Abstract
OBJECTIVE
To compare the prevalence of and characteristics associated with early intervention (EI) program enrollment among infants born late preterm (34–36 weeks’ gestation), early term (37–38 weeks’ gestation), and term (39–41 weeks’ gestation).
METHODS
A Massachusetts cohort of 554 974 singleton infants born during 1998 through 2005 and survived the neonatal period was followed until the third birthday of each infant. Data came from the Pregnancy to Early Life Longitudinal Data System that linked birth certificates, birth hospital discharge reports, death certificates, and EI program enrollment records. We calculated prevalence and adjusted risk ratios to compare differences and understand associations.
RESULTS
The prevalence of EI program enrollment increased with each decreasing week of gestation before 41 weeks (late preterm [23.5%], early term [14.9%], and term [11.9%]. In adjusted analyses, the strongest predictors of EI enrollment (adjusted risk ratio ≥1.20) for all gestational age groups were male gender, having a congenital anomaly, and having mothers who were ≥40 years old, nonhigh school graduates, and recipients of public insurance.
CONCLUSIONS
Infants born late preterm and early term have higher prevalence of EI program services enrollment than infants born at term, and may benefit from more frequent monitoring for developmental delays or disabilities.
Keywords: late preterm, early intervention, developmental outcomes, developmental disabilities, prematurity, gestational age, postnatal development, follow-up studies
Preterm birth is a leading cause of mortality, morbidity, and disability.1 Late preterm births (340/7 through 366/7 weeks’ completed gestation) account for approximately three-quarters of all preterm births in the United States.2 Adverse health outcomes among infants born late preterm are well documented and include temperature instability, respiratory difficulties, hypoglycemia, and hyperbilirubinemia during the newborn period resulting in prolonged hospitalization, as well as higher rates of neonatal mortality.3-7 Since the early 1990s, the proportion of late preterm births among US singletons has increased by 19%, from 6.8% in 1990 to 1991 to 8.1% in 2005 to 20068; although data from 2007 to 2010 suggest a decline in late pretermbirths.9 In Massachusetts (MA), the rate of late preterm birth increased more substantially by 43%, from 4.8% in 1990 to 1991 to 6.8% in 2005 to 2006.8 An increase in early term births, those occurring from 370/7 through 386/7 weeks’ completed gestation, has also been observed. These early term infants are also physiologically immature and have increased risk for mortality and short-term morbidity compared with those born at 39 weeks’ completed gestation and later.10,11 Although the risk of short-term morbidity is well established, knowledge about longer-term morbidity and disability among infants born late preterm and early term is limited but needed.12-19 In 2007, the American Academy of Pediatrics Committee on Fetus and Newborn called for large population studies that evaluate the long-term neurodevelopmental and behavioral outcomes of children born late preterm given their premature nervous systems.20
Developmental delay and disability are important indicators of adverse neurodevelopmental outcomes. Early intervention (EI) program services are available at no cost to any MA child meeting program eligibility for developmental delay or disability and in need of such services. Given the limited knowledge about the risk for long-term developmental morbidity among infants born late preterm and early term, and given the increasing rate of late preterm and early term births, we wanted to know if infants born late preterm and early term have higher prevalences of EI program services enrollment than infants born at term. If such differences exist, these infants may benefit from more frequent monitoring for developmental delays or disabilities. Also, it may further discourage early obstetric intervention in the absence of medical indications.
Our study objectives were to (1) investigate the prevalence of and characteristics associated with EI program enrollment in MA, comparing infants born late preterm (340/7–366/7 weeks’ completed gestation) to infants born early term (370/7–386/7 weeks’ completed gestation) and at term (390/7–416/7 weeks’ completed gestation); (2) examine the association between gestational age and EI program enrollment; and (3) describe EI program services utilization by gestational age groups.
METHODS
Study Population and Design
We analyzed a cohort of singleton children born late preterm, early term, and term in MA hospitals to resident mothers and followed children via records from birth untilage 3 years. Data were derived from the MA Pregnancy to Early Life Longitudinal Data System (PELL). PELL is a longitudinally linked and relational data system with information on mothers and their children from delivery and birth through early childhood. The PELL data system uses deterministic and probabilistic methodologies to link vital statistics records (birth and death certificates), hospital utilization, and public health programs’ participation data by using LinkPro software (InfoSoft, Inc, Winnipeg, Manitoba, Canada). PELL is a public–university partnership among the Boston University School of Public Health, the MA Department of Public Health, and the Centers for Disease Control and Prevention, the funding agency.
