Abstract
Phosphorus is a vital nutrient for living organisms and is obtained by bacteria primarily via phosphate uptake. However, phosphate is often scarcely accessible in nature, and there is evidence that in many areas of the ocean, its concentration limits bacterial growth. Surprisingly, the phosphate starvation response has been extensively investigated in different model organisms (e.g., Escherichia coli), but there is a dearth of studies on heterotrophic marine bacteria. In this work, we describe the response of Pseudovibrio sp. strain FO-BEG1, a metabolically versatile alphaproteobacterium and potential symbiont of marine sponges, to phosphate limitation. We compared the physiology, protein expression, and secondary metabolite production under phosphate-limited conditions to those under phosphate surplus conditions. We observed that phosphate limitation had a pleiotropic effect on the physiology of the strain, triggering cell elongation, the accumulation of polyhydroxyalkanoate, the degradation of polyphosphate, and the exchange of membrane lipids in favor of phosphorus-free lipids such as sulfoquinovosyl diacylglycerols. Many proteins involved in the uptake and degradation of phospho-organic compounds were upregulated, together with subunits of the ABC transport system for phosphate. Under conditions of phosphate limitation, FO-BEG1 secreted compounds into the medium that conferred an intense yellow coloration to the cultures. Among these compounds, we identified the potent antibiotic tropodithietic acid. Finally, toxin-like proteins and other proteins likely involved in the interaction with the eukaryotic host were also upregulated. Altogether, our data suggest that phosphate limitation leads to a pronounced reorganization of FO-BEG1 physiology, involving phosphorus, carbon, and sulfur metabolism; cell morphology; secondary metabolite production; and the expression of virulence-related genes.
INTRODUCTION
Phosphorus (P) is an essential macronutrient for all living organisms, since it is an important component of biomolecules and a fundamental element in cellular regulatory processes. The preferential source of P for bacteria is phosphate (Pi), even though organic molecules containing P, such as phosphoesters (molecules with C—O—P bonds) and phosphonates (molecules with C—P bonds), which together are components of the dissolved organic phosphorus pool (DOP), can also be utilized (1). Pi is often scarcely accessible in nature. In many marine environments, the concentration of Pi can be in the nanomolar range, and there is growing evidence that P limits bacterial growth and productivity in many areas of the ocean, at least during part of the year (2–5). In addition, unlike nitrogen, P cannot be fixed from the atmosphere; thus, over geological time scales, it is considered to be the ultimate limiting macronutrient in marine ecosystems (6).
Due to its crucial role in cell metabolism and its scarcity in natural environments, bacteria evolved several mechanisms to sense Pi concentrations and regulate P metabolism accordingly. Pi starvation induces the expression of the so-called Pi starvation-inducible (psi) genes, which encode several proteins involved in P uptake and metabolism. Among these genes are those coding for high-affinity transporters for Pi (ABC transporters) and enzymes involved in the uptake and degradation of organic molecules containing P. These genes are members of the Pho regulon, a global regulatory circuit involved in bacterial P management (7). The Pho regulon is controlled by a two-component regulatory system (TCRS), composed of a transmembrane histidine kinase, PhoR, and a response regulator, PhoB. When the concentration of environmental Pi is low, PhoR undergoes autophosphorylation using ATP and acts as a kinase for the response regulator. PhoB then binds to a specific target sequence, called the Pho box, in the upstream regions of the genes of the Pho regulon, regulating their transcription (7). In Escherichia coli, the Pi limitation response can involve the regulation of up to 400 genes, which represent almost 10% of the E. coli genome (8). This high number of genes reflects the importance of Pi in the regulation of cellular physiology, including processes not directly correlated with P metabolism, such as the production of secondary metabolites (9). Additionally, it is well documented that the PhoR-PhoB system is involved in the regulation of virulence-related genes (10).
The Pho regulon and the Pi limitation response have been extensively studied in E. coli, and the responses to Pi limitation of other important pathogenic bacteria, plant symbionts, and bacteria of biotechnological interest have also been investigated (11–15). Strangely, despite growing evidence suggesting that Pi limitation is common in many areas of the ocean, among marine microorganisms, the Pi limitation response was investigated mainly in phototrophs (16, 17), and studies on the Pi limitation response of heterotrophic marine bacteria are scarce. In this study, we investigated the response to Pi limitation of the chemorganoheterotrophic strain FO-BEG1, an alphaproteobacterium closely related to Pseudovibrio denitrificans. Bacteria belonging to this genus have been isolated worldwide, often from marine sponges, and are therefore assumed to be their symbionts (18).
In a previous study, we analyzed the genome of strain FO-BEG1 and showed that it is metabolically versatile and possesses several genes that could play a role in prokaryote-eukaryote interactions, indicating that FO-BEG1 is well adapted to both free-living and symbiotic life-styles (18). Furthermore, we identified genes and gene clusters involved in the synthesis of bioactive secondary metabolites, in accordance with previously reported experimental data that showed the production of these compounds in many strains belonging to the Pseudovibrio genus (19).
In this study, we compared the physiology, protein expression, and secondary metabolite production of strain FO-BEG1 under Pi-limited (−Pi) conditions to those under Pi surplus (+Pi) conditions, and we integrated these data with a bioinformatic analysis to identify putative Pho boxes in the strain genome. We observed that Pi limitation leads to a severe physiological reorganization, which influences not only P metabolism but also many other metabolic and structural traits. We discovered that Pi limitation triggers the production of secondary metabolites and influences the expression of virulence-related genes, which could play a crucial role in the establishment and maintenance of interactions with eukaryotic hosts.
MATERIALS AND METHODS
Growth conditions.
Strain FO-BEG1 was cultivated under −Pi and +Pi conditions by using the carbohydrate-mineral (CM) medium, as described previously (20). The medium contained glucose (10 mmol liter−1) as the only carbon source, ammonia (10 mmol liter−1) as the only nitrogen source, and final Pi concentrations of 1.4 mmol liter−1 and 0.1 mmol liter−1 under +Pi and −Pi conditions, respectively. This resulted in ratios of C to N to P of 43:7:1 under +Pi conditions and 600:100:1 under −Pi conditions. The medium was buffered with Tris-HCl, and the pH was adjusted to 8. Erlenmeyer flasks (250 ml) were filled with 100 ml of medium, inoculated with 100 μl of a preculture grown under +Pi conditions, incubated at 28°C in the dark, and shaken at 120 rpm. Bacterial growth was monitored by measurement of optical density at 600 nm (OD600) using an Eppendorf BioPhotometer (Eppendorf AG). The OD600 was correlated with the cell number determined by using a Thoma counting chamber (Brand GmbH) (data not shown). Micrographs were taken throughout the growth period by using an Axioplan universal microscope (Carl Zeiss GmbH), and at least 100 cells for each time point and under each condition were measured by using the AxioVision Rel. 4.8 imagining system program (Carl Zeiss GmbH). During the growth period, the UV-visible (UV-Vis) spectra of the cell-free supernatants were recorded by using a Beckman DU 640 spectrophotometer (Beckman Coulter).
Chemical analyses, determination of alkaline phosphatase activity, and detection of inclusions.
Glucose and acetate concentrations were determined by using a high-performance liquid chromatography (HPLC) system (Sykam GmbH), as described previously by Bondarev et al. (18). Pi concentrations were determined colorimetrically by the ascorbic acid method (21), using a SpectroDirect spectrophotometer (Aqualytic). The activity of alkaline phosphatase (AP) during bacterial growth was evaluated by monitoring the degradation of 4-para-nitrophenyl phosphate bis(tris) salt (pNPP) (purity, ≥97.00%; Sigma-Aldrich). Experiments were performed independently twice, always analyzing biological triplicates. The level of tropodithietic acid (TDA) was measured by means of reverse-phase HPLC (RP-HPLC), using pure TDA as a standard (purity of ≥98%; BioViotica Naturstoffe GmbH). The presence and quantification of polyphosphate were investigated by staining cells with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), performed according to the method described previously by Kulakova et al. (22). Nile red was used for staining of polyhydroxyalkanoate (PHA) inclusions. Polar lipid analysis was carried out by the identification service of the DSMZ and B. J. Tindall (DSMZ, Braunschweig, Germany). Detailed information about materials and protocols can be found in the supplemental material.
Proteome analysis.
