Abstract
Using coarse-grained molecular dynamics simulations we have explored the effect of α-Synuclein (αSyn) on the structural and mechanical properties of small unilamellar vesicles in the fluid-phase. The study is motivated by observations that a high density of membrane-bound αSyn inhibits the fusion of synthetic small unilamellar vesicles. By combining three-dimensional pressure tensor calculations with our recently developed spherical harmonics fluctuation analysis approach, we show a reduction in membrane surface tension and increased membrane undulations when αSyn is bound to the vesicle’s outer leaflet at a 200:1 L/P. The protein effects these changes by decreasing the negative pressure in the headgroup region of the outer leaflet and increasing the positive pressure throughout the hydrocarbon core.
Main Text
Multiple in vivo studies have shown that membrane-bound α-Synuclein (αSyn), an amphipathic α-helix that associates with synaptic vesicles (SVs) (1), can disrupt SV trafficking (2). A recent in vitro study showed that αSyn inhibits fusion of small unilamellar vesicles (SUVs) (3), whose size closely matches that of SVs (∼30–40 nm diameter). This effect was seen both in DPPC SUVs in the gel-phase and, importantly, in a more physiologically relevant quaternary lipid mixture in the fluid-phase (DOPC/DOPE/SM/Chol). This mixture is highly fusogenic and was originally conceived to closely mimic the lipid composition of synaptic vesicles (4). DeWitt and Rhoades (5) have recently shown that αSyn inhibits SNARE-mediated fusion of fluid-phase SUVs without interacting directly with those proteins.
Collectively, these findings have led to the hypothesis that αSyn inhibits fusion through direct alteration of the lipid bilayer’s physical properties (2,3,5–7). Once biophysical mechanisms for these inhibitory effects are understood, the impact is expected to extend to other proteins with similar amphipathic characteristics. For example, apolipoprotein A-I, a related, but larger membrane binding protein that shares αSyn’s amphipathic 11-mer repeat sequence, was also shown to inhibit vesicle fusion. On the other hand, a short (but still amphipathic) segment of αSyn has no inhibitory effect (3). Exactly what bestows αSyn with its antifusogenic activity, be it sequence-specific or a more generic feature of all amphipathic helices, remains unknown.
Because of their small size, SUVs are under high curvature stress, imparting an intrinsic driving force for fusion. In the case of gel-phase SUVs, thermodynamic measurements prompted the hypothesis that αSyn anneals defect zones in the lipid matrix, thus inhibiting SUV fusion by relieving curvature stress (3,7). However, no direct experimental measurements to test this hypothesis have yet been made. Our early molecular dynamics (MD) simulations of αSyn bound to SDS micelles were consistent with this idea, showing that the protein relaxes the highly curved spherical micelle into an ellipsoidal shape (6).
Relatedly, multiple biophysical studies have demonstrated αSyn’s capacity to induce membrane curvature in giant (flat and rigidless) vesicles, leading to tubules of diameter <40 nm (8). Using coarse-grained MD simulations, we recapitulated this finding, showing that αSyn induces positive mean curvature fields (∼30–40 nm) in flat bilayers. This curvature-effect stems from the protein’s specific insertion depth (1–4 Å beneath the headgroup phosphates (6,9,10)), a highly specific location that has been predicted to induce positive curvature (11,12). Of note, an amphipathic segment of apolipoprotein A-I has been shown to partition to the same approximate location (13). These findings leave open important questions: what impact does αSyn have, if any, on the curvature-related properties of a 30–40 nm vesicle? And, can these effects explain αSyn’s inhibition of SUV fusion?
The goal of this study is to explore whether αSyn reduces curvature stress in fluid-phase SUVs, and if so what underlies this change. Using coarse-grained MD simulations, employing the MARTINI force field (14), we have examined DPPC SUVs (∼35 nm in diameter) with and without αSyn prebound at the experimental lipid-protein ratio (200:1) (3). Details of the simulation methodology are provided in the Supporting Material. The protein is simulated as a single, extended α-helix whose secondary structure is fixed. While conformational heterogeneity of the protein has recently been noted (15), we have shown that it has little effect on protein partition depth or membrane curvature (10). We have simulated DPPC vesicles in the fluid-phase, rather than the more complex quaternary mixture, for several reasons (discussed at length in the Supporting Material). Principally, this choice eliminates prohibitive equilibration issues in multicomponent mixtures that, given current computational limitations, preclude faithful reporting of tensions.
Fig. 1 shows a snapshot from the simulations of the αSyn-bound vesicle that illustrates the surface density of the bound protein. Bilayer structural properties were determined using our recently developed algorithm (see the Supporting Material for details) (16). As expected, the headgroup density is higher in the inner leaflet due to compaction in the convex vesicle. The protein has very little effect on bilayer structure, partitioning to a similar depth as previous studies (6,9,10,17), with no notable change in bilayer thickness (Fig. S1 in the Supporting Material). Additionally, αSyn increases the average acyl-chain order parameter by ∼5% (Fig. S2), although the chains remain fluid.