For this analysis, we used MA birth certificates from January 1, 1998, through December 31, 2005, linked to their corresponding infant death certificates, infant birth hospital discharge records, and EI program participation data from January 1, 1998, through December 31, 2008, to allow for 3 years of potential EI enrollment. We restricted our analysis to singleton births, 34 to 41 weeks’ gestation who survived the neonatal period. Neonatal deaths were excluded because we wanted to examine factors associated with EI enrollment among surviving infants. Because obstetric decision-making and practices around age at delivery is different for twins and singletons, we excluded twins from the analysis. Also, in MA, nearly 40% of twins are conceived with assisted reproductive technology; twins who are conceived via in vitro fertilization have increased rates of preterm birth and other morbidity compared with spontaneously conceived twins.21
The PELL data system linked over 99% of MA birth certificates to MA birth hospitalization records and infant death certificates, and 83% of EI program records could be linked to birth certificate records. Children of families that moved to MA after the child’s birth could not be linked to the MA PELL data system, because linkage to EI program participant records depends on MA residency at the time of participation, not on MA birth. Approximately 5% of MA residents (0–5 years old) moved in or out of state annually.
We classified births as late preterm(34–36 weeks), early term (37–38 weeks), and term (39–41 weeks) based on gestational age. We estimated gestational age by using both last menstrual period (LMP) and clinical estimate as reported on the birth certificate (in completed weeks). The clinical estimate of gestational age was substituted for the LMP-based estimate of gestational age when the measures differed by >2 weeks.22
As defined by Clements et al,23 children were classified as enrolled in the MA EI program if they had completed an Individual Family Service Plan, regardless of whether they received services. An Individual Family Service Plan is completed after referral and formal evaluation for eligibility. Using the service delivery records and claims data set, we examined type of EI program services used (eg, developmental specialist, occupational therapist, and speech language pathologist); number of EI program services used; and number of calendar years enrolled.
Covariates selected were known risk factors for developmental delay or disability and for preterm birth and were available in the PELL data system. Variables derived from birth certificate responses included infant gender, maternal education, maternal age, parity, race-ethnicity, pregnancy complications, labor and delivery complications, congenital anomalies, mother’s language preference, maternal nativity, and the Adequacy of Prenatal Care Utilization Index.24 Payer at time of delivery was derived from variables from birth certificate and hospital discharge records.
First, to investigate infants at highest risk for enrollment in EI program services, we compared the prevalence of children enrolled during the first 3 years after birth among infants who were born term, early term, and late preterm by maternal and infant characteristics. Prevalence risk ratios describe independent associations between these characteristics and enrollment in EI program services by gestational age groups. Second, we examined the association between gestational age and enrollment in EI program services by calculating crude and adjusted risk ratios (aRRs) and 95%confidence intervals (95% CIs).25 Finally, we compared EI program service-type usage, frequency of service, and number of calendar years enrolled by gestational age group. Service-type usage was compared with percentages, whereas service frequency and years of use were compared with means and SDs. Due to confidentiality, the exact date of service was unavailable to calculate the actual length of enrollment, and thus we calculated calendar years.
To examine the association between gestational age and EI program enrollment, we estimated risk ratios by using generalized estimating equations with a modified Poisson regression.25 Unlike logistic regression, this modified Poisson approach allowed for direct estimation of the risk ratio. This is important as the risk ratio, as opposed to the odds ratio, is the preferred measure when an outcome under evaluation is frequent. We fit both unadjusted and covariate-adjusted risks on the log scale, together with robust variance estimators. In addition, because some children in our study were siblings, using generalized estimating equations allowed us to account for within-family correlation in our data. All data were analyzed by using SAS software, version 9.2 for Windows (SAS Institute, Inc, Cary, NC). Because of our large sample, we chose not to include P values as even small differences would be statistically significant.
RESULTS
Our final study population comprised 554 947 infants: 27 345 late preterm, 116 035 early term, and 411 567 term. Figure 1 outlines the study selection process revealing inclusion and exclusion criteria. The distributions of selected characteristics of our participants are shown by gestational age group in Table 1. The highest proportions of infants were boys and those without congenital anomalies. The highest proportions of mothers were 25 to 34 years old, had an education level beyond high school, and were non-Hispanic white, privately insured, US-born, preferred speaking English, nulliparous before the index child, and received adequate prenatal care. Pregnancy and labor and delivery complications were more common among infants born late preterm than those born early term or term.
FIGURE 1.