To investigate the effect of Pi limitation on protein expression, we chose a mass spectrometry (MS) shotgun proteomic approach coupled with isotope-coded protein labeling (ICPL) (23), performed on cytoplasmatic, membrane, and extracellular proteins. Biological duplicates were analyzed for the cytoplasmatic proteome, whereas data for the membrane and the extracellular proteomes were obtained after combining biological triplicates. In all proteomic analyses, samples collected during the logarithmic phase (T1) under −Pi conditions (−Pi T1) were used as the reference for calculation of the relative protein abundances. For the cytoplasmatic proteome, we compared the protein expression levels of cells growing under −Pi and +Pi conditions collected during the logarithmic phase (−Pi T1 versus +Pi T1) (OD600 of 0.51 ± 0.03 for −Pi T1; OD600 of 0.78 ± 0.04 for +Pi T1). Additionally, in order to monitor the effect of prolonged Pi limitation, we compared the protein expression levels of logarithmic-phase and stationary-phase (T2) cells growing under −Pi conditions (−Pi T1 versus −Pi T2) (OD600 of 1.29 ± 0.04 for −Pi T2). Cells growing logarithmically under Pi limitation were collected at the same growth stage where we previously detected the first increase in AP activity. The proteome analyses conducted on the membrane and extracellular proteins were performed according to the same rationale in an independent experiment. In addition, for these two proteomes, a comparison of the protein expression levels between logarithmic-phase cells growing under −Pi conditions and stationary-phase cells growing under +Pi conditions was performed (−Pi T1 versus +Pi T2) (OD600 of 2.16 ± 0.01 for +Pi T2). An overview of the ICPL scheme and of the sample comparisons can be found in Table S1 in the supplemental material.
Proteins were labeled at the free amino groups with one of the four isotopic forms of nicotinoyloxysuccinamide. The complexity of the samples was then reduced via SDS-PAGE, and the proteins were digested into peptides and analyzed via liquid chromatography-electrospray ionization-tandem MS (LC-ESI-MS/MS). Since peptides with identical amino acid sequences derived from the four differentially labeled samples differ in mass, they appeared as sister peaks in the acquired MS spectra (multiplets; complete quadruplets if the peptides were detected in all four samples and incomplete quadruplets if not), with mass shifts corresponding to the difference between the masses of the labels (23). Peptides were then assigned to proteins by using the MASCOT suite, and the fold change of each protein was calculated as the median of the abundance ratios of the sister peptides. A fold change of ≤−1.75 or ≥1.75 was regarded as significant regulation. The regulated proteins were then divided into 21 categories according to their Clusters of Orthologous Groups (COG) classification. All upregulated proteins were used to perform a gene set enrichment analysis using their respective Gene Ontology (GO) annotations. Finally, for selected proteins of interest, we performed either a phylogenetic reconstruction or an analysis to identify their domain content. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium (24) via the PRIDE partner repository with the data set identifiers PXD001621 and 10.6019/PXD00162 for the extracellular and membrane proteomes and PXD001624 and 10.6019/PXD001624 for the cytoplasmatic proteome. Detailed descriptions of the experimental procedures and the data analyses are given in the supplemental material.
Identification of potential Pho boxes in the genome of strain FO-BEG1.
The in silico screening of the Pho box in the genome of strain FO-BEG1 was based on methods described previously by Yuan et al. (25). The Pho box sequences proposed by those authors were used to build a position weight matrix (26) utilized to scan the intergenic regions of strain FO-BEG1. From this first scan, an initial gene list was obtained, and from this list, a total of 29 genes (see Table S2 in the supplemental material), with the best alignments against the position weight matrix and upregulated as determined by the proteomic approach, were selected and used to build a new position weight matrix specific for Pseudovibrio sp. strain FO-BEG1. The latter was then used to obtain the final list of genes that present a putative Pho box in their upstream regions. A detailed description of the method is provided in the supplemental material.
RESULTS
Effect of Pi limitation on bacterial growth, cell morphology, and nutrient uptake.
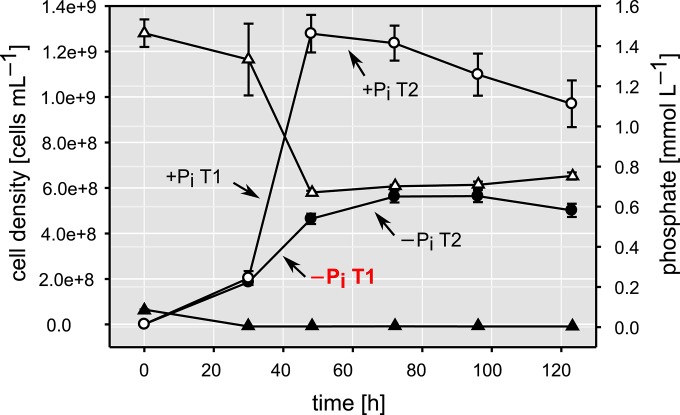
Pi limitation significantly depressed bacterial growth, leading to a final cell density 2.5 to 3.5 times lower than that observed under +Pi conditions (Fig. 1). The cultures growing under conditions of Pi surplus were characterized by a doubling time (td) of 4.33 h and a growth rate (μ) of 0.16 h−1. Pi-limited cultures had similar growth during the first half of the logarithmic phase (td of 4.77 h; μ of 0.15 h−1), but in the second half, when Pi was completely taken up by the cells (Fig. 1), the td increased to 7.68 h, and the μ dropped to 0.09 h−1. Cells growing under +Pi conditions did not metabolize all Pi provided, whereas Pi was completely taken up under −Pi conditions during the first 30 h of growth (Fig. 1). After this growth stage, we detected a considerable increase in the activity of AP (see Fig. S1 in the supplemental material), which was negligible in cultures growing under +Pi conditions (data not shown).
FIG 1.
Graph showing cell densities (circles) and phosphate concentrations (triangles) over time for cultures grown under +Pi (empty symbols) and −Pi (filled symbols) conditions. Error bars represent the standard deviations of data from biological triplicates. The arrows indicate the growth stages where samples for the proteomic analyses were taken. For all proteome samples, −Pi T1 (shown in red) was used as a reference.
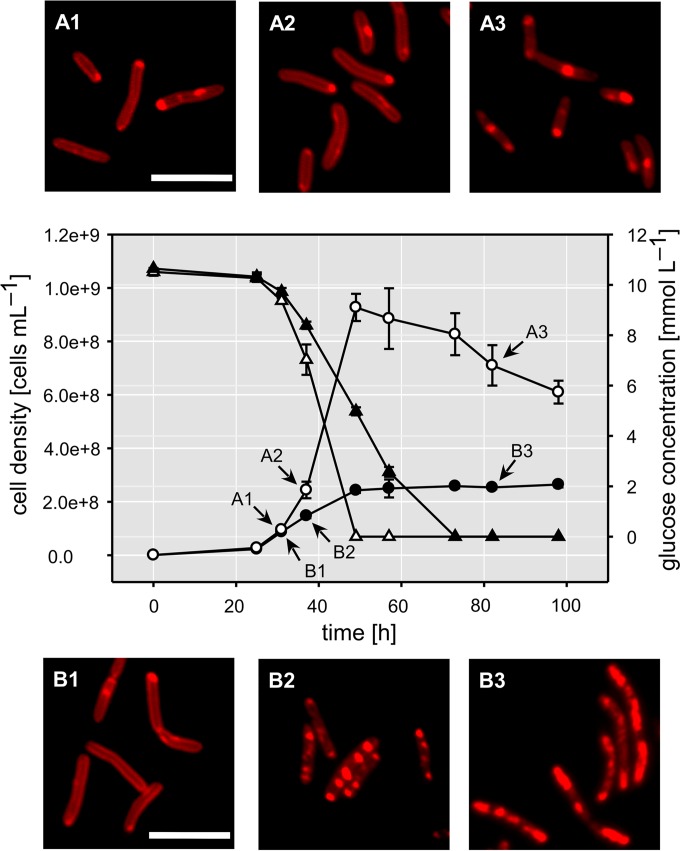
Regardless of the difference in growth, glucose was metabolized completely under both conditions (Fig. 2). Under Pi limitation, uptake continued during the nonproliferating period, indicating a possible accumulation of carbon storage compounds. Consistently, we detected the presence of lipophilic inclusions, likely polyhydroxyalkanoate (PHA), starting after ∼37 h of growth (Fig. 2). Cells growing under Pi limitation underwent a progressive increase in cell length throughout the growth period. We detected cells up to 12 μm in length, and this elongation led to a pronounced increase in the cell surface (from 8.95 ± 1.59 μm2 to 15.34 ± 3.49 μm2) (see Fig. S2 in the supplemental material).
FIG 2.
Cell densities (circles) and glucose concentrations (triangles) over time for cultures grown under +Pi (empty symbols) and −Pi (filled symbols) conditions. Error bars represent the standard deviations of data from biological triplicates. Micrographs show the presence of polyhydroxyalkanoates detected by Nile red staining. (A) Cells growing under +Pi conditions; (B) cells growing under −Pi conditions. The micrographs were taken after 24 h (A1 and B1), 37 h (A2 and B2), and 81 h (A3 and B3) of growth, as indicated by the arrows. Bar, 5 μm.
