environ Health 213:79–87. doi: 10.1016/j.ijheh.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Engelhart S, Saborowski F, Krakau M, Scherholz-Schlosser G, Heyer I, Exner M. 2003. Severe Serratia liquefaciens sepsis following vitamin C infusion treatment by a naturopathic practitioner. J Clin Microbiol 41:3986–3988. doi: 10.1128/JCM.41.8.3986-3988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald P, Drew JH, Kruszelnicki I. 1984. Serratia: a problem in a neonatal nursery. Aust Paediatr J 20:205–207. [DOI] [PubMed] [Google Scholar]
- 7.Grohskopf LA, Roth VR, Feikin DR, Arduino MJ, Carson LA, Tokars JI, Holt SC, Jensen BJ, Hoffman RE, Jarvis WR. 2001. Serratia liquefaciens bloodstream infections from contamination of epoetin alfa at a hemodialysis center. N Engl J Med 344:1491–1497. doi: 10.1056/NEJM200105173442001. [DOI] [PubMed] [Google Scholar]
- 8.Mossad SB. 2000. The world's first case of Serratia liquefaciens intravascular catheter-related suppurative thrombophlebitis and native valve endocarditis. Clin Microbiol Infect 6:559–560. doi: 10.1046/j.1469-0691.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- 9.Pinna A, Usai D, Sechi LA, Carta A, Zanetti S. 2011. Detection of virulence factors in Serratia strains isolated from contact lens-associated corneal ulcers. Acta Ophthalmol 89:382–387. doi: 10.1111/j.1755-3768.2009.01689.x. [DOI] [PubMed] [Google Scholar]
- 10.Roth VR, Arduino MJ, Nobiletti J, Holt SC, Carson LA, Wolf CF, Lenes BA, Allison PM, Jarvis WR. 2000. Transfusion-related sepsis due to Serratia liquefaciens in the United States. Transfusion 40:931–935. doi: 10.1046/j.1537-2995.2000.40080931.x. [DOI] [PubMed] [Google Scholar]
- 11.Martincic I, Mastronardi C, Chung A, Ramírez-Arcos S. 2008. Unexplained agglutination of stored red blood cells in Alsever's solution caused by the gram-negative bacterium Serratia liquefaciens. Immunohematology 24:39–44. [PubMed] [Google Scholar]
- 12.Traub WH. 2000. Antibiotic susceptibility of Serratia marcescens and Serratia liquefaciens. Chemotherapy 46:315–321. doi: 10.1159/000007304. [DOI] [PubMed] [Google Scholar]
- 13.Rice SA, Koh KS, Queck SY, Labbate M, Lam KW, Kjelleberg S. 2005. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J Bacteriol 187:3477–3485. doi: 10.1128/JB.187.10.3477-3485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbate M, Queck SY, Koh KS, Rice SA, Givskov M, Kjelleberg S. 2004. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J Bacteriol 186:692–698. doi: 10.1128/JB.186.3.692-698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Houdt R, Givskov M, Michiels CW. 2007. Quorum sensing in Serratia. FEMS Microbiol Rev 31:407–424. doi: 10.1111/j.1574-6976.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- 16.Espinal P, Marti S, Vila J. 2012. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Trampuz A, Widmer AF. 2006. Infections associated with orthopedic implants. Curr Opin Infect Dis 19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 18.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 19.Vickery K, Deva A, Jacombs A, Allan J, Valente P, Gosbell IB. 2012. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J Hosp Infect 80:52–55. doi: 10.1016/j.jhin.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Donlan RM. 2001. Biofilms and device-associated infections. Emerg Infect Dis 7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143(Part 12):3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 22.Someya N, Morohoshi T, Okano N, Otsu E, Usuki K, Sayama M, Sekiguchi H, Ikeda T, Ishida S. 2009. Distribution of N-acylhomoserine lactone-producing fluorescent pseudomonads in the phyllosphere and rhizosphere of potato (Solanum tuberosum L.). Microbes Environ 24:305–314. doi: 10.1264/jsme2.ME09155. [DOI] [PubMed] [Google Scholar]
- 23.Vivas J, Razquin BE, Lopez-Fierro P, Naharro G, Villena A. 2004. Correlation between production of acyl homoserine lactones and proteases in an Aeromonas hydrophila aroA live vaccine. Vet Microbiol 101:167–176. doi: 10.1016/j.vetmic.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Romero M, Muras A, Mayer C, Bujan N, Magarinos B, Otero A. 2014. In vitro quenching of fish pathogen Edwardsiella tarda AHL production using marine bacterium Tenacibaculum sp. strain 20J cell extracts. Dis Aquat Organ 108:217–225. doi: 10.3354/dao02697. [DOI] [PubMed] [Google Scholar]
