Abstract
Understanding of the colonization process of epithelial bacteria attached to the rumen tissue during rumen development is very limited. Ruminal epithelial bacterial colonization is of great significance for the relationship between the microbiota and the host and can influence the early development and health of the host. MiSeq sequencing of 16S rRNA genes and quantitative real-time PCR (qPCR) were applied to characterize ruminal epithelial bacterial diversity during rumen development in this study. Seventeen goat kids were selected to reflect the no-rumination (0 and 7 days), transition (28 and 42 days), and rumination (70 days) phases of animal development. Alpha diversity indices (operational taxonomic unit [OTU] numbers, Chao estimate, and Shannon index) increased (P < 0.01) with age, and principal coordinate analysis (PCoA) revealed that the samples clustered together according to age group. Phylogenetic analysis revealed that Proteobacteria, Firmicutes, and Bacteroidetes were detected as the dominant phyla regardless of the age group, and the abundance of Proteobacteria declined quadratically with age (P < 0.001), while the abundances of Bacteroidetes (P = 0.088) and Firmicutes (P = 0.009) increased with age. At the genus level, Escherichia (80.79%) dominated at day zero, while Prevotella, Butyrivibrio, and Campylobacter surged (linearly; P < 0.01) in abundance at 42 and 70 days. qPCR showed that the total copy number of epithelial bacteria increased linearly (P = 0.013) with age. In addition, the abundances of the genera Butyrivibrio, Campylobacter, and Desulfobulbus were positively correlated with rumen weight, rumen papilla length, ruminal ammonia and total volatile fatty acid concentrations, and activities of carboxymethylcellulase (CMCase) and xylanase. Taking the data together, colonization by ruminal epithelial bacteria is age related (achieved at 2 months) and might participate in the anatomic and functional development of the rumen.
INTRODUCTION
Recent years have witnessed growing interest in the diversity and function of ruminal epithelial bacteria. It has been demonstrated that ruminal epithelial bacteria are involved in oxygen scavenging, tissue recycling, and urea digestion (1). Furthermore, in steers, the ruminal epithelial bacterial communities of acidosis-resistant and acidosis-susceptible groups were different during subacute ruminal acidosis development, and this difference could be recognized by the host TLRs (Toll-like receptors), which are associated with changes in the function of the rumen epithelial tissue barrier (2). Similarly, in the mouse colon, epithelial bacterial diversity correlated with TLR2 and TLR4 gene expression (3). These findings reveal that since epithelial bacteria are directly attached to the epithelial surface, their end products may play a direct or indirect role in host immune responses and tissue barrier function.
Ruminal epithelial bacteria are distinctly different at the taxonomic level from bacteria associated with rumen contents (4, 5). By use of culture-based techniques, the ruminal epithelial communities have been found to comprise predominantly Gram-positive, facultatively anaerobic flora, among which Butyrivibrio spp. (31.1%), Bacteroides spp. (22.4%), Selenomonas ruminantium (9.9%), Succinivibrio dextrinosolvens (8.7%) and Streptococcus bovis (8.1%) predominate (6). At the class level, ruminal epithelial bacteria were found by pyrosequencing to be composed mainly of Clostridia (67%), Bacteroidia (9%), Deltaproteobacteria (4%), and Erysipelotrichia (3%) (7).
Diet is an indispensable determinant of the structure and function of the diverse microbial populations in rumen contents and attached to rumen tissue. In the rumen contents of dairy cows, deep amplicon sequencing of the 16S rRNA gene revealed a higher abundance of members of the Fibrobacteraceae family in total mixed-ration samples and a lower abundance of members of the propionate-producing Veillonellaceae than in pasture samples (8). Moreover, based on sequence information from the reference marker of PCR-denaturing gradient gel electrophoresis (DGGE), abundances of the phyla Firmicutes and Proteobacteria in the heifer ruminal epithelial bacterial community decreased in response to a rapid transition from a diet containing 97% hay to a diet containing 8% hay (9).
The process of bacterial colonization in the developing rumen is important for the achievement of rumen functions. Bacterial colonization can influence the early development and productive efficiency of the mature animal (10). In cow rumen contents, the bacterial composition underwent dynamic changes during the first 2 years; levels of members of the phylum Proteobacteria decreased with age, while those of Bacteroidetes and Firmicutes increased with age (11). Our earlier study showed that during the three typical phases of the rumen development process (nonrumination, 0 to 3 weeks; transition, 3 to 8 weeks; rumination, from 8 weeks on) in goats, microbial colonization of the rumen contents occurs at 1 month, functional achievement at 2 months, and anatomic development after 2 months (12). Nonetheless, research into age-related dynamic colonization by ruminal epithelial bacterial populations has been very limited. Therefore, we aimed to characterize the sequential dynamics of ruminal epithelial bacterial colonization and to explore its relationship with previously published rumen anatomic and functional parameters.
MATERIALS AND METHODS
Sample collection.
This study was conducted using samples collected from 17 selected goat kids offered supplemental feeding (a feed concentrate in addition to forage after 20 days postbirth) in our previous study (12). The use of animals, including welfare, husbandry, experimental procedures, and the collection of rumen samples used for this study, was approved by the Animal Care Committee, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China. Immediately after birth, each goat kid was placed in an individual pen so as to avoid direct contact with other adult animals and other kids. The kids were then maintained, each within an individual pen, for the duration of the study. From 0 to 20 days, the kids were offered goat milk (1 liter per meal) twice, at 0800 and 1700 h. Between days 20 and 40, feed was provided individually twice daily, at 0800 and 1700 h; at each meal, the kids received a bucket of goat milk (0.5 liter) and a separate bucket containing a mixture of forage (fresh grass; 0.04 kg [dry matter {DM}]/meal) and a starter concentrate (0.12 kg [DM]/meal). The kids were weaned at 40 days, and from then until 70 days, they were fed the starter concentrate (0.17 kg [DM]/meal) and forage (0.06 kg [DM]/meal). The starter concentrate contained, per kilogram (DM), 74.1 g whey powder, 211 g corn flour, 320 g bean meal, 65 g fish meal, 220 g fat powder, 51 g milk powder, 8.6 g CaCO3, 25.3 g CaHPO4, 5 g NaCl, and 20 g premix. The starter concentrate and forage were offered ad libitum. Four kids were slaughtered at 0 and 42 days, and three kids were slaughtered at 7, 28, and 70 days. Immediately after the kids were slaughtered, the rumen tissue was scraped to remove attached feed particles and was rinsed three times with sterile phosphate-buffered saline (pH 7.4) to remove nonadherent bacteria. The samples were then transferred into RNAlater solution (Invitrogen, Carlsbad, CA) and were stored at −20°C until molecular analysis.