General results of proteome analyses and in silico screening for identification of potential Pho boxes.
During the proteomic analyses, we identified 1,862 and 1,866 proteins in the two replicates used for the cytoplasmatic proteome. In addition to these proteins, 1,809 and 258 proteins were identified for the membrane and the extracellular proteomes, respectively. For each proteome, the false discovery rate (27, 28) was always <0.5%. In the two replicates of the cytoplasmatic proteome, 655 and 651 proteins were quantified, and in total, according to the criteria we applied, 315 proteins were found to be regulated. In the extracellular and membrane proteomes, 80 and 364 proteins were quantified, respectively. Considering the low number of quantified proteins in the extracellular fraction and considering that the extracellular and membrane proteome analyses were performed by using the same bacterial cultures, we decided to combine these two data sets. In these two proteomes, 311 proteins were found to be regulated. The complete list of regulated proteins can be found in Tables S3 to S7 in the supplemental material.
Overall, in the comparison between −Pi T1 and +Pi T1, the higher number of upregulated proteins was observed for COG categories E, G, C, and O (Fig. 3A and C), whereas in the comparison between −Pi T1 and −Pi T2, the majority of upregulated proteins belonged to COG category J (Fig. 3B and D). The gene set enrichment analyses performed by using the Gene Ontology classification of the upregulated proteins underlined that many processes related to phosphorus metabolism were significantly enriched in the logarithmic phase under −Pi conditions (T1) (see Fig. S3 in the supplemental material). Additionally, in both proteomic data sets, many genes involved in transmembrane active transport were significantly enriched under −Pi T1 conditions. Also, the categories referring to proteins involved in protein synthesis were significantly enriched in the comparison between −Pi T1 and −Pi T2, reflecting the decline of cell proliferation in the stationary phase (T2) (see Fig. S3 in the supplemental material).
FIG 3.

Histograms illustrating the distribution of the regulated proteins among the Clusters of Orthologous Groups (COG). The absolute number of proteins belonging to each COG is reported. (A and B) Data for the cytoplasmatic proteome; (C to E) data for membrane and extracellular proteomes. COG categories are indicated as follows: C, “energy production and conversion”; D, “cell cycle control, cell division, and chromosome partitioning”; E, “amino acid transport and metabolism”; F, “nucleotide transport and metabolism”; G, “carbohydrate transport and metabolism”; H, “coenzyme transport and metabolism”; I, “lipid transport and metabolism”; J, “translation, ribosomal structure, and biogenesis”; K, “transcription”; L, “replication, recombination, and repair”; M, “cell wall/membrane/envelop biogenesis”; N, “cell motility”; O, “posttranslational modification, protein turnover, and chaperone functions”; P, “inorganic ion transport and metabolism”; Q, “secondary metabolite biosynthesis, transport, and catabolism”; R, “general functional prediction only”; S, “function unknown”; T, “signal transduction mechanism”; U, “intracellular trafficking, secretion, and vesicular transport”; V, “defense mechanisms”; N.A., “not assigned.”
The in silico analysis of the PhoB binding site revealed that strain FO-BEG1 possesses an 18-nucleotide Pho box, characterized by two 5′-CTGTCAT-3′ repetitions separated by a region of 4 nucleotides (see Fig. S4 in the supplemental material). In the first repetition, the first position does not show a high degree of conservation. However, due to the absence of molecular data that could elucidate the Pho box structure of this bacterium, we assumed that it resembled the well-conserved 18-nucleotide sequence known for E. coli and Sinorhizobium meliloti (25). The list of all genes exhibiting a putative Pho box in their upstream regions is reported in Table S8 in the supplemental material.
Pi limitation leads to a profound reorganization of P metabolism.
In order to decrease the bias due to the growth phase and focus on the effect of Pi limitation on Pseudovibrio sp. FO-BEG1 physiology, in the rest of this report, we refer to the comparison between −Pi T1 and +Pi T1, unless otherwise stated. As reported previously for other bacteria (7, 13), in strain FO-BEG1, Pi limitation triggered the upregulation of several genes coding for proteins involved in P uptake and metabolism (COG category P; GO categories “phosphorelay response regulatory activity,” “organophosphate ester,” “organophosphonate,” and “inorganic phosphate transmembrane transport activity”) (Fig. 3A, C, and E; see also Fig. S3 and Tables S4 and S5 in the supplemental material). Among these proteins, we detected three out of four subunits (PSE_1688, PSE_1690, and PSE_1691) of the Pst high-affinity ABC transport system for Pi. In proximity to its gene cluster, there is a gene (PSE_1692) encoding a protein homologous to PhoU, expressed only under −Pi conditions. This gene belongs to the pst operon E. coli, and the encoded protein appears to play a role in signal transduction (7). Consistently, a PhoH-like protein (PSE_0499), described to be part of the Pho regulon in E. coli (7), was highly upregulated under Pi limitation. Finally, we detected a >11-fold upregulation of PhoB (PSE_1693), which is the transcription factor of the two-component regulatory system (TCRS) PhoR-PhoB, which governs the phosphate starvation response. A putative Pho box was identified in the upstream region of the first gene of the pst gene cluster, PSE_1688 (see Tables S4 and S5 in the supplemental material).
After the complete consumption of the provided Pi in −Pi cultures and the consequent increase in AP activity, we still observed cell proliferation (Fig. 1; see also Fig. S1 in the supplemental material). Since no other source of P was provided, we quantified the stored polyphosphate (poly-Pi), previously detected in the cells, in order to verify whether it was used as a P source. Indeed, under −Pi conditions, the amount of stored poly-Pi decreased from 87.3 ± 3.4 fg cell−1 to 30.2 ± 2.4 fg cell−1 immediately after complete Pi uptake (see Fig. S5 in the supplemental material). Consistently, under these conditions, we detected a >3-fold-upregulated protein homologous to a poly-Pi kinase 1 (Ppk1 [PSE_2769]), which catalyzes the reversible synthesis of poly-Pi from ATP (29).
Two proteins, PhnD (PSE_3629) and PhnC (PSE_3630), of the phosphonate ABC transport system were strongly upregulated, together with two proteins involved in the degradation of these molecules (PhnJ [PSE_4852] and PhnM [PSE_4857]) and three subunits of the ABC transport system for sn-glycerol-3-phosphate (UgpB [PSE_0472 and PSE_0680] and UgpC [PSE_0683]). Potential Pho boxes were identified in the upstream regions of the PSE_0680, PSE_3629, and PSE_3630 genes. Finally, we detected a strong upregulation of three nucleosidases (PSE_1783, PSE_1587, and PSE_2573), and for two of these nucleosidases (PSE_1587 and PSE_2573), a potential Pho box was identified within the promoter region of their genes.
Effect of Pi limitation on cell envelope composition.
Thin-layer chromatography (TLC) analysis of the cellular polar lipids revealed that under +Pi conditions, most lipids contained P, whereas under Pi limitation, amino lipids (ALs) and glycolipids (GLs) were dominant (Fig. 4). Consistently, under Pi limitation, several proteins involved in cell envelope biogenesis and lipid metabolism were upregulated (COG categories I and M) (Fig. 3A, C, and E; see also Tables S4 and S5 in the supplemental material). Among these proteins, we detected a >2-fold-upregulated protein containing a phosphatidic acid phosphatase type 2 domain (PSE_2249) that is conserved in enzymes involved in the catabolism of phospholipids. Additionally, in the upstream region of its gene, a potential Pho box was identified (see Tables S4 and S5 in the supplemental material). Also, a protein homologous to PlsX (PSE_3572), described to be involved in membrane lipid and phospholipid metabolism (30), was significantly upregulated in one replicate of the cytoplasmatic proteome. Finally, we detected an upregulated protein homologous to acyl coenzyme A (acyl-CoA) dehydrogenase (PSE_0035) and two upregulated proteins homologous to enoyl-CoA hydratase (PSE_0406 and PSE_3595) (COG category I) (Fig. 3; see also Tables S4 and S5 in the supplemental material), both of which are involved in the oxidation of fatty acids and could be required for the degradation of the exchanged lipids.
FIG 4.
TLC analysis of polar lipids of cells growing under +Pi (A) and −Pi (B) conditions. Spots represent phospholipids (PL), phosphatidylglycerol (PG), phosphatidylmonomethylethanolamine (PMME), phosphatidylethanolamine (PE), amino lipids (AL), glycolipids (GL1 to GL4), and general lipids (L).