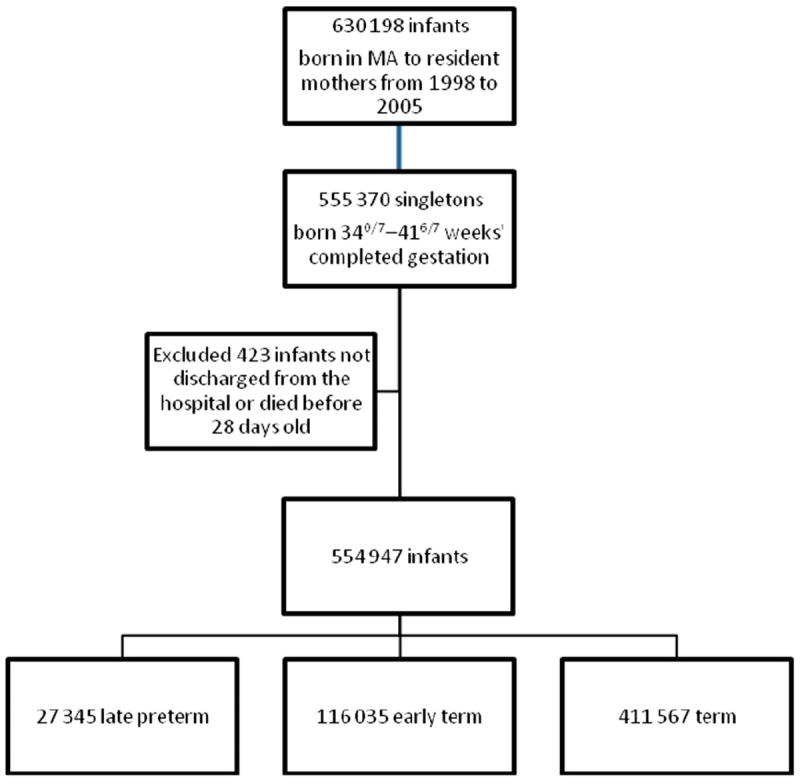
Flow diagram of study selection process revealing inclusion and exclusion criteria.
TABLE 1.
Distribution of Infant and Maternal Characteristics of Study Population at Birth by Late Preterm, Early Term, and Term Birth, MA, 1998–2008
| Distribution of Study Population at Birth, %
|
|||
|---|---|---|---|
| Late Preterm (N = 27 345) | Early Term (N = 116 035) | Term (N = 411 567) | |
| Infant characteristics | |||
| Infant gender | |||
| Boy | 54.2 | 52.8 | 50.6 |
| Girl | 45.8 | 47.2 | 49.4 |
| Congenital anomaliesa | |||
| Yes | 8.1 | 6.1 | 5.1 |
| No | 91.0 | 93.4 | 94.4 |
| Maternal demographics | |||
| Maternal age, y | |||
| <20 | 8.1 | 6.2 | 6.2 |
| 20–24 | 15.5 | 14.7 | 15 |
| 25–29 | 23.2 | 23.1 | 24.3 |
| 30–34 | 30.3 | 32.2 | 33.2 |
| 35–39 | 18.1 | 19.2 | 17.7 |
| 40–44 | 4.5 | 4.3 | 3.4 |
| 45+ | 0.2 | 0.1 | 0.1 |
| Maternal education | |||
| No HS diploma or GED | 13.1 | 10.8 | 9.9 |
| HS diploma or GED | 42.1 | 40.7 | 39.6 |
| Any post HS | 44.6 | 48.3 | 50.2 |
| Maternal race/ethnicity | |||
| White, non-Hispanic | 66.6 | 69.5 | 73.4 |
| African American, non-Hispanic | 10.6 | 8.3 | 6.8 |
| Hispanic | 14.1 | 12.8 | 11.6 |
| Asian/Pacific Islander | 6.4 | 7.3 | 6.0 |
| Other | 2.3 | 2.0 | 2.0 |
| Delivery payer source | |||
| Private | 61.2 | 65.7 | 68 |
| Public | 37.7 | 33.2 | 30.9 |
| Other | 0.6 | 0.6 | 0.6 |
| Mother’s language preference | |||
| English | 88.6 | 88.6 | 89.4 |
| Not English | 11.4 | 11.4 | 10.6 |
| Maternal nativity | |||
| US-born | 74.1 | 73.1 | 74.8 |
| Non US-born | 25.9 | 26.9 | 25.2 |
| Reproductive history | |||
| Parity | |||
| 1 | 48.7 | 40.3 | 44.6 |
| 2 | 29.6 | 35.9 | 34.6 |
| 3 | 13.2 | 15.9 | 14.2 |
| 4 or more | 8.5 | 7.9 | 6.6 |
| Pregnancy complications | |||
| Yes | 59.9 | 47.0 | 39.2 |
| No | 39.8 | 52.7 | 60.6 |
| Labor and delivery complications | |||
| Yes | 58.1 | 41.1 | 41.1 |
| No | 41.6 | 58.6 | 58.7 |
| APNCU index | |||
| Adequate/adequate plus | 69.9 | 76.1 | 78.6 |
| Intermediate | 22.5 | 17.8 | 15.5 |
| Inadequate | 4.0 | 3.0 | 2.8 |
| Unknown | 3.1 | 2.8 | 3.0 |
| No prenatal