DNA extraction and preparation of amplicons for high-throughput sequencing.
Total DNA was extracted from the 17 rumen mucosal tissue samples using the bead-beating method as suggested by Chen et al. (9). The quality and quantity of DNA were measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). The total DNA of the rumen tissue was then diluted to 50 ng/μl and was used in the preparation of amplicons for high-throughput sequencing. Conventional PCR was performed to amplify the V2 and V3 regions of the 16S rRNA gene using universal primers 104F (5′-NNNNNN GGCGVACGGGTGAGTAA-3′) and 530R (5′-CCGCNGCNGCTGGCAC-3′) (13). The forward primer, 104F, contained 6-base barcodes [represented by the italicized poly(N) section of the primer sequence] at the 5′ terminus. All the 104F primer barcodes used are presented in Table S1 in the supplemental material. PCR mixtures (50 μl) contained 30 ng DNA template, 5 μl of 10× Pfx amplification buffer, 2 μl of a 10 mM deoxynucleoside triphosphate (dNTP) mixture, 2 μl of 50 mM MgSO4, 1 μl of Platinum Pfx DNA polymerase, 0.25 μl of bovine serum albumin (20 mg/ml), and 2 μl of each primer (10 μM) (Invitrogen, Life Sciences, USA). Reaction conditions consisted of an initial cycle of 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s, and a final extension of 72°C for 10 min. PCR products were excised from a 1.5% agarose gel and were purified using the Wizard SV Gel and PCR Clean-Up system (Promega, Madison, WI, USA). Bar-coded V2–V3 amplicons were mixed at equal molar ratios and were subjected to Illumina paired-end library preparation, cluster generation, and 300-bp paired-end sequencing on an Illumina MiSeq PE300 instrument.
Data analysis.
Raw data were filtered through a quality control pipeline using the Quantitative Insight into Microbial Ecology (QIIME) tool kit (14) and mothur (15). Specifically, the 300-bp reads were truncated at any site of >3 sequential bases receiving a quality score of <Q20, and any read containing ambiguous base calls or barcode/primer errors was discarded, as were reads with <80% (of total read length) consecutive high-quality base calls. Sequences were deconvoluted into individual samples based on their barcodes. For paired-end reads, only sequences that overlapped >10% and had <10% mismatches were assembled according to their overlap sequence using COPE (Connecting Overlapped Pair-End) (16). The assembled sequences were then trimmed of primers and were assigned to operational taxonomic units (OTUs) at a 97% identity threshold using UPARSE (17). Chimeric sequences were identified and removed using UCHIME (18). Taxonomy classifications were assigned against the latest Greengenes database (May 2013 release) (19) using the mothur-based implementation of the RDP Bayesian classifier with a 0.80 confidence threshold. Sequences were aligned by PyNAST, and a phylogenetic tree was constructed using FastTree (20). Taxonomic identification and comparisons were performed at the phylum and genus levels.
Alpha diversity values for ruminal epithelial bacterial communities of kids at different ages (0, 7, 28, 42, and 70 days) were obtained using various diversity indices (observed species, Chao estimate, abundance-based coverage estimator [ACE], Shannon and Simpson diversity indices) and using the alpha rarefaction pipeline at a sequence depth of 27,000 as the number nearest to the minimum number of sequence reads per sample for all 17 samples (27,120). Principal coordinate analysis (PCoA) of microbial communities (jackknifed beta diversity from resampling 100 times at a depth of 20,250 sequences) was performed using Bray-Curtis distance. Within-group similarity was calculated as the average of the pairwise similarity between each two paired samples within each group using the Bray-Curtis metric.
A transformation-based principal components analysis (tb-PCA) (21) using the Hellinger distance was used to ordinate the 17 samples according to OTU abundance data. Only the top 10 OTUs for which differences in abundance among 17 samples contributed most to the clustering obtained from the tb-PCA were selected and represented in the tb-PCA. The OTUs were identified by using the largest contributors to the first two principal components as selection criteria.
Quantification of total bacteria and selected bacterial genera.
Absolute quantitative real-time PCR (qPCR) was performed to determine the copy numbers of the 16S rRNA genes of total bacteria and of two selected bacterial genera (Prevotella and Bacteroides). The primers, which were validated previously (22–24), are listed in Table S2 in the supplemental material. qPCR was performed using SYBR Premix Ex Taq (Perfect Real Time) (TaKaRa, Japan) on an ABI 7900HT Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA). The standard curves for total bacteria and for each bacterial genus were prepared using plasmid DNA containing each unique 16S rRNA insert. The copy number of the 16S rRNA gene per gram of fresh tissue was calculated as described by Zhou et al. (25). The relative abundances of bacterial genera were calculated by dividing the copy number of the 16S rRNA gene for each genus by the copy number of the 16S rRNA gene for total bacteria.
Statistical analysis.
The apparent relative abundances of communities at the phylum and genus levels, the alpha diversity indices (MiSeq sequencing), and the qPCR results for total ruminal epithelial bacteria and abundant bacterial genera were analyzed in a completely randomized design with the MIXED procedure of SAS (SAS Institute, Inc., Cary, NC). The model included the fixed effect of age and the random effect of the animal within age, with the individual animal as the experimental unit. Orthogonal contrasts were used to test for the linear and quadratic effects of age. Statistical significance was accepted at a P value of <0.05, and a P value of <0.10 was considered to indicate a trend. All data presented are expressed as least-squares means.
Spearman's rank correlations between the ruminal bacterial community (MiSeq relative abundance) and anatomic and functional variables were analyzed using the PROC CORR procedure of SAS. Only bacterial groups that represented >2% of the total community in at least one sample and that were detected in >50% of the rumen tissue samples were included in the analysis.