Among the cellular polar lipids detected under Pi limitation, four different types of GL were identified (GL1 to GL4) (Fig. 4). One GL (GL4) had a retention factor consistent with the S-containing lipid sulfoquinovosyl diacylglycerol (SQDG). In one of the proposed pathways for the synthesis of SQDG, the biosynthetic process starts from UDP-glucose, which is then converted into SQDG via the intermediate formation of UDP-sulfoquinovose (31). All genes required for the biosynthesis of these lipids were found in the genome of Pseudovibrio sp. FO-BEG1. Consistently, under −Pi conditions, we detected a >2-fold-upregulated protein homologous to a UTP-glucose-1-phosphate uridylyltransferase (PSE_3207) that is responsible for the synthesis of UDP-glucose and a 22-fold-upregulated protein homologous to SqdB that is responsible for the formation of UDP-sulfoquinovose (PSEp_0373) (see Tables S4 and S5 in the supplemental material). Intriguingly, a potential Pho box was identified upstream of a second copy of the sqdB gene, PSE_2321 (see Table S8 in the supplemental material).
Consistent with the rearrangement of the membrane composition, we detected the upregulation of two proteins homologous to the phage shock protein PspA (PSE_4192 and PSE_4593). These proteins are members of the phage shock regulon that is induced under membrane stress conditions, and they regulate the transcription of enzymes involved in the maintenance of membrane stability, proton motive force, and protein secretion (32). Under −Pi conditions, we detected an upregulated protein homologous to TolB (PSE_4678) belonging to the Tol-Pal complex and a bacterial outer membrane protein containing an OmpA domain (PSE_1259) described to interact with the Tol-Pal system. Different functions were proposed for this system, but it seems to be involved mainly in the maintenance of cell envelope stability (33). Additionally, several proteins likely to be involved in the biosynthesis of lipopolysaccharide and peptidoglycan were also upregulated (PSE_4236, PSE_3471, PSE_3402, and PSE_3559) (see Tables S4 and S5 in the supplemental material).
Effect of Pi limitation on cellular carbon metabolism.
In accordance with the lipophilic inclusions detected via Nile red staining, under −Pi conditions, two phasin-like proteins (PhaP [PSE_1257 and PSE_3807]) were upregulated. These proteins are associated with PHA granules and have been shown to affect polymer synthesis (34). We identified a Pho box in the upstream region of the PSE_3807 gene, indicating that its transcription is likely under the direct control of PhoB. Consistently, the formation of inclusions started after 37 h of growth, when Pi in the medium was completely taken up by the cells (Fig. 1 and 2). Additionally, a protein homologous to PhaR (PSE_0173) was >3-fold upregulated under −Pi conditions. PhaR is a transcription regulator, and there is evidence suggesting that the expression of the phasin genes (phaP) and phaR genes is derepressed under PHA-accumulating conditions (35). Finally, a protein homologous to an acetyl-CoA acetyltransferase (PSE_0174) was also upregulated. This protein shared 68% similarity (99% query coverage; E value of 0.0) with the PhbA protein of S. meliloti strain Rm41 that is involved in the first steps of PHA synthesis (36).
Pi limitation also triggered significant changes in the central metabolism. Under −Pi conditions, citrate synthase (PSE_3391), the first enzyme in the tricarboxylic acid (TCA) cycle, was downregulated (see Tables S4 and S5 in the supplemental material). Instead, two enzymes involved in the acetate kinase-phosphotransacetylase (Pta-AckA) pathway (PSE_1086 and PSE_1087), in which acetyl-CoA is converted into acetate via acetyl-Pi, were upregulated. In agreement with this, we observed acetate production starting during the second half of the exponential phase (0.15 ± 0.02 mmol liter−1) and reaching maximum levels (0.73 ± 0.09 mmol liter−1) at the beginning of stationary phase. The acetate was subsequently taken up by the cells during the rest of the growth period (data not shown). A potential PhoB binding site was identified in the upstream region of the PSE_1086 gene, suggesting that the Pta-AckA pathway is directly regulated in response to Pi limitation.
Control of oxidative stress.
As outlined by the GO enrichment analysis where the category “peroxidase activity” was significantly enriched among the upregulated proteins under Pi limitation (see Fig. S3 in the supplemental material), several proteins potentially involved in the oxidative stress response were upregulated under −Pi conditions. For example, we detected an upregulated protein having an alkyl hydroperoxide reductase (AhpC)/thiol-specific antioxidant (TSA) family domain (PSE_0180) that can control peroxide levels, and in Mycobacterium tuberculosis, it was described to in turn be reduced by an alkylhydroperoxidase (AhpD) (37). Consistently, a protein homologous to the latter was also upregulated under −Pi conditions (PSE_0181). Interestingly, Ahp proteins were shown to be specifically required for E. coli viability under conditions of prolonged Pi starvation (38). Two proteins homologous to bacterioferritin (Bfr [PSE_1030 and PSE_3844]), which are responsible for iron storage and protection against oxidative stress via iron detoxification (39), were also upregulated, and both their genes presented a Pho box in their upstream regions.
Under −Pi conditions, two proteins homologous to PotD and PotA (PSE_1679 and PSE_1681), which belong to the Pot ABC transporter system for putrescine and spermidine, were highly upregulated. The latter molecules are polyamines and play an important role in the protection of macromolecules against radicals (40). In addition, the potD gene presented a Pho box in its upstream region (see Tables S4 and S5 in the supplemental material), which is consistent with data from previous studies conducted on S. meliloti (25). Finally, a protein responsible for the synthesis of glutathione (PSE_0381), a molecule which can help cells protect macromolecules via scavenging reactive oxygen species (ROS), was also upregulated under −Pi conditions. Protection against oxidative stress also requires a secondary line of defense consisting of enzymes able to repair damaged macromolecules (41). Indeed, in strain FO-BEG1, Pi limitation also triggered the upregulation of several proteases, peptidases, chaperone-like proteins, and proteins involved in DNA repair (COG categories O and L) (Fig. 3A, C, and E; see also Tables S4 and S5 in the supplemental material).
Effect of Pi limitation on secondary metabolite production and expression of proteins involved in prokaryote-eukaryote interactions.
Surprisingly, Pi limitation triggered the secretion of compounds that gave a yellow coloration to the cultures and the cell-free supernatants. The latter showed a characteristic UV-visible absorption spectrum, with a maximum absorbance at ∼320 nm and a broad shoulder at 400 nm (see Fig. S6 in the supplemental material). The color appeared when cells entered stationary phase, and its intensity increased during the remaining incubation time. RP-HPLC analysis revealed the presence of a compound with a retention time and a spectrum consistent with those of tropodithietic acid (TDA), an antibiotic that is produced, together with a yellow-brown pigment, by many bacteria belonging to the Roseobacter clade (42). Its production was detected only under −Pi conditions (1.55 mg liter−1) and only during the stationary phase.
Pi limitation also influenced the expression of protein homologues, described for other bacteria, to be involved in secretion, cell-cell interactions, and virulence. A protein (PSE_2475) annotated as a transcription regulator belonging to a TCRS was upregulated >2-fold. This protein shared 44% similarity (query coverage, 96%; E value, ≤1e−54) with the transcriptional regulator PhoP from E. coli and Salmonella enterica, and the phylogenetic analysis that we performed showed that PSE_2475 clusters together with PhoP sequences of S. enterica (see Fig. S7 in the supplemental material). PhoP is a member of the TCRS PhoP-PhoQ, which has been well studied in Salmonella strains and was shown to be activated by different environmental cues, and it regulates the expression of virulence genes (43).
A protein homologous to TolC (PSE_3720) was found to be >2-fold upregulated under −Pi conditions. These proteins are versatile outer membrane transport proteins described to be involved in the efflux of noxious molecules and the secretion of antibiotics or virulence factors in other bacteria (44). The tolC gene is located in a genomic region encoding proteins for a type 1 secretion system (T1SS). Adjacent to this region is the largest gene of the whole genome (PSE_3716), containing five T1SS-143 repeat domains. This domain of about 143 amino acids is found in proteins of the genera Vibrio and Legionella, which share properties with RTX (repeats in toxin) proteins. These proteins are secreted by Gram-negative bacteria via the T1SS and are described to be virulence factors, having cytotoxic functions, proteolytic and lipolytic activities, or roles in adhesion and biofilm formation (45). Interestingly, PSE_3716 was upregulated in one replicate under −Pi conditions, and in agreement with data reported previously by Yoshida et al. (46), the whole genomic region seems to be under the direct control of PhoB, since the tolC gene had a potential Pho box within its promoter region (see Table S8 in the supplemental material). Another member of the RTX protein family, a hemolysin-like protein (PSE_4352), was upregulated >3-fold, and a Pho box was identified in the upstream region of its gene. Similarly, this protein was upregulated >9-fold in the comparison between −Pi T1 and +Pi T2 (see Table S7 in the supplemental material). In this comparison, a second hemolysin-like toxin, PSEp_0144, was also upregulated >2-fold. Both hemolysin-like proteins and the RTX-like protein contained one or more serralysin-like metalloprotease domains (see Fig. S8 in the supplemental material). Serralysins are endopeptidases that act as virulence factors and can cause tissue damage. A similar domain was also present in another upregulated protein, PSE_4952, which, like the large toxin PSE_3716, also presented a cadherin domain (see Fig. S8 in the supplemental material). Cadherins are an important family of calcium-dependent cell-cell adhesion molecules that play key roles in developmental processes and maintenance of tissue architecture, and they are implicated in the regulation of signaling events throughout the animal kingdom (47). Finally, we detected a 16-fold upregulation of a protein homologous to invasion-associated locus B (IalB [PSE_3194]), which is known to be a major virulence factor in Bartonella bacilliformis and is upregulated in response to environmental cues signaling vector-to-host transmission (48).