Figure 1.

Snapshot of αSyn bound to a fluid-phase DPPC vesicle. αSyn (red); N-terminus (black); and C-terminus (green). Solvent beads were removed for clarity. To see this figure in color, go online.
Fig. 2 shows the bilayer lateral pressure profiles, PLat(r) (see Eq. S1 in the Supporting Material), determined using the three-dimensional pressure tensor method of Ollila et al. (18) (see the Supporting Material and Fig. S3 for details). Integration of the lateral pressure profiles gives the membrane surface tension, γ (see Eq. S2 in the Supporting Material). Addition of αSyn causes a dramatic reduction in γ (from 149.2 to 46.3 mN/m, a drop of ∼70%; see Fig. S4 and Table S1 in the Supporting Material). While we note that these values, especially in the pure DPPC vesicle, are quite high (from an experimental stability perspective), the tensions do agree roughly with previous MARTINI results (18) (see Discussion in the Supporting Material).
Figure 2.
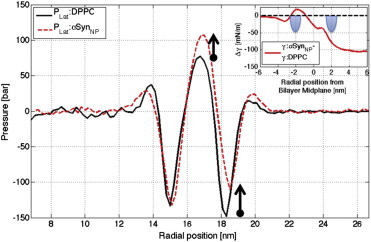
Lateral pressure profiles, PLat, for pure DPPC (black solid line) and αSynNP (red dashed line) systems. Surface tension differences (γαSyn,NP-γDPPC) with lipid headgroups (inset). To see this figure in color, go online.
Dissecting the differences in the lateral pressure profiles in Fig. 2 reveals the source of this surface tension difference. Negative (compressive) pressure peaks in lipid bilayers reflect attraction in the headgroups, whereas the positive pressure peaks correspond to chain repulsion in the hydrocarbon core. Across a flat, tensionless bilayer these opposing pressures perfectly compensate. The positive surface tension in the pure DPPC vesicle arises from the following: 1) a substantial increase in the negative pressure in the outer leaflet headgroups relative to the inner leaflet headgroups (this predictability reflects the convexity of the curved bilayer in the SUV whereby the outer leaflet lipid head-head spacing is increased relative to the inner leaflet and thus experiences a larger, i.e., negative restoring force); and 2) a weak expansive pressure in the lipid chains that fails to offset the pressure in the headgroups.
Regarding the protein-induced reduction in γ, the data suggest important differences in both the headgroup region of the outer (protein-containing) leaflet and across the entirety of the hydrocarbon core (both leaflets). These changes are consistent with a recent investigation of a different peptide inserted in a lamellar bilayer (12). In the outer-leaflet headgroups, the pressure is less negative than in the inner leaflet headgroup region (thus reversing the trend seen in the pure vesicle). This likely reflects the fact that the protein fills the space between the stressed headgroups, thus relieving the restoring force between them. In the hydrocarbon core, the protein causes an increase in the positive pressure (chain-chain repulsion). This effect starts in the inner leaflet at the point where the inner leaflet’s carbonyl density is zero, and extends outward throughout the outer-leaflet chains (Figs. S1 and S5).
Changes in surface tension should be reflected in the mechanical properties of the SUV. Specifically, reduced γ should manifest as increased undulations in the vesicle. To test this, we analyzed bilayer fluctuation intensity using our spherical harmonics algorithm (see Methods in the Supporting Material for details) (16). Fig. 3 presents the fluctuations spectra, alm, for each system (see also Fig. S6). Lower degree fluctuations (L < 25 for a vesicle with a radius of 17 nm) correspond to long wave undulations that propagate along the surface of the sphere. In a tensionless system we have shown how to use these profiles to extract an experimentally quantified measure of membrane bending rigidity using Helfrich continuum theory (16,19). In the case of a vesicle with protein bound, no theory yet exists to reliably quantify the bending rigidity. Nonetheless, we can clearly see from the spectra that αSyn increases the undulation intensity relative to pure DPPC. Increased fluctuations suggest a floppier, less rigid vesicle. To quantify the spectra, we summed all undulation degrees, and show a near-threefold increase in fluctuations when αSyn is bound (Table S1).
Figure 3.

Fluctuations spectra, alm, for pure DPPC (black solid line) and αSynNP (red dashed line) vesicle systems. Increased alm intensity corresponds to a less rigid membrane. To see this figure in color, go online.