Nucleotide sequence accession numbers.
The sequencing data obtained in this study were deposited in the Metagenomics RAST (MG-RAST) server under identification numbers (IDs) 4613702.3 to 4613735.3 and in the NCBI Sequence Read Archive (SRA) under accession numbers SRR1823055, SRR1823127, SRR1823131, SRR1823138, SRR1823141, SRR1830869, SRR1830870, and SRR1830872 to SRR1830881.
RESULTS
Sampling depth, coverage, and alpha diversity.
After data filtering, quality control, assembling of paired-end reads, and removal of primers, chimeras, and low-confidence singletons, a total of 1,064,895 V2–V3 16S rRNA sequence reads from the 17 samples, with an average of 62,641 sequence reads for each sample (the minimum value for one sample was 27,120 sequence reads, and the maximum was 130,437), were used in this project. The average length of sequence reads after primer removal was 383 bp. The overall number of OTUs detected by the analysis reached 2,409 based on 97% nucleotide sequence identity between reads.
With a subsample of 27,000 reads for every sample, the rarefaction curves (see Fig. S1 in the supplemental material) showed that most of our sampling effort provided sufficient OTU coverage to accurately describe the bacterial composition of each group. Alpha diversity measures (Table 1) indicated that age did not affect coverage (P > 0.05) but showed a trend toward increasing values (P = 0.058) on the ACE index. Moreover, the number of OTUs, the Chao estimate, and the Shannon index increased linearly with age (P < 0.05), while the Simpson index declined linearly with age (P = 0.001).
TABLE 1.
Alpha diversity measures of the ruminal epithelial bacterial communities in kids at different agesa
| Alpha diversity index | Value at the following age (day): |
SEM |
P valueb |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 28 | 42 | 70 | L | Q | ||
| OTUs | 272.3 | 279.7 | 430.3 | 527.5 | 453.0 | 73.24 | 0.032 | 0.123 |
| Chao | 437.1 | 466.9 | 566.9 | 726.9 | 640.2 | 75.51 | 0.025 | 0.166 |
| ACE | 581.1 | 609.3 | 565.2 | 790.7 | 661.2 | 69.81 | 0.210 | 0.436 |
| Shannon | 1.61 | 1.37 | 2.91 | 2.96 | 3.22 | 0.383 | 0.002 | 0.158 |
| Simpson | 0.37 | 0.56 | 0.20 | 0.17 | 0.10 | 0.065 | 0.001 | 0.326 |
| Coverage | 0.995 | 0.995 | 0.995 | 0.993 | 0.994 | 0.001 | 0.151 | 0.456 |
From 27,000 reads.
L, linear; Q, quadratic.
Taxonomic composition of ruminal epithelial bacterial populations across different age groups.
In total, 26 phyla were identified within the ruminal epithelial microbiota. The abundance of 15 phyla in all the samples was <0.5%; these were Acidobacteria, Cyanobacteria, Elusimicrobia, Gemmatimonadetes, Lentisphaerae, NC10, Nitrospirae, OD1, OP11, Planctomycetes, Spirochaetes, Synergistetes, TM6, Tenericutes, and Verrucomicrobia. Among the 26 phyla, the Proteobacteria, Firmicutes, and Bacteroidetes were detected as the dominant phyla regardless of the age group (Table 2), but their ratio and composition differed considerably among the groups. The phylum Proteobacteria was the most abundant phylum in samples from 0 and 7 days, reaching proportions as high as >85%. Afterwards, the abundance of Proteobacteria declined quadratically (P < 0.001) with age. The phylum Bacteroidetes exhibited low values during the first week (<5%), and its abundance increased significantly afterwards (approximately 10%). The Firmicutes increased quadratically (P = 0.009) with age, becoming dominant at 42 and 70 days (54.71% and 40.29%, respectively). The Actinobacteria (P = 0.096) and TM7 tended to increase linearly (P = 0.067) with age, while age had quadratic effects on the abundances of Chloroflexi (P = 0.014) and Thermi (P = 0.051). Furthermore, the Fibrobacteres and Fusobacteria peaked at relative abundances of 0.49% at 42 days and 1.18% at day zero, respectively.
TABLE 2.
Phylum-level taxonomic composition of the ruminal epithelial bacterial communities at different ages in kidsa
| Phylum | % of sequences at the following age (days): |
SEM |
P valueb |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 28 | 42 | 70 | L | Q | ||
| Actinobacteria | 0.24 | 0.22 | 1.86 | 1.18 | 1.28 | 0.51 | 0.096 | 0.139 |
| Bacteroidetes | 1.57 | 3.31 | 12.80 | 8.61 | 10.57 | 3.77 | 0.088 | 0.227 |
| Chloroflexi | 0.00 | 0.01 | 0.80 | 0.22 | 0.42 | 0.11 | 0.013 | 0.014 |
| Fibrobacteres | 0.00 | 0.01 | 0.06 | 0.49 | 0.18 | 0.24 | 0.344 | 0.453 |
| Firmicutes | 1.94 | 7.06 | 16.99 | 54.71 | 40.29 | 4.84 | <0.0001 | 0.009 |
| Fusobacteria | 1.18 | 0.85 | 0.04 | 0.00 | 0.00 | 0.61 | 0.151 | 0.363 |
| Proteobacteria | 90.13 | 86.29 | 60.65 | 28.64 | 45.42 | 4.74 | <0.0001 | 0.0003 |
| TM7 | 0.03 | 0.01 | 0.01 | 0.29 | 0.17 | 0.08 | 0.067 | 0.562 |
| Thermi | 0.23 | 1.21 | 1.13 | 1.31 | 0.00 | 0.49 | 0.511 | 0.051 |
| Unclassified | 4.63 | 0.81 | 0.74 | 4.06 | 1.12 | 1.73 | 0.565 | 0.891 |
| Others (<0.5%) | 0.06 | 0.23 | 4.94 | 0.50 | 0.54 | 0.15 | 0.025 | 0.600 |
Data for the top 9 phyla are shown.
L, linear; Q, quadratic.