Effect of prolonged Pi limitation on protein expression.
By comparing the protein expression levels between the logarithmic and the exponential phases under −Pi conditions, we observed that only a minority of proteins (14%) were upregulated during the stationary phase (Fig. 3B and D). Consistent with the reduction of cell proliferation (Fig. 1), many of these proteins were involved in the repression of DNA replication and gene transcription (COG categories K and L) (Fig. 3B and D; see also Tables S3 and S6 in the supplemental material). The lipid rearrangement seemed to continue during stationary phase, as a protein containing a phosphatidic acid phosphatase type 2 domain (PSE_2249) and a protein homologous to phospholipase D (PSE_0624), both likely involved in the degradation of the phospholipids, were more abundant than in the logarithmic phase. Similar fold changes were also observed for the two phasin-like proteins (PSE_1257 and PSE_3807). Interestingly, a protein homologous to a major ferric iron binding protein (FbpA [PSE_1253]) was >10-fold upregulated during the stationary phase. These proteins are part of the ABC system for the transport of iron(III) that was also shown to be significantly regulated in response to Pi limitation in S. meliloti (13).
DISCUSSION
Reduced growth and rearrangement of P metabolism.
Pi has essential metabolic, structural, and regulatory roles in all bacteria (11, 49). The environmental Pi concentration influences not only P metabolism but also the bacterial life-style and interspecies and interkingdom relations, by affecting secondary metabolite production and virulence gene expression (9, 10). Previous studies have addressed the effect of Pi limitation on, e.g., the antibiotic production of Streptomyces spp., on bacteria pathogenic for humans, or on plant-associated bacteria that are of agricultural interest (9, 13, 25). Much less is known about the response of heterotrophic marine bacteria to Pi limitation, despite growing evidence suggesting that this condition is common in many areas of the ocean (2, 3).
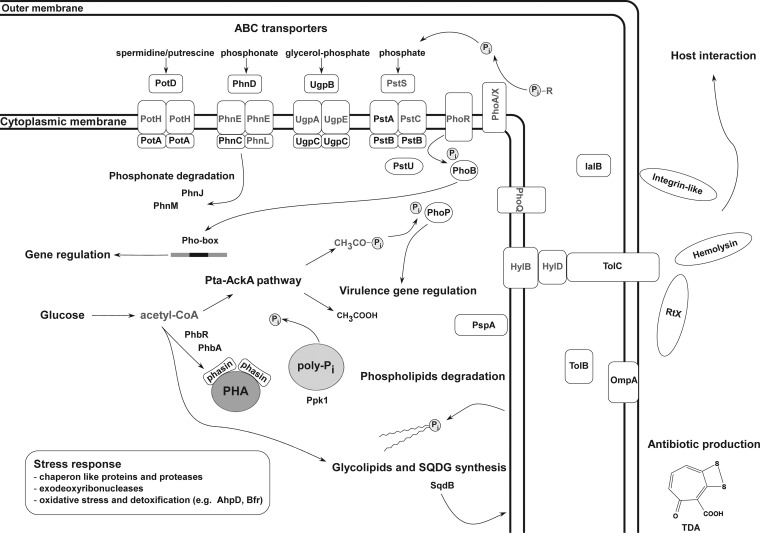
Figure 5 shows a schematic overview of the physiological adaptations observed in Pseudovibrio during growth under Pi limitation. As described previously for other strains (8, 13, 14), Pi limitation significantly depressed bacterial growth, and this was reflected in the downregulation of proteins involved in DNA replication and protein synthesis (COG categories C and E) (Fig. 3A, C, and E; see also Tables S4 and S5 in the supplemental material). The growth rate dropped concomitantly with the complete consumption of Pi (Fig. 1), and a consistent increase in AP activity was observed immediately afterwards (see Fig. S1 in the supplemental material). Different APs were identified in the genome of FO-BEG1 (e.g., PSE_1012 and PSE_2813), but none were quantified during the proteomic analysis. APs are enzymes that cleave Pi groups in organic molecules containing phosphoester bonds, and their expression has been widely used as an indicator of P limitation (50). Considering these data together, it is reasonable to state that in FO-BEG1, the Pi limitation response is activated at this growth stage, with an environmental Pi concentration of ∼4 μmol liter−1 (see Fig. S1 in the supplemental material). These data are consistent with what has been previously described for E. coli (7). The strategy used by FO-BEG1 to scavenge Pi from the environment is characterized by the upregulation of ABC transporters for Pi and proteins involved in the uptake and degradation of organic molecules containing P. Genes encoding these proteins are typically present and expressed in bacteria adapted to thriving in marine regions with limited amounts of Pi (51). Even though the Pi concentration can be far below 4 μmol liter−1 in these regions, the respective C to N to P ratios are often comparable to the values used in our experiments (4, 52). Altogether, these data underline the versatility of Pseudovibrio, and as far as P metabolism is concerned, they indicate that it is well adapted to thrive as a free-living organism in the open ocean, where Pi availability is often limited and the DOP can represent the only bioavailable P pool (1).
FIG 5.
Schematic overview of the major physiological adaptation of strain FO-BEG1 to Pi limitation. The proposed model was inferred by considering the proteins and the physiological traits induced under −Pi conditions (shown in black). Proteins and metabolites not directly detected during the experiments are shown in gray. The position of each protein in the scheme was based on localization predictions performed as described in the supplemental material.
The cell proliferation observed after the consumption of Pi was likely sustained by the degradation of stored poly-Pi, known to be an optimal intracellular P source (53). In the genome of strain FO-BEG1, several genes encoding proteins involved in poly-Pi degradation are present (e.g., PSE_2770). However, none could be quantified during the proteomic analyses. Instead, we detected upregulated Ppk1, which is an enzyme involved in the reversible synthesis of poly-Pi (29), and as described previously for other species, it can also be responsible for degradation (54, 55).
In E. coli and S. meliloti, the Pi limitation response is controlled by the TCRS PhoR-PhoB. Both genes were detected in the genome of FO-BEG1 (PSE_1693 and PSE_1687), and PhoB was highly upregulated under −Pi conditions. Moreover, we detected many similarities to other organisms concerning the regulated proteins and the genes that presented a potential Pho box in their promoter regions. Therefore, it is reasonable to state that in Pseudovibrio, the response to Pi limitation is controlled by the PhoR-PhoB system.
Adjustment of carbon metabolism, membrane lipid rearrangement, and protection against oxidative stress.
We showed that Pi limitation also greatly affects the carbon metabolism of Pseudovibrio sp. FO-BEG1, confirming the importance of P in the overall regulation of cell physiology. Pi-limited cells took up all glucose provided, and due to the lower cell density, the rate of consumption of carbon per cell was consequently higher than that in +Pi cultures. Our data suggest that some carbon might have been used for the synthesis of the inclusion PHAs, which might be accumulated by bacteria to increase survival in changing environments or as a sink for redundant reducing power under conditions of nutrient limitation and carbon surplus (56, 57). The observed PHA accumulation and the upregulation of genes involved in PHA synthesis, accompanied by the presence of a Pho box in the promoter region of one of these genes (PSE_1257, PSE_3807, PSE_0173, and PSE_0174) (see Tables S4 and S5 in the supplemental material), suggest that, as observed for Acinetobacter species (58), PHA accumulation can also be part of the Pho regulon in marine organisms.
Pseudovibrio underwent pronounced cell elongation under Pi limitation. This phenomenon has been observed frequently for Pi-starved cells and can be due to the activation of the SOS stress response (59–61). Longer cells would have an increased surface area for nutrient uptake but also increased cellular P demand for the synthesis of membrane phospholipids. However, we showed a rearrangement of the cellular lipid composition in favor of P-free lipids (Fig. 4). Changes of the membrane phospholipids were suggested to activate the psp response, which controls enzymes involved in membrane stability and the maintenance of proton motive force (32). Therefore, the upregulation of PspA and other proteins involved in the maintenance of cell envelope stability supports the hypothesis that Pseudovibrio experiences membrane stress during growth under Pi limitation, likely due to lipid rearrangement.