- 25.Milton DL, Chalker VJ, Kirke D, Hardman A, Camara M, Williams P. 2001. The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J Bacteriol 183:3537–3547. doi: 10.1128/JB.183.12.3537-3547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 27.Padilla D, Acosta F, Garcia JA, Real F, Vivas JR. 2009. Temperature influences the expression of fimbriae and flagella in Hafnia alvei strains: an immunofluorescence study. Arch Microbiol 191:191–198. doi: 10.1007/s00203-008-0442-y. [DOI] [PubMed] [Google Scholar]
- 28.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Engelborghs Y, Michiels CW. 2006. Role of quorum sensing and antimicrobial component production by Serratia plymuthica in formation of biofilms, including mixed biofilms with Escherichia coli. Appl Environ Microbiol 72:7294–7300. doi: 10.1128/AEM.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei JR, Lai HC. 2006. N-Acylhomoserine lactone-dependent cell-to-cell communication and social behavior in the genus Serratia. Int J Med Microbiol 296:117–124. doi: 10.1016/j.ijmm.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Di Bonaventura G, Piccolomini R, Paludi D, D'Orio V, Vergara A, Conter M, Ianieri A. 2008. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J Appl Microbiol 104:1552–1561. doi: 10.1111/j.1365-2672.2007.03688.x. [DOI] [PubMed] [Google Scholar]
- 31.Martí S, Rodríguez-Baño J, Catel-Ferreira M, Jouenne T, Vila J, Seifert H, Dé E. 2011. Biofilm formation at the solid-liquid and air-liquid interfaces by Acinetobacter species. BMC Res Notes 4:5. doi: 10.1186/1756-0500-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivas J, Padilla D, Real F, Bravo J, Grasso V, Acosta F. 2008. Influence of environmental conditions on biofilm formation by Hafnia alvei strains. Vet Microbiol 129:150–155. doi: 10.1016/j.vetmic.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Labbate M, Zhu H, Thung L, Bandara R, Larsen MR, Willcox MD, Givskov M, Rice SA, Kjelleberg S. 2007. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J Bacteriol 189:2702–2711. doi: 10.1128/JB.01582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanks RM, Stella NA, Kalivoda EJ, Doe MR, O'Dee DM, Lathrop KL, Guo FL, Nau GJ. 2007. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol 189:7262–7272. doi: 10.1128/JB.00859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schembri MA, Klemm P. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect Immun 69:1322–1328. doi: 10.1128/IAI.69.3.1322-1328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroll C, Barken KB, Krogfelt KA, Struve C. 2010. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol 10:179. doi: 10.1186/1471-2180-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayliss CD. 2009. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol Rev 33:504–520. doi: 10.1111/j.1574-6976.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 38.Snyder JA, Lloyd AL, Lockatell CV, Johnson DE, Mobley HL. 2006. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect Immun 74:1387–1393. doi: 10.1128/IAI.74.2.1387-1393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Struve C, Bojer M, Krogfelt KA. 2008. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. Infect Immun 76:4055–4065. doi: 10.1128/IAI.00494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Woude MW, Bäumler AJ. 2004. Phase and antigenic variation in bacteria. Clin Microbiol Rev 17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai HC, Soo PC, Wei JR, Yi WC, Liaw SJ, Horng YT, Lin SM, Ho SW, Swift S, Williams P. 2005. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J Bacteriol 187:3407–3414. doi: 10.1128/JB.187.10.3407-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco-Stewart VS, Brown EE, Parr C, Kalab M, Jacobs MR, Yomtovian RA, Ramirez-Arcos SM. 2012. Serratia marcescens strains implicated in adverse transfusion reactions form biofilms in platelet concentrates and demonstrate reduced detection by automated culture. Vox Sang 102:212–220. doi: 10.1111/j.1423-0410.2011.01550.x. [DOI] [PubMed] [Google Scholar]
- 43.Greco CA, Zhang JG, Kalab M, Yi QL, Ramirez-Arcos SM, Gyongyossy-Issa MI. 2010. Effect of platelet additive solution on bacterial dynamics and their influence on platelet quality in stored platelet concentrates. Transfusion 50:2344–2352. doi: 10.1111/j.1537-2995.2010.02726.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.