Of the 26 genera observed with >0.5% of relative abundance, 15 changed significantly with age (Table 3), while the others remained relatively stable. Moreover, a great proportion of sequences (ranging from 13.38% to 82.99%) remained unclassified at the genus level. Within the Bacteroidetes phylum, the genus Bacteroides peaked at 2.46% (7 days), and Porphyromonas abundance decreased linearly (P = 0.011) with age, while Prevotella abundance increased linearly (P = 0.022) with age. In the Firmicutes phylum, the genus Butyrivibrio exhibited low values during the first week (<1%) but became dominant afterwards, peaking at 41.38% relative abundance at 42 days. The abundance of Mogibacterium increased linearly (P = 0.003) with age, and that of p-75-a5 tended to increase quadratically (P = 0.077) with age. In contrast, the relative proportions of Oscillospira and Streptococcus declined with age. In the Proteobacteria phylum, the genus Escherichia was dominant at day zero (80.79%), decreased quadratically (P < 0.001) with age, and reached stable values (about 1%) after 28 days. In contrast, the proportion of Campylobacter was very minor during the first week (<1%) but surged afterwards, peaking at 29.99% at 70 days. Age had linearly decreasing effects on the genera Arcobacter (P = 0.038) and Enterobacter (P = 0.077) but, conversely, had increasing linear effects on Desulfobulbus (P = 0.033) and quadratic effects on Paracoccus (P = 0.075). Of the other minor genera that did not belong to these three phyla, Actinomyces, SHD-231, Fibrobacter, Fusobacterium, and Thermus peaked at 0.21% (70 days), 0.80% (28 days), 0.49% (42 days), 1.18% (0 days), and 1.31% (42 days), respectively.
TABLE 3.
Genus-level taxonomic composition of the ruminal epithelial bacterial communities at different ages in kids
| Phylum | Genus | % of sequences at the following age (days): |
SEM |
P valuea |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 28 | 42 | 70 | L | Q | |||
| Bacteroidetes | Bacteroides | 0.12 | 2.46 | 0.80 | 0.03 | 0.01 | 0.591 | 0.107 | 0.759 |
| Porphyromonas | 0.70 | 0.44 | 0.02 | 0.00 | 0.00 | 0.178 | 0.011 | 0.083 | |
| Prevotella | 0.47 | 0.20 | 4.86 | 5.76 | 5.18 | 1.718 | 0.022 | 0.149 | |
| Firmicutes | Bulleidia | 0.00 | 0.01 | 0.24 | 0.05 | 0.07 | 0.093 | 0.576 | 0.240 |
| Butyrivibrio | 0.10 | 0.56 | 8.90 | 41.38 | 30.13 | 5.606 | 0.000 | 0.086 | |
| Lysinibacillus | 0.00 | 0.00 | 0.55 | 0.00 | 0.00 | 0.213 | 0.947 | 0.197 | |
| Mogibacterium | 0.00 | 0.01 | 0.42 | 0.17 | 0.51 | 0.102 | 0.003 | 0.648 | |
| Oscillospira | 0.02 | 0.91 | 0.12 | 0.04 | 0.04 | 0.095 | 0.005 | 0.839 | |
| Ruminococcus | 0.02 | 0.05 | 0.82 | 0.52 | 0.30 | 0.351 | 0.441 | 0.158 | |
| Streptococcus | 0.38 | 0.12 | 0.35 | 0.00 | 0.00 | 0.117 | 0.056 | 0.964 | |
| Succiniclasticum | 0.00 | 0.01 | 0.28 | 0.04 | 0.12 | 0.107 | 0.468 | 0.353 | |
| p-75-a5 | 0.00 | 0.00 | 0.02 | 0.11 | 0.82 | 0.182 | 0.006 | 0.077 | |
| Proteobacteria | Acidovorax | 0.21 | 0.16 | 0.14 | 0.30 | 0.00 | 0.106 | 0.343 | 0.286 |
| Acinetobacter | 0.08 | 0.05 | 0.11 | 0.72 | 0.01 | 0.322 | 0.752 | 0.255 | |
| Actinobacillus | 0.49 | 0.01 | 0.01 | 0.00 | 0.00 | 0.219 | 0.259 | 0.332 | |
| Arcobacter | 0.37 | 0.75 | 0.08 | 0.02 | 0.00 | 0.206 | 0.038 | 0.406 | |
| Burkholderia | 0.00 | 0.01 | 0.26 | 0.00 | 0.00 | 0.084 | 0.901 | 0.129 | |
| Campylobacter | 0.02 | 0.50 | 4.27 | 20.33 | 29.99 | 4.467 | <0.0001 | 0.560 | |
| Desulfobulbus | 0.00 | 0.01 | 0.00 | 0.40 | 3.78 | 1.154 | 0.033 | 0.160 | |
| Enterobacter | 0.27 | 0.01 | 0.02 | 0.02 | 0.00 | 0.071 | 0.077 | 0.178 | |
| Escherichia | 80.79 | 7.48 | 1.65 | 0.23 | 0.06 | 3.578 | <0.0001 | <0.0001 | |
| Herbaspirillum | 0.00 | 0.06 | 1.09 | 0.00 | 0.00 | 0.376 | 0.896 | 0.156 | |
| Janthinobacterium | 0.00 | 0.01 | 0.33 | 0.00 | 0.00 | 0.110 | 0.902 | 0.141 | |
| Mannheimia | 0.31 | 0.01 | 0.01 | 0.00 | 0.00 | 0.141 | 0.269 | 0.366 | |
| Paracoccus | 0.00 | 0.00 | 0.13 | 0.21 | 0.00 | 0.089 | 0.631 | 0.075 | |
| Succinivibrio | 0.00 | 0.04 | 3.27 | 0.01 | 0.00 | 1.142 | 0.938 | 0.156 | |
| Variovorax | 0.00 | 0.01 | 0.26 | 0.00 | 0.00 | 0.087 | 0.915 | 0.151 | |
| Actinobacteria | Actinomyces | 0.03 | 0.01 | 0.03 | 0.00 | 0.21 | 0.078 | 0.116 | 0.184 |
| Chloroflexi | SHD-231 | 0.00 | 0.01 | 0.80 | 0.21 | 0.42 | 0.111 | 0.013 | 0.014 |
| Fibrobacteres | Fibrobacter | 0.00 | 0.01 | 0.06 | 0.49 | 0.18 | 0.237 | 0.344 | 0.453 |
| Fusobacteria | Fusobacterium | 1.18 | 0.85 | 0.04 | 0.00 | 0.00 | 0.612 | 0.151 | 0.363 |
| Thermi | Thermus | 0.23 | 1.21 | 1.13 | 1.31 | 0.00 | 0.490 | 0.510 | 0.051 |
| Unclassified | 13.38 | 82.99 | 66.02 | 26.00 | 27.13 | 7.535 | 0.055 | 0.014 | |
| Others (<0.5%) | 0.83 | 1.05 | 2.91 | 1.65 | 1.00 | 0.812 | 0.845 | 0.107 | |
L, linear; Q, quadratic.