In oceanic surface waters, P uptake for phospholipid synthesis can represent an important part of the total bacterial Pi incorporation (62), since Pi present in phospholipids can constitute up to 36% of the total cellular P (63). During Pi limitation, levels of phospholipids can decrease by up to 97% (64, 65); therefore, membrane lipid rearrangements provide Pseudovibrio with an additional P source and at the same time decrease its cellular demand, resulting in a competitive advantage in environments depleted of this nutrient. SQDGs can play an important role in membrane restructuring under Pi-limited conditions (17, 66), but to the best of our knowledge, this is the first study where SQDG production was observed as a direct response to Pi limitation in heterotrophic marine bacteria. Our data support recent molecular evidence suggesting that the ability of heterotrophic marine bacteria to produce SQDG might be more widespread than previously thought (67).
As observed for other bacterial strains (14, 38, 68), under −Pi conditions, we detected several proteins potentially involved in protection against oxidative stress (see Tables S4 and S5 in the supplemental material). So far, it has not been clarified why cells growing under Pi limitation experience increased oxidative stress. It can be speculated that, as also suggest by Yuan et al. (68), the physiological changes induced by Pi limitation, especially the membrane lipid rearrangements, could increase cellular sensitivity to ROS, requiring the activation of defense mechanisms.
Secretion of secondary metabolites and expression of virulence-related genes.
Pi limitation greatly influenced the metabolites secreted by Pseudovibrio sp. FO-BEG1 (see Fig. S6 in the supplemental material). We previously showed that Pi limitation drastically affected both the amount and composition of compounds secreted by the strain during growth (20). Among these compounds, in the present work we identified the potent antibiotic TDA, which is a broad-spectrum antibiotic for which stable resistant mutants have been found to be difficult to select (69). Consequently, TDA production confers an ecological advantage to Pseudovibrio when competing for limited resources. Moreover, the production of the antibiotic can also be beneficial for the hosts, providing protection against potentially pathogenic bacteria. It is worth considering that the presence of sulfur in the molecules of TDA and SQDGs suggests that Pi limitation also affects the overall sulfur metabolism and sulfur demand of Pseudovibrio sp. FO-BEG1. This speculation is supported by the higher number of compounds containing sulfur detected in the exometabolome of this strain growing under Pi limitation (20).
The PhoR-PhoB system and the Pho regulon have been shown repeatedly to influence bacterial virulence (10, 70, 71). Accordingly, we detected several upregulated protein homologues, which have been described to be involved in prokaryote-eukaryote interactions in other bacteria. For example, TolB, OmpA, and PspA can be involved in adhesion to and invasion of host cells and interaction with the host defense mechanism and were reported to be upregulated during macrophage infection and biofilm formation (72–74). Several virulence factors are regulated by the TCRS PhoQ-PhoP, which has been well characterized in Salmonella species (43). The upregulated PSE_2475 protein formed a unique cluster with the transcription regulator PhoP from Salmonella enterica in the phylogenetic reconstruction that we performed (see Fig. S7 in the supplemental material), suggesting a similarity in function. In FO-BEG1, PhoP could have been activated by acetyl-Pi, derived from the upregulated Pta-AckA pathway. Acetyl-Pi can activate the response regulator proteins of TCRSs, independently of the respective histidine kinases, and was shown to directly activate PhoP (75). Therefore, it was suggested that the overexpression of the Pta-AckA pathway is a way to connect Pi limitation with the general induction of a large number of genes (76, 77).
The upregulation of TolC and the RTX-like toxin (PSE_3716) and the presence of a Pho box in the promoter region of the tolC gene are strong indications that the whole genomic region, which also contains two subunits required for a functional T1SS (hlyD [PSE_3718] and hlyB [PSE_3719]), is under the direct control of PhoB and is upregulated under Pi limitation. The RTX-like toxin possesses a serralysin-like metalloprotease domain and two galactose binding domains (see Fig. S8 in the supplemental material), which could interact with glycosylated groups of proteins present on the eukaryotic cell surface, such as cadherins and integrins. Consistently, integrin- and cadherin-like proteins were also described previously for sponges and corals (78, 79), which are the two major hosts of Pseudovibrio strains. This suggests that, as observed for other bacteria (80), FO-BEG1 could use this type of toxin to adhere to the host cell surface and penetrate via disruption of the host tissue. Serralysin-like domains were identified also in other proteins upregulated under −Pi conditions, the hemolysins PSE_4352 and PSEp_0144 and the PSE_4952 protein (see Fig. S8 in the supplemental material). Additionally, cadherin- and integrin-like domains were identified in both RTX-like proteins and the PSE_4952 protein, respectively. Cadherins and integrins have been shown to mediate cell-cell and cell-matrix interactions in metazoans, and proteins containing these domains have also been identified in different bacterial strains (81, 82). As in strain FO-BEG1, cadherins and integrins are found in bacterial proteins that have additional enzymatic and adhesion domains (81). Interestingly, it was shown previously that proteins containing these domains are involved in bacterial cell interactions, biofilm formation, adherence, interactions with the extracellular matrix, and eukaryotic cell toxicity (82–85). These data support our hypothesis that Pseudovibrio sp. FO-BEG1 could use these proteins, together with IalB, to adhere to and interact with the host cell.
Altogether, these data indicate that Pi limitation and the general stress response induced by Pi limitation enhance the expression of proteins involved in the establishment and maintenance of a symbiotic relationship with marine invertebrates such as sponges. Sponges are filter-feeding organisms capable of pumping hundreds of liters of seawater per day (86). Consequently, the colonization of such organisms might represent a strategy adopted by Pseudovibrio to overcome nutrient limitation encountered as a free-living organism in the surrounding water, since the association with the sponge will expose the bacterium to a continuous flow of water and nutrients.
Conclusions.
In this work, we describe for the first time in detail the response of a marine heterotrophic bacterium to Pi limitation. We show that this nutrient regime has a pleiotropic effect on the physiology of Pseudovibrio sp. FO-BEG1. This suggests that in marine systems, such conditions could also shape the biogeochemical cycles of other nutrients by drastically affecting bacterial physiology. Additionally, our data show that Pi limitation has a significant influence on the production of secondary metabolites. Considering the interest in new bioactive molecules of marine origin and in the Pseudovibrio genus for exploitation of novel compounds, our work indicates that Pi limitation can represent a promising starting condition for triggering the production of these molecules. Finally, the proteomic analyses that we performed pointed out how Pi limitation, or the general stress response induced by it, also affects the expression of virulence-related genes in Pseudovibrio. Further molecular work could unravel the regulatory connections between the environmental nutrient regimes and the ability of these bacteria to colonize their hosts.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to M. Meyer, S. Menger, C. Probian, and R. Appel for technical support. We thank John P. Phelan for proofreading the manuscript. We are indebted to F. Garcia-Prado, S. Lehnert, A. Brunner, and L. Wöhlbrand for help and suggestion on technical aspects of the proteomic analysis.
This study was funded by European Research Council grant no. 203364 and the Max Planck Society. J.M.G. was supported by grant CTM2013-48292-C3-3-R from the Spanish Ministry of Economy and Competitiveness.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.04167-14.
