Abstract
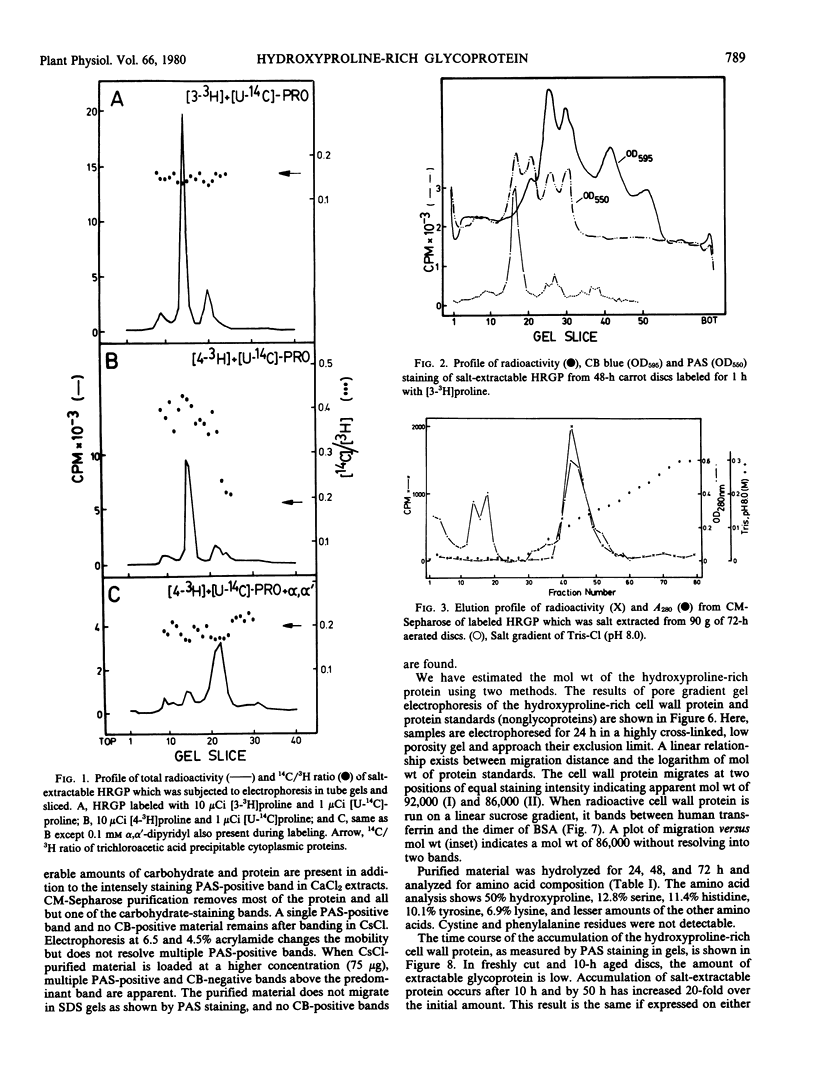
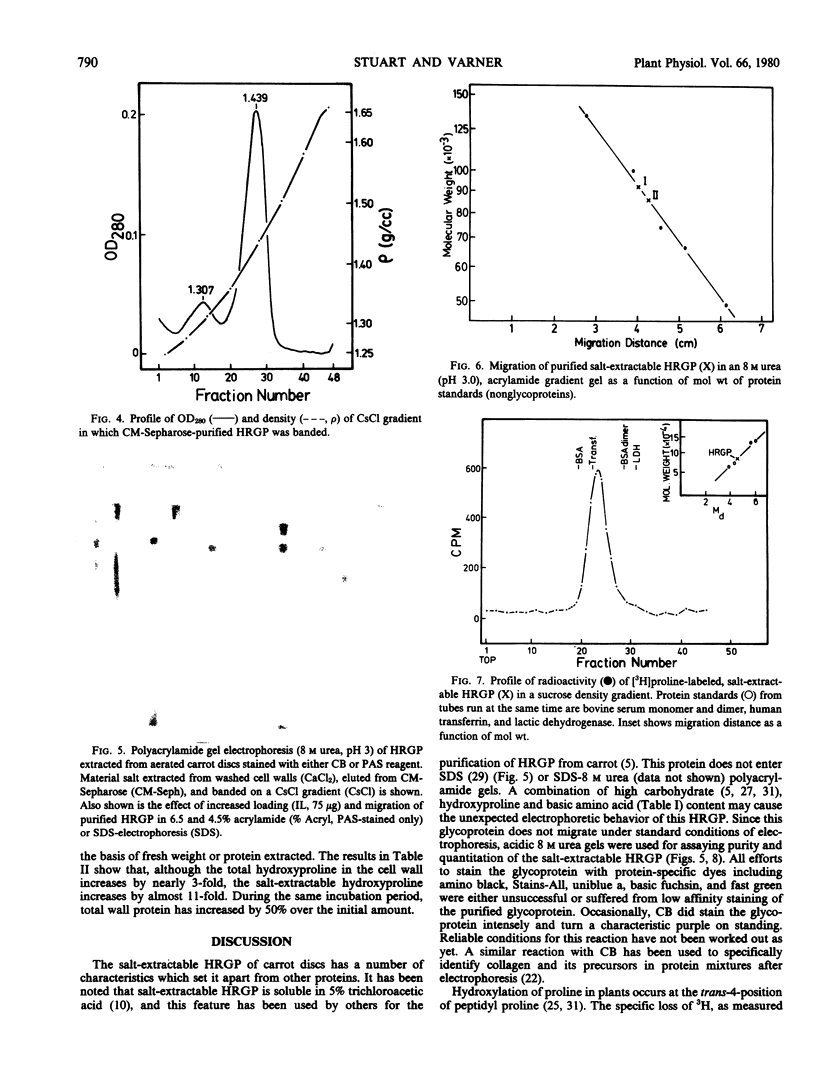
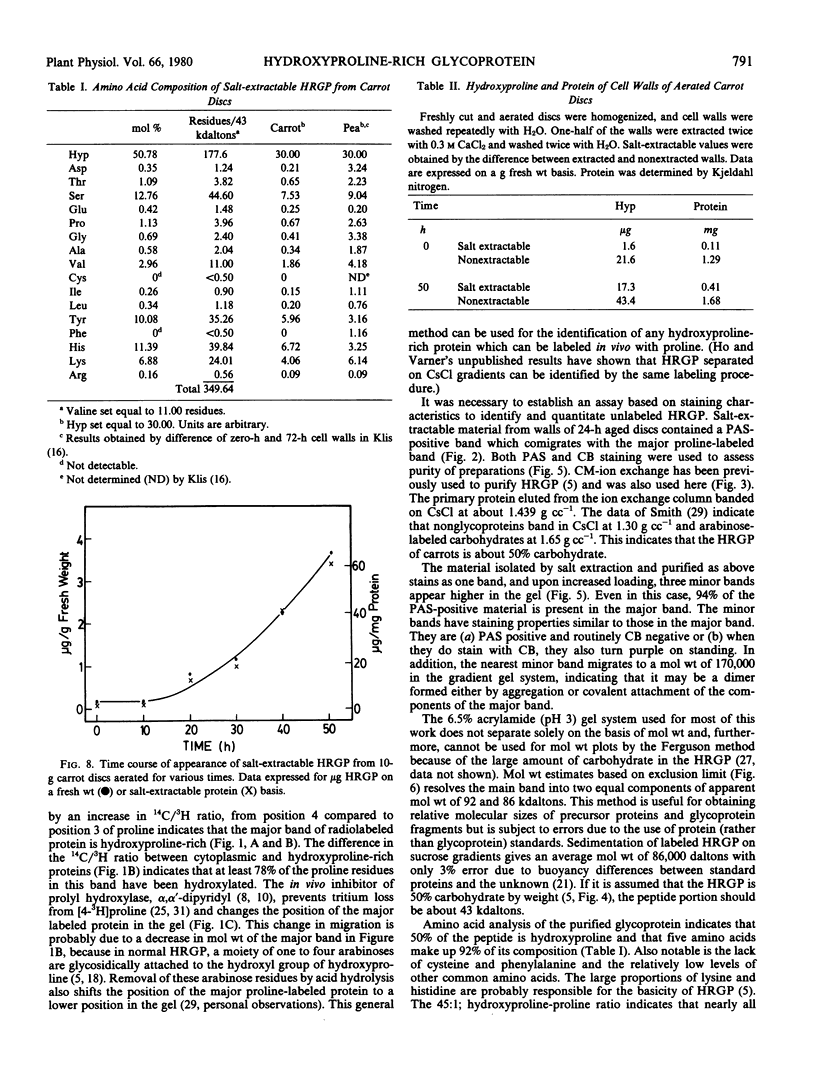
The salt-extractable hydroxyproline-rich glycoprotein (HRGP) of the cell wall of aerated carrot root discs has been studied by polyacrylamide gel electrophoresis. The predominant proline-labeled protein extractable from the cell wall is rich in hydroxyproline as shown by its specific loss of 3H from proline labeled in position 4 and its shift in electrophoretic mobility after labeling in the presence of an inhibitor of hydroxyproline synthesis. Unlabeled HRGP can be identified by staining gels for carbohydrate. The HRGP has been purified by ion exchange chromatography and CsCl gradient centrifugation. The HRGP consists of about 50% protein and 50% carbohydrate with an overall molecular weight of 86,000. The amino acid composition of the protein portion consists of 50% hydroxyproline, 19% basic amino acids, 12% serine, and 10% tyrosine. This glycoprotein accumulates in a salt-extractable pool in the cell wall beginning between 10 and 20 hours of aeration and may also become incorporated into the nonextractable portion of the cell wall.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and Secretion of Hydroxyproline-containing Macromolecules in Carrots: II. In vivo Conversion of Peptidyl Proline to Peptidyl Hydroxyproline. Plant Physiol. 