OTU diversity and similarity analysis.
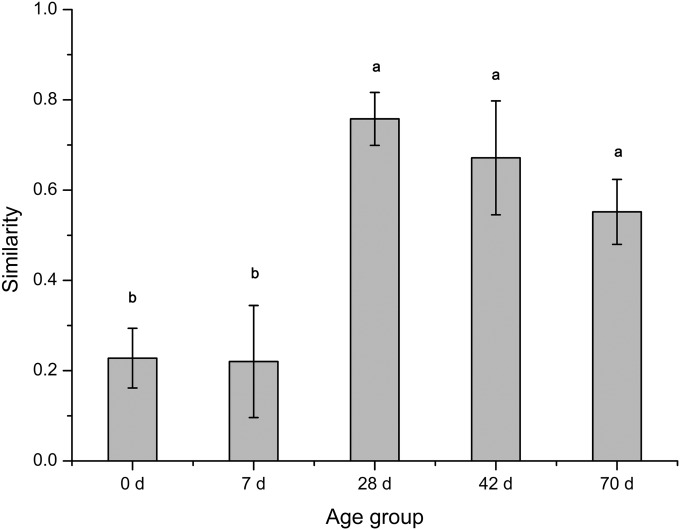
To minimize the variations created by different sample depths, jackknife subsampling (resampling 100 times at a depth of 20,250 sequences) was used in our study before beta diversity analysis was performed. PCoA analysis using the Bray-Curtis similarity metric revealed that the samples clustered according to age group (Fig. 1). The average within-group similarity showed a significant difference between different age groups; the values at 28, 42, and 70 days exceeded those at 0 and 7 days (Fig. 2).
FIG 1.
Principal coordinate analysis (PCoA) of the ruminal epithelial bacterial OTUs at different ages in kids. d, days.
FIG 2.
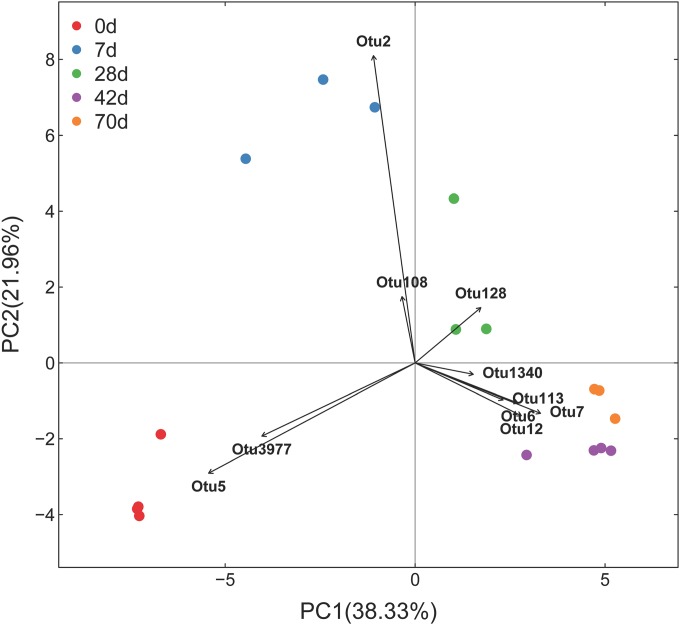
Within-group similarity, calculated as the average of the pairwise similarity between each two paired samples within each group using the Bray-Curtis metric. The closer the similarity is to 1, the higher the average similarity within a group. Letters above the bars indicate the significance of differences between groups; groups with different letters have significantly different similarity values (P < 0.05).
Ten dominant OTUs that contributed to the separation of the ruminal bacterial community at five different ages were identified (Fig. 3). The two dominant OTUs that separated samples at day zero from others were identified as Escherichia spp. Two OTUs in the Neisseriaceae and Ruminococcaceae families separated samples at 7 days from others. Likewise, for the separation of samples at 28 days, one OTU was identified (Ruminococcus spp.). The four OTUs contributing to the separation of samples at 42 days and 70 days from others displayed high identity to Butyrivibrio spp. and Campylobacter spp.
FIG 3.
Transformation-based PCA of ruminal epithelial bacterial OTUs at different ages in kids. The top 10 OTUs contributing to the separation of samples are displayed. OTU2, Neisseriaceae family; OTU108, Ruminococcaceae family; OTU5, Escherichia genus; OTU3977, Escherichia genus; OTU128, Ruminococcus genus; OTU6, Butyrivibrio genus; OTU7, Campylobacter genus; OTU12, Butyrivibrio genus; OTU113, Campylobacter genus; OTU1340, Butyrivibrio genus.
Quantification of total ruminal epithelial bacteria and abundant bacterial genera.
As illustrated by qPCR, the total ruminal epithelial bacterial copy numbers (P = 0.013) and the abundance of the genus Prevotella increased linearly (P = 0.049) with age (Table 4). Age did not affect (P > 0.05) the abundance of the genus Bacteroides.
TABLE 4.
qPCR results for total bacteria and two genera of the ruminal epithelial bacterial communities at different ages in kids
| Taxon | Abundancea at the following age (days): |
SEM |
P valueb |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 28 | 42 | 70 | L | Q | ||
| Bacteria | 1.37 | 1.43 | 3.72 | 3.09 | 4.40 | 0.838 | 0.013 | 0.510 |
| Prevotella | 2.36 | 4.29 | 3.75 | 8.25 | 7.26 | 1.820 | 0.049 | 0.556 |
| Bacteroides | 2.39 | 3.23 | 1.13 | 2.91 | 4.71 | 1.396 | 0.314 | 0.259 |
The abundance of total bacteria is expressed as 108 copy numbers per gram of rumen tissue. The relative abundances of the Prevotella and Bacteroides genera are expressed as percentages, calculated by dividing the 16S rRNA gene copy number of each genus by the 16S rRNA gene copy number of total bacteria.