REFERENCES
- 1.Dyhrman ST, Ammerman JW, Van Mooy BAS. 2007. Microbes and the marine phosphorus cycle. Oceanography 20:110–116. doi: 10.5670/oceanog.2007.54. [DOI] [Google Scholar]
- 2.Thingstad TF, Krom MD, Mantoura RFC, Flaten GAF, Groom S, Herut B, Kress N, Law CS, Pasternak A, Pitta P, Psarra S, Rassoulzadegan F, Tanaka T, Tselepides A, Wassmann P, Woodward EMS, Riser CW, Zodiatis G, Zohary T. 2005. Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 309:1068–1071. doi: 10.1126/science.1112632. [DOI] [PubMed] [Google Scholar]
- 3.Wu JF, Sunda W, Boyle EA, Karl DM. 2000. Phosphate depletion in the western North Atlantic Ocean. Science 289:759–762. doi: 10.1126/science.289.5480.759. [DOI] [PubMed] [Google Scholar]
- 4.Cotner JB, Ammerman JW, Peele ER, Bentzen E. 1997. Phosphorus-limited bacterioplankton growth in the Sargasso Sea. Aquat Microb Ecol 13:141–149. doi: 10.3354/ame013141. [DOI] [Google Scholar]
- 5.Rivkin RB, Anderson MR. 1997. Inorganic nutrient limitation of oceanic bacterioplankton. Limnol Oceanogr 42:730–740. doi: 10.4319/lo.1997.42.4.0730. [DOI] [Google Scholar]
- 6.Paytan A, McLaughlin K. 2007. The oceanic phosphorus cycle. Chem Rev 107:563–576. doi: 10.1021/cr0503613. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh YJ, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13:198–203. doi: 10.1016/j.mib.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanBogelen RA, Olson ER, Wanner BL, Neidhardt FC. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J Bacteriol 178:4344–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martín JF. 2004. Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the PhoR-PhoP system: an unfinished story. J Bacteriol 186:5197–5201. doi: 10.1128/JB.186.16.5197-5201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 11.Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 12.Rodríguez-García A, Barreiro C, Santos-Beneit F, Sola-Landa A, Martín JF. 2007. Genome-wide transcriptomic and proteomic analysis of the primary response to phosphate limitation in Streptomyces coelicolor M145 and in a ΔphoP mutant. Proteomics 7:2410–2429. doi: 10.1002/pmic.200600883. [DOI] [PubMed] [Google Scholar]
- 13.Krol E, Becker A. 2004. Global transcriptional analysis of the phosphate starvation response in Sinorhizobium meliloti strains 1021 and 2011. Mol Genet Genomics 272:1–17. doi: 10.1007/s00438-004-1030-8. [DOI] [PubMed] [Google Scholar]
- 14.Ishige T, Krause M, Bott M, Wendisch VF, Sahm H. 2003. The phosphate starvation stimulon of Corynebacterium glutamicum determined by DNA microarray analyses. J Bacteriol 185:4519–4529. doi: 10.1128/JB.185.15.4519-4529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Krüger WMA, Lery LMS, Soares MR, de Neves-Manta FS, Batista e Silva CM, da Costa Neves-Ferreira AG, Perales J, Bisch PM. 2006. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics 6:1495–1511. doi: 10.1002/pmic.200500238. [DOI] [PubMed] [Google Scholar]
- 16.Tetu SG, Brahamsha B, Johnson DA, Tai V, Phillippy K, Palenik B, Paulsen IT. 2009. Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. ISME J 3:835–849. doi: 10.1038/ismej.2009.31. [DOI] [PubMed] [Google Scholar]
- 17.Dyhrman ST, Jenkins BD, Rynearson TA, Saito MA, Mercier ML, Alexander H, Whitney LP, Drzewianowski A, Bulygin VV, Bertrand EM, Wu ZJ, Benitez-Nelson C, Heithoff A. 2012. The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS One 7:e33768. doi: 10.1371/journal.pone.0033768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondarev V, Richter M, Romano S, Piel J, Schwedt A, Schulz-Vogt HN. 2013. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ Microbiol 15:2095–2113. doi: 10.1111/1462-2920.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Halloran JA, Barbosa TM, Morrissey JP, Kennedy J, O'Gara F, Dobson ADW. 2011. Diversity and antimicrobial activity of Pseudovibrio spp. from Irish marine sponges. J Appl Microbiol 110:1495–1508. doi: 10.1111/j.1365-2672.2011.05008.x. [DOI] [PubMed] [Google Scholar]
- 20.Romano S, Dittmar T, Bondarev V, Weber RJ, Viant MR, Schulz-Vogt HN. 2014. Exo-metabolome of Pseudovibrio sp. FO-BEG1 analyzed by ultra-high resolution mass spectrometry and the effect of phosphate limitation. PLoS One 9:e96038. doi: 10.1371/journal.pone.0096038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen HP, Karoleff F. 1999. Determination of nutrients, p 159–226. In Grasshoff K, Kremling K, Ehrhardt M (ed), Methods of seawater analysis. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 22.Kulakova AN, Hobbs D, Smithen M, Pavlov E, Gilbert JA, Quinn JP, McGrath JW. 2011. Direct quantification of inorganic polyphosphate in microbial cells using 4′-6-diamidino-2-phenylindole (DAPI). Environ Sci Technol 45:7799–7803. doi: 10.1021/es201123r. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt A, Kellermann J, Lottspeich F. 2005. A novel strategy for quantitative proteomics using isotope-coded protein labels. Proteomics 5:4–15. doi: 10.1002/pmic.200400873. [DOI] [PubMed] [Google Scholar]
- 24.Vizcaíno JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolomé S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H. 2014. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol 32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan ZC, Zaheer R, Morton R, Finan TM. 2006. Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Res 34:2686–2697. doi: 10.1093/nar/gkl365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stormo GD. 2000. DNA binding sites: representation and discovery. Bioinformatics 16:16–23. doi: 10.1093/bioinformatics/16.1.16. [DOI] [PubMed] [Google Scholar]
- 27.Peng JM, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. 2003. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J Proteome Res 2:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 28.Elias JE, Haas W, Faherty BK, Gygi SP. 2005. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods 2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 29.Rao NN, Gómez-García MR, Kornberg A. 2009. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y, Li H, Binkowski TA, Holzle D, Joachimiak A. 2009. Crystal structure of fatty acid/phospholipid synthesis protein PlsX from Enterococcus faecalis. J Struct Funct Genomics 10:157–163. doi: 10.1007/s10969-008-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benning C. 1998. Biosynthesis and function of the sulfolipid sulfoquinovosyl diacylglycerol. Annu Rev Plant Physiol Plant Mol Biol 49:53–75. doi: 10.1146/annurev.arplant.49.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Joly N, Engl C, Jovanovic G, Huvet M, Toni T, Sheng X, Stumpf MPH, Buck M. 2010. Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol Rev 34:797–827. doi: 10.1111/j.1574-6976.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- 33.Lloubès R, Cascales E, Walburger A, Bouveret E, Lazdunski C, Bernadac A, Journet L. 2001. The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res Microbiol 152:523–529. doi: 10.1016/S0923-2508(01)01226-8. [DOI] [PubMed] [Google Scholar]
- 34.de Almeida A, Nikel PI, Giordano AM, Pettinari MJ. 2007. Effects of granule-associated protein PhaP on glycerol-dependent growth and polymer production in poly(3-hydroxybutyrate)-producing Escherichia coli. Appl Environ Microbiol 73:7912–7916. doi: 10.1128/AEM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maehara A, Taguchi S, Nishiyama T, Yamane T, Doi Y. 2002. A repressor protein, PhaR, regulates polyhydroxyalkanoate (PHA) synthesis via its direct interaction with PHA. J Bacteriol 184:3992–4002. doi: 10.1128/JB.184.14.3992-4002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tombolini R, Povolo S, Buson A, Squartini A, Nuti MP. 1995. Poly-beta-hydroxybutyrate (Phb) biosynthetic genes in Rhizobium meliloti 41. Microbiology 141:2553–2559. doi: 10.1099/13500872-141-10-2553. [DOI] [PubMed] [Google Scholar]
- 37.Guimarães BG, Souchon H, Honoré N, Saint-Joanis B, Brosch R, Shepard W, Cole ST, Alzari PM. 2005. Structure and mechanism of the alkyl hydroperoxidase AhpC, a key element of the Mycobacterium tuberculosis defense system against oxidative stress. J Biol Chem 280:25735–25742. doi: 10.1074/jbc.M503076200. [DOI] [PubMed] [Google Scholar]
- 38.Moreau PL, Gérard F, Lutz NW, Cozzone P. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol Microbiol 39:1048–1060. doi: 10.1046/j.1365-2958.2001.02303.x. [DOI] [PubMed] [Google Scholar]
- 39.Carrondo MA. 2003. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. EMBO J 22:1959–1968. doi: 10.1093/emboj/cdg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wortham BW, Patel CN, Oliveira MA. 2007. Polyamines in bacteria: pleiotropic effects yet specific mechanisms. Adv Exp Med Biol 603:106–115. doi: 10.1007/978-0-387-72124-8_9. [DOI] [PubMed] [Google Scholar]
- 41.Cabiscol E, Piulats E, Echave P, Herrero E, Ros J. 2000. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J Biol Chem 275:27393–27398. doi: 10.1074/jbc.M003140200. [DOI] [PubMed] [Google Scholar]
- 42.Geng H, Bruhn JB, Nielsen KF, Gram L, Belas R. 2008. Genetic dissection of tropodithietic acid biosynthesis by marine roseobacters. Appl Environ Microbiol 74:1535–1545. doi: 10.1128/AEM.02339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Federici L, Walas F, Luisi B. 2004. The structure and mechanism of the TolC outer membrane transport protein. Curr Sci 87:190–196. [Google Scholar]