We have provided a compelling correlation between αSyn’s inhibition of SUV fusion and a reduction in surface tension and rigidity. The molecular composition of SV membranes is far more complex than any model systems that have been studied to date (20). The likelihood of an asymmetric distribution of phospholipids and cholesterol (determined by their individual spontaneous curvatures), and asymmetric (conical-shaped) membrane proteins, inevitably determines the energy stored to drive fusion. The seminal study of the DOPC/DOPE/SM/Chol mixture provides important evidence that SV lipids are stressed (4). However, the extent to which SVs in a neuron are actually stressed, and to what extent αSyn alters their mechanical properties, remains an important avenue of research.
Author Contributions
A.R.B. and J.N.S. contributed equally in research design, analysis, and writing for this article.
Acknowledgments
Simulations and analysis were completed at the Minnesota Supercomputing Institute.
This work was supported by National Institutes of Health grant No. RO1 NS084998 (to J.N.S.) and National Research Service Award Fellowship No. F31 NS077634 (to A.R.B.).
Supporting Material
References
- 1.Burré J., Sharma M., Südhof T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auluck P.K., Caraveo G., Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu. Rev. Cell Dev. Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- 3.Kamp F., Exner N., Haass C. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque M.E., McIntosh T.J., Lentz B.R. Influence of lipid composition on physical properties and PEG-mediated fusion of curved and uncurved model membrane vesicles: “nature’s own” fusogenic lipid bilayer. Biochemistry. 2001;40:4340–4348. doi: 10.1021/bi002030k. [DOI] [PubMed] [Google Scholar]
- 5.DeWitt D.C., Rhoades E. α-Synuclein can inhibit SNARE-mediated vesicle fusion through direct interactions with lipid bilayers. Biochemistry. 2013;52:2385–2387. doi: 10.1021/bi4002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlmutter J.D., Braun A.R., Sachs J.N. Curvature dynamics of α-synuclein familial Parkinson disease mutants: molecular simulations of the micelle- and bilayer-bound forms. J. Biol. Chem. 2009;284:7177–7189. doi: 10.1074/jbc.M808895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuscher B., Kamp F., Beyer K. Alpha-synuclein has a high affinity for packing defects in a bilayer membrane: a thermodynamics study. J. Biol. Chem. 2004;279:21966–21975. doi: 10.1074/jbc.M401076200. [DOI] [PubMed] [Google Scholar]
- 8.Varkey J., Isas J.M., Langen R. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J. Biol. Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jao C.C., Der-Sarkissian A., Langen R. Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc. Natl. Acad. Sci. USA. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun A.R., Sevcsik E., Sachs J.N. α-Synuclein induces both positive mean curvature and negative Gaussian curvature in membranes. J. Am. Chem. Soc. 2012;134:2613–2620. doi: 10.1021/ja208316h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campelo F., McMahon H.T., Kozlov M.M. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodt A.J., Pastor R.W. Molecular modeling of lipid membrane curvature induction by a peptide: more than simply shape. Biophys. J. 2014;106:1958–1969. doi: 10.1016/j.bpj.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hristova K., Wimley W.C., White S.H. An amphipathic α-helix at a membrane interface: a structural study using a novel x-ray diffraction method. J. Mol. Biol. 1999;290:99–117. doi: 10.1006/jmbi.1999.2840. [DOI] [PubMed] [Google Scholar]
- 14.Marrink S.J., Risselada H.J., de Vries A.H. The MARTINI force field: coarse-grained model for biomolecular simulations. J. Phys. Chem. B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 15.Vermaas J.V., Tajkhorshid E. Conformational heterogeneity of α-synuclein in membrane. Biochim. Biophys. Acta. 2014;1838:3107–3117. doi: 10.1016/j.bbamem.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun A.R., Sachs J.N. Determining structural and mechanical properties from molecular dynamics simulations of lipid vesicles. J. Chem. Theory Comput. 2014;10:4160–4168. doi: 10.1021/ct500460u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun A.R., Lacy M.M., Sachs J.N. α-Synuclein-induced membrane remodeling is driven by binding affinity, partition depth, and interleaflet order asymmetry. J. Am. Chem. Soc. 2014;136:9962–9972. doi: 10.1021/ja5016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ollila O.H., Risselada H.J., Marrink S.J. 3D pressure field in lipid membranes and membrane-protein complexes. Phys. Rev. Lett. 2009;102:078101. doi: 10.1103/PhysRevLett.102.078101. [DOI] [PubMed] [Google Scholar]
- 19.Brandt E.G., Braun A.R., Edholm O. Interpretation of fluctuation spectra in lipid bilayer simulations. Biophys. J. 2011;100:2104–2111. doi: 10.1016/j.bpj.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsch J.W., Kelly R.B. Lipids of synaptic vesicles: relevance to the mechanism of membrane fusion. Biochemistry. 1981;20:378–385. doi: 10.1021/bi00505a024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