L, linear; Q, quadratic.
Relationship between the bacterial community and anatomic and functional variables.
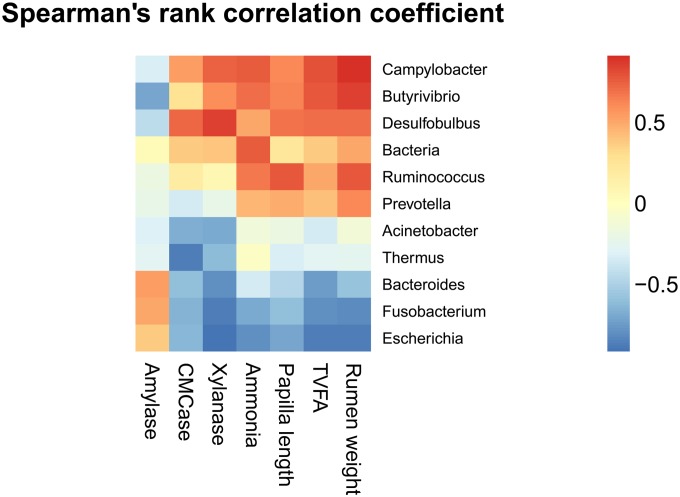
The abundance of the bacterial community at the genus level and anatomic and functional variables were regarded as correlated with each other if their correlation coefficients were above 0.55. The relative abundances of the genera Bacteroides, Escherichia, and Fusobacterium were negatively correlated with rumen weight, while the relative abundances of Butyrivibrio, Campylobacter, Desulfobulbus, Prevotella, and Ruminococcus were positively correlated with rumen weight (Fig. 4). The rumen papilla length was positively correlated with the relative abundances of Butyrivibrio, Campylobacter, Desulfobulbus, and Ruminococcus but was negatively correlated with the relative abundances of Escherichia and Fusobacterium. The ammonia concentration was positively correlated with the total bacterial copy number and the abundances of Butyrivibrio, Campylobacter, and Ruminococcus but was negatively correlated with the abundances of Escherichia and Fusobacterium. The total volatile fatty acid (TVFA) concentration was positively correlated with Butyrivibrio, Campylobacter, and Desulfobulbus abundances but negatively correlated with Bacteroides, Escherichia, and Fusobacterium abundances. Furthermore, xylanase activity was positively correlated with Butyrivibrio, Campylobacter, and Desulfobulbus abundances but negatively correlated with Acinetobacter, Bacteroides, Escherichia, Fusobacterium, and Thermus abundances. Carboxymethylcellulase (CMCase) activity was positively correlated with Desulfobulbus abundance but negatively correlated with Acinetobacter, Bacteroides, Escherichia, Fusobacterium, and Thermus abundances. Amylase activity was negatively correlated with Butyrivibrio abundance.
FIG 4.
Coefficients of correlation between relative abundances of the ruminal epithelial bacterial genera, on the one hand, and anatomic and functional variables, on the other.
DISCUSSION
Relationships between gastrointestinal microbial communities and their hosts have been shown in recent years to play an important role in the host's well-being and proper function. The objective of this study was to evaluate how bacteria colonized the rumen epithelium during normal development. Using MiSeq sequencing, we obtained 62,641 reads on average for each sample (with a minimum of 27,120 reads) with good coverage (>99.6%). Furthermore, our results suggest that each age group has its own distinct epithelial microbiota, as reflected by the clustering of samples by age group using PCoA and tb-PCA. Meanwhile, most of the alpha diversity indices (except ACE and the Simpson index) increased with age, suggesting that the microbiota in older age groups is more diverse than that in earlier age groups. This is similar to the process of microbial colonization of the rumen contents (11, 26).
Individual variation is an indispensable determinant of microbiota taxa. Although the experimental conditions, diet, and sampling procedures were similar for bovine rumen samples, a large fraction of the OTUs (50%) occurred in only a small number of samples (0 to 30%), implying that bacterial taxa differed considerably between individual bovine rumen contents (27). Similarly, within-group similarity in the rumen epithelial bacterial community in our study during the first week was quite low (around 0.2). Despite this, we observed a drastic increase in within-group similarity with age. This suggests that the rumen epithelium in older groups is a more restricted environment, housing a bacterial community more homogeneous than the earlier, heterogeneous community.
Host genetics is another factor affecting gastrointestinal microbial adaptation and evolution (28). In newborn calves, the bacterial communities of fecal content samples revealed higher similarity in PCR–single-strand conformation polymorphism (PCR-SSCP) profiles for twins than for their coresidents of the same age, indicating that individual microbial diversity might be genetically influenced (29). In our study, the effects of age on rumen epithelial bacterial diversity were compared using different groups of kids, which also accounted for the variation. Moreover, total epithelial bacterial populations for alfalfa hay- and oat-fed dairy calves (30), hay-fed sheep (31), and concentrate- and fresh-grass-fed goat kids (this study) ranged from 4.4 × 109 to 4.2 × 1010, 4.4 × 107 to 2.2 × 108, and 1.4 × 108 to 4.4 × 107 copy numbers per g of wet tissue, respectively. Besides the differences in model animals, diet, the DNA extraction method, and PCR primers and conditions, the host might also be an important determinant in regulating bacterial density.
In all age groups, Firmicutes, Bacteroidetes, and Proteobacteria were the dominant phyla in the epithelial microbiota, and their abundances and genus compositions differed remarkably, in line with previous studies (7, 32). Similarly, at the genus level, although the abundances of some genera changed with age, they were present in all groups, representing a core microbiome in the ruminal epithelial microbiota of goats. The current study showed that after 20 days, the epithelial microbiota harbored more members of the genera Butyrivibrio and Campylobacter than the bacterial population in the rumen contents (11), indicating that these two genera might be uniquely abundant epithelial bacteria.