- 45.Satchell KJF. 2011. Structure and function of MARTX toxins and other large repetitive RTX proteins. Annu Rev Microbiol 65:71–90. doi: 10.1146/annurev-micro-090110-102943. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Makino K. 2010. Identification and characterization of novel phosphate regulon genes, ecs0540-ecs0544, in Escherichia coli O157:H7. Mol Genet Genomics 284:197–205. doi: 10.1007/s00438-010-0559-y. [DOI] [PubMed] [Google Scholar]
- 47.Juliano RL. 2002. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 48.Coleman SA, Minnick MF. 2003. Differential expression of the invasion-associated locus B (ialB) gene of Bartonella bacilliformis in response to environmental cues. Microb Pathog 34:179–186. doi: 10.1016/S0882-4010(03)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stock JB, Ninfa AJ, Stock AM. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev 53:450–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoppe HG. 2003. Phosphatase activity in the sea. Hydrobiologia 493:187–200. doi: 10.1023/A:1025453918247. [DOI] [Google Scholar]
- 51.Martiny AC, Huang Y, Li W. 2011. Adaptation to nutrient availability in marine microorganisms by gene gain and loss, p 269–276. In de Bruijn FJ. (ed), Handbook of molecular microbial ecology II: metagenomics in different habitats. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 52.Pinhassi J, Gómez-Consarnau L, Alonso-Sáez L, Sala MM, Vidal M, Pedrós-Alió C, Gasol JM. 2006. Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquat Microb Ecol 44:241–252. doi: 10.3354/ame044241. [DOI] [Google Scholar]
- 53.Kulaev I, Kulakovskaya T. 2000. Polyphosphate and phosphate pump. Annu Rev Microbiol 54:709–734. doi: 10.1146/annurev.micro.54.1.709. [DOI] [PubMed] [Google Scholar]
- 54.Ghorbel S, Smirnov A, Chouayekh H, Sperandio B, Esnault C, Kormanec J, Virolle MJ. 2006. Regulation of ppk expression and in vivo function of Ppk in Streptomyces lividans TK24. J Bacteriol 188:6269–6276. doi: 10.1128/JB.00202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geißdörfer W, Ratajczak A, Hillen W. 1998. Transcription of ppk from Acinetobacter sp. strain ADP1, encoding a putative polyphosphate kinase, is induced by phosphate starvation. Appl Environ Microbiol 64:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malmcrona-Friberg K, Tunlid A, Mårdén P, Kjelleberg S, Odham G. 1986. Chemical changes in cell envelope and poly-β-hydroxybutyrate during short-term starvation of a marine bacterial isolate. Arch Microbiol 144:340–345. doi: 10.1007/BF00409882. [DOI] [Google Scholar]
- 57.Anderson AJ, Dawes EA. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schembri MA, Bayly RC, Davies JK. 1995. Phosphate concentration regulates transcription of the Acinetobacter polyhydroxyalkanoic acid biosynthetic genes. J Bacteriol 177:4501–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Løvdal T, Skjoldal EF, Heldal M, Norland S, Thingstad TF. 2008. Changes in morphology and elemental composition of Vibrio splendidus along a gradient from carbon-limited to phosphate-limited growth. Microb Ecol 55:152–161. doi: 10.1007/s00248-007-9262-x. [DOI] [PubMed] [Google Scholar]
- 60.van der Veen S, van Schalkwijk S, Molenaar D, de Vos WM, Abee T, Wells-Bennik MHJ. 2010. The SOS response of Listeria monocytogenes is involved in stress resistance and mutagenesis. Microbiology 156:374–384. doi: 10.1099/mic.0.035196-0. [DOI] [PubMed] [Google Scholar]
- 61.Goclaw-Binder H, Sendersky E, Shimoni E, Kiss V, Reich Z, Perelman A, Schwarz R. 2012. Nutrient-associated elongation and asymmetric division of the cyanobacterium Synechococcus PCC 7942. Environ Microbiol 14:680–690. doi: 10.1111/j.1462-2920.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- 62.Van Mooy BAS, Moutin T, Duhamel S, Rimmelin P, Van Wambeke F. 2008. Phospholipid synthesis rates in the eastern subtropical South Pacific Ocean. Biogeosciences 5:133–139. doi: 10.5194/bg-5-133-2008. [DOI] [Google Scholar]
- 63.Geider RJ, La Roche J. 2002. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17. doi: 10.1017/S0967026201003456. [DOI] [Google Scholar]
- 64.Benning C, Huang ZH, Gage DA. 1995. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch Biochem Biophys 317:103–111. doi: 10.1006/abbi.1995.1141. [DOI] [PubMed] [Google Scholar]
- 65.Minnikin DE, Baddiley J, Abdolrah H. 1972. Variation of polar lipid composition of Bacillus subtilis (Marburg) with different growth conditions. FEBS Lett 27:16–18. doi: 10.1016/0014-5793(72)80398-3. [DOI] [PubMed] [Google Scholar]
- 66.Benning C, Beatty JT, Prince RC, Somerville CR. 1993. The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron-transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation. Proc Natl Acad Sci U S A 90:1561–1565. doi: 10.1073/pnas.90.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villanueva L, Bale N, Hopmans EC, Schouten S, Damsté JSS. 2014. Diversity and distribution of a key sulpholipid biosynthetic gene in marine microbial assemblages. Environ Microbiol 16:774–787. doi: 10.1111/1462-2920.12202. [DOI] [PubMed] [Google Scholar]
- 68.Yuan ZC, Zaheer R, Finan TM. 2005. Phosphate limitation induces catalase expression in Sinorhizobium meliloti, Pseudomonas aeruginosa and Agrobacterium tumefaciens. Mol Microbiol 58:877–894. doi: 10.1111/j.1365-2958.2005.04874.x. [DOI] [PubMed] [Google Scholar]
- 69.Porsby CH, Webber MA, Nielsen KF, Piddock LJV, Gram L. 2011. Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select. Antimicrob Agents Chemother 55:1332–1337. doi: 10.1128/AAC.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J. 2008. Depletion of intestinal phosphate after operative injury activates the virulence of P. aeruginosa causing lethal gut-derived sepsis. Surgery 144:189–197. doi: 10.1016/j.surg.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaborin A, Romanowski K, Gerdes S, Holbrook C, Lepine F, Long J, Poroyko V, Diggle SP, Wilke A, Righetti K, Morozova I, Babrowski T, Liu DC, Zaborina O, Alverdy JC. 2009. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc Natl Acad Sci U S A 106:6327–6332. doi: 10.1073/pnas.0813199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowe F, Lipps CJ, Tsolis RM, Groisman E, Heffron F, Kusters JG. 1998. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect Immun 66:3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith SGJ, Mahon V, Lambert MA, Fagan RP. 2007. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett 273:1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 74.Karlinsey JE, Maguire ME, Becker LA, Crouch MLV, Fang FC. 2010. The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol 78:669–685. doi: 10.1111/j.1365-2958.2010.07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCleary WR, Stock JB. 1994. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem 269:31567–31572. [PubMed] [Google Scholar]
- 76.Summers ML, Elkins JG, Elliott BA, McDermott TR. 1998. Expression and regulation of phosphate stress inducible genes in Sinorhizobium meliloti. Mol Plant Microbe Interact 11:1094–1101. doi: 10.1094/MPMI.1998.11.11.1094. [DOI] [PubMed] [Google Scholar]
- 77.Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ. 2007. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol 189:5574–5581. doi: 10.1128/JB.00564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brower DL, Brower SM, Hayward DC, Ball EE. 1997. Molecular evolution of integrins: genes encoding integrin beta subunits from a coral and a sponge. Proc Natl Acad Sci U S A 94:9182–9187. doi: 10.1073/pnas.94.17.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. 2012. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/beta-catenin complex. Proc Natl Acad Sci U S A 109:13046–13051. doi: 10.1073/pnas.1120685109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linhartová I, Bumba L, Mašín J, Basler M, Osička R, Kamanová J, Procházková K, Adkins I, Hejnová-Holubová J, Sadílková L, Morová J, Šebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao L, Yan X, Borysenko CW, Blair HC, Wu C, Yu L. 2005. CHDL: a cadherin-like domain in Proteobacteria and Cyanobacteria. FEMS Microbiol Lett 251:203–209. doi: 10.1016/j.femsle.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Rao SP, Gehlsen KR, Catanzaro A. 1992. Identification of a beta 1 integrin on Mycobacterium avium-Mycobacterium intracellulare. Infect Immun 60:3652–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraiberg M, Borovok I, Weiner RM, Lamed R. 2010. Discovery and characterization of cadherin domains in Saccharophagus degradans 2-40. J Bacteriol 192:1066–1074. doi: 10.1128/JB.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdian PL, Caramelo JJ, Ausmees N, Zorreguieta A. 2013. RapA2 is a calcium-binding lectin composed of two highly conserved cadherin-like domains that specifically recognize Rhizobium leguminosarum acidic exopolysaccharides. J Biol Chem 288:2893–2904. doi: 10.1074/jbc.M112.411769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chatterjee R, Nag S, Chaudhuri K. 2008. Identification of a new RTX-like gene cluster in Vibrio cholerae. FEMS Microbiol Lett 284:165–171. doi: 10.1111/j.1574-6968.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 86.Vogel S. 1977. Current-induced flow through living sponges in nature. Proc Natl Acad Sci U S A 74:2069–2071. doi: 10.1073/pnas.74.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.