1970 Feb;45(2):223–227. doi: 10.1104/pp.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Distribution and metabolism of protein-bound hydroxyproline in an elongating tissue, the Avena coleoptile. Plant Physiol. 1968 Jun;43(6):865–870. doi: 10.1104/pp.43.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall D. K., Shimbayashi K. Factors Affecting Growth of Tobacco Callus Tissue and Its Incorporation of Tyrosine. Plant Physiol. 1960 May;35(3):396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Klis F. M. Glycosylated seryl residues in wall protein of elongating pea stems. Plant Physiol. 1976 Feb;57(2):224–226. doi: 10.1104/pp.57.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCormick P. J., Chandrasekhar S., Millis A. J. Direct visualization of collagens and procollagens in polyacrylamide gels. Anal Biochem. 1979 Sep 1;97(2):359–366. doi: 10.1016/0003-2697(79)90086-1. [DOI] [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. Crystalline glycoprotein cell walls of algae: their stucture, composition and assembly. Philos Trans R Soc Lond B Biol Sci. 1974 Jul 25;268(891):129–146. doi: 10.1098/rstb.1974.0021. [DOI] [PubMed] [Google Scholar]
- SCHRAM E., MOORE S., BIGWOOD E. J. Chromatographic determination of cystine as cysteic acid. Biochem J. 1954 May;57(1):33–37. doi: 10.1042/bj0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]