The ruminal epithelial bacterial composition of goat kids undergoes sequential changes during the rumen development process. On the day of birth, the phylum Proteobacteria (90.13%) far exceeded other phyla, with the majority belonging to the genus Escherichia. This microbiota might derive from the microbiota of the mother's vagina (33), skin, colostrum (34), and environment. The genus Escherichia comprises facultatively anaerobic bacteria. Some commensal strains of Escherichia coli can reduce intestinal epithelial inflammatory signaling in vitro (35), while some pathogenic E. coli strains can invade host cells (36). As reflected by tb-PCA, two dominant OTUs that separated samples at day zero from others also displayed high identity to this genus. These facultative anaerobes could create the reduced environment that is required for anaerobic microbes (37). Further work would be needed to ascertain the functions of this genus in the rumen. Moreover, this genus declined considerably in abundance at 7 days, and a great many genera belonged to unclassified Proteobacteria. The remarkable changes observed in ruminal epithelial bacterial communities during the first week after birth indicated a rapid change in the rumen environment.
A noticeable change occurring at 28 days after solid food was offered (at 20 days) was the increase in ruminal epithelial bacterial density, which was reflected by qPCR (almost three times the copy numbers). Similar observations have been reported for rumen and gut contents (37). Moreover, the changes in rumen tissues were similar to the compositional changes in rumen contents (11): the genus Bacteroides declined in abundance in rumen tissues, while the proportions of the genera Prevotella, Butyrivibrio, Campylobacter, and Succinivibrio surged. Taking the findings together, solid food caused disruption in the ruminal epithelial microbiota by selecting bacterial taxa that were more specific and were adapted to new substrates. Members of the genus Prevotella, numerically predominant in ruminants, are capable of utilizing starches, other noncellulosic polysaccharides, and simple sugars as energy sources (24). Members of the Butyrivibrio group (including the genera Butyrivibrio and Pseudobutyrivibrio) represented 12.98% of total bacteria in the rumina of goats (38) and were involved in the biohydrogenation of unsaturated C18 fatty acids (39). Although some species of Campylobacter, such as Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species, have been implicated in the initiation of gastrointestinal diseases (40), they represent persistent residents of rumen microbial communities (10). Some species of the genus Succinivibrio could ferment maltose and glucose, thus participating in the last stage of starch digestion (41). From 28 to 70 days, the abundances of the genera Prevotella, Butyrivibrio, and Campylobacter still increased with age, implying that they were dominant genera in ruminal epithelial microbiota after the provision of solid feed. However, what role these genera played and how they interacted with the host have not been explored and remain to be elucidated.
Based on the data already published on rumen functional variables in the same goat kids (12), we explored the relationship between ruminal epithelial microbiota and rumen functions. The abundances of the genera Butyrivibrio, Campylobacter, Desulfobulbus, and Ruminococcus were positively correlated with rumen weight, rumen papilla length, ammonia concentration, and total volatile fatty acid concentration, suggesting that they might be involved in ammonia and volatile fatty acid metabolism as well as in the anatomic development of the rumen. Similarly, the genera Butyrivibrio, Campylobacter, and Desulfobulbus might also participate in fibrolytic enzyme secretion, and the genus Fusobacterium is related to starch degradation. Furthermore, specific commensal microbes have been reported to modulate host immune responses via proinflammatory and anti-inflammatory pathways, as well as epithelial-cell-mediated signals (42, 43). Therefore, further investigation to better explain the symbiotic relationship between the ruminal epithelial microbiota and the host is warranted.
Conclusion.
In conclusion, ruminal epithelial bacterial communities were more diverse in older groups than in earlier groups, and each age group had its distinct microbiota. Of the three dominant phyla, Proteobacteria declined in abundance with age, while Bacteroidetes and Firmicutes increased in abundance with age. The genus Escherichia dominated at the day of birth, while Prevotella, Butyrivibrio, and Campylobacter surged in abundance at around 2 months. Furthermore, the correlations between particular genera of the ruminal epithelial bacteria and anatomic and functional variables might indicate different types of relationships with the host. This study provides some preliminary information on the potential role of the ruminal epithelial bacteria, and further investigations are required to determine their actual roles and interactions with the host.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the financial support received from the National Natural Science Foundation of China (grant 31320103917), “Strategic Priority Research Program—Climate Change: Carbon Budget and Relevant Issues” (grant XDA05020700), “CAS Visiting Professorship for Senior International Scientists” (grants 2010T2S13 and 2012T1S0009), and Hunan Provincial Creation Development Project (2013TF3006).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00203-15.
REFERENCES
- 1.Cheng KJ, McCowan RP, Costerton JW. 1979. Adherent epithelial bacteria in ruminants and their roles in digestive-tract function. Am J Clin Nutr 32:139–148. [DOI] [PubMed] [Google Scholar]
- 2.Chen YH, Oba M, Guan LL. 2012. Variation of bacterial communities and expression of Toll-like receptor genes in the rumen of steers differing in susceptibility to subacute ruminal acidosis. Vet Microbiol 159:451–459. doi: 10.1016/j.vetmic.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Devkota S, Musch MW, Jabri B, Nagler C, Antonopoulos DA, Chervonsky A, Chang EB. 2010. Regional mucosa-associated microbiota determine physiological expression of TLR2 and TLR4 in murine colon. PLoS One 5:e13607. doi: 10.1371/journal.pone.0013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei CX, Mao SY, Cheng YF, Zhu WY. 2010. Diversity, abundance and novel 16S rRNA gene sequences of methanogens in rumen liquid, solid and epithelium fractions of Jinnan cattle. Animal 4:20–29. doi: 10.1017/S1751731109990681. [DOI] [PubMed] [Google Scholar]
- 5.Cho SJ, Cho KM, Shin EC, Lim WJ, Hong SY, Choi BR, Kang JM, Lee SM, Kim YH, Kim H, Yun HD. 2006. 16S rDNA analysis of bacterial diversity in three fractions of cow rumen. J Microbiol Biotechnol 16:92–101. [Google Scholar]
- 6.Mead LJ, Jones GA. 1981. Isolation and presumptive identification of adherent epithelial bacteria (“epimural” bacteria) from the ovine rumen wall. Appl Environ Microbiol 41:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petri RM, Schwaiger T, Penner GB, Beauchemin KA, Forster RJ, McKinnon JJ, McAllister TA. 2013. Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl Environ Microbiol 79:3744–3755. doi: 10.1128/AEM.03983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Menezes AB, Lewis E, O'Donovan M, O'Neill BF, Clipson N, Doyle EM. 2011. Microbiome analysis of dairy cows fed pasture or total mixed ration diets. FEMS Microbiol Ecol 78:256–265. doi: 10.1111/j.1574-6941.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Penner GB, Li M, Oba M. 2011. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl Environ Microbiol 77:5770–5781. doi: 10.1128/AEM.00375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li RW, Connor EE, Li C, Baldwin RL, Sparks ME. 2012. Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ Microbiol 14:129–139. doi: 10.1111/j.1462-2920.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 11.Jami E, Israel A, Kotser A, Mizrahi I. 2013. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J 7:1069–1079. doi: 10.1038/ismej.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao J, Li X, Beauchemin KA, Tan Z, Tang S, Zhou C. 2015. Rumen development process in goats as affected by supplemental feeding v. grazing: age-related anatomic development, functional achievement and microbial colonisation. Br J Nutr 113:888–900. doi: 10.1017/S0007114514004413. [DOI] [PubMed] [Google Scholar]
- 13.Hristov AN, Callaway TR, Lee C, Dowd SE. 2012. Rumen bacterial, archaeal, and fungal diversity of dairy cows in response to ingestion of lauric or myristic acid. J Anim Sci 90:4449–4457. doi: 10.2527/jas.2011-4624. [DOI] [PubMed] [Google Scholar]
- 14.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Yuan J, Yiu SM, Li Z, Xie Y, Chen Y, Shi Y, Zhang H, Li Y, Lam TW, Luo R. 2012. COPE: an accurate k-mer-based pair-end reads connection tool to facilitate genome assembly. Bioinformatics 28:2870–2874. doi: 10.1093/bioinformatics/bts563. [DOI] [PubMed] [Google Scholar]
- 17.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 18.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legendre P, Gallagher E. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. doi: 10.1007/s004420100716. [DOI] [PubMed] [Google Scholar]
- 22.Denman SE, McSweeney CS. 2006. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol 58:572–582. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 23.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. 2006. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson DM, Weimer PJ. 2007. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 75:165–174. doi: 10.1007/s00253-006-0802-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Hernandez-Sanabria E, Guan LL. 2009. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl Environ Microbiol 75:6524–6533. doi: 10.1128/AEM.02815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey M, Enjalbert F, Combes S, Cauquil L, Bouchez O, Monteils V. 2014. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J Appl Microbiol 116:245–257. doi: 10.1111/jam.12405. [DOI] [PubMed] [Google Scholar]
- 27.Jami E, Mizrahi I. 2012. Composition and similarity of bovine rumen microbiota across individual animals. PLoS One 7:e33306. doi: 10.1371/journal.pone.0033306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. 2008. Evolution of mammals and their gut microbes. Science 320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer M, Abenthum A, Matthes J, Kleeberger D, Ege M, Hölzel C, Bauer J, Schwaiger K. 2012. Development and genetic influence of the rectal bacterial flora of newborn calves. Vet Microbiol 161:179–185. doi: 10.1016/j.vetmic.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Malmuthuge N, Li MJ, Chen YH, Fries P, Griebel PJ, Baurhoo B, Zhao X, Guan LL. 2012. Distinct commensal bacteria associated with ingesta and mucosal epithelium in the gastrointestinal tracts of calves and chickens. FEMS Microbiol Ecol 79:337–347. doi: 10.1111/j.1574-6941.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 31.Wallace RJ, Cheng KJ, Dinsdale D, Orskov ER. 1979. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature 279:424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]
- 32.Sadet S, Martin C, Meunier B, Morgavi DP. 2007. PCR-DGGE analysis reveals a distinct diversity in the bacterial population attached to the rumen epithelium. Animal 1:939–944. doi: 10.1017/S1751731107000304. [DOI] [PubMed] [Google Scholar]
- 33.Mändar R, Mikelsaar M. 1996. Transmission of mother's microflora to the newborn at birth. Biol Neonate 69:30–35. doi: 10.1159/000244275. [DOI] [PubMed] [Google Scholar]
- 34.Wise GH, Anderson GW. 1939. Factors affecting the passage of liquids into the rumen of the dairy calf. I. Method of administering liquids: drinking from open pail versus sucking through a rubber nipple. J Dairy Sci 22:697–705. [Google Scholar]
- 35.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. 2005. Bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol 175:4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 36.Kalita A, Hu J, Torres AG. 2014. Recent advances in adherence and invasion of pathogenic Escherichia coli. Curr Opin Infect Dis 27:459–464. doi: 10.1097/QCO.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jost T, Lacroix C, Braegger CP, Chassard C. 2012. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One 7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Z, Hang S, Mao S, Zhu W. 2014. Diversity of Butyrivibrio group bacteria in the rumen of goats and its response to the supplementation of garlic oil. Asian-Australas J Anim Sci 27:179–186. doi: 10.5713/ajas.2013.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckaert C, Vlaeminck B, Fievez V, Maignien L, Dijkstra J, Boon N. 2008. Accumulation of trans C18:1 fatty acids in the rumen after dietary algal supplementation is associated with changes in the Butyrivibrio community. Appl Environ Microbiol 74:6923–6930. doi: 10.1128/AEM.01473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK, Norman J, Day AS, Zhang L, Mitchell HM. 2010. Host attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J Infect Dis 202:1855–1865. doi: 10.1086/657316. [DOI] [PubMed] [Google Scholar]
- 41.O'Herrin SM, Kenealy WR. 1993. Glucose and carbon dioxide metabolism by Succinivibrio dextrinosolvens. Appl Environ Microbiol 59:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. 2012. The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 12:728–734. doi: 10.1038/nri3312. [DOI] [PubMed] [Google Scholar]
- 43.Thomas LV, Ockhuizen T. 2012. New insights into the impact of the intestinal microbiota on health and disease: a symposium report. Br J Nutr 107(Suppl 1):S1–S13. doi: 10.1017/S0007114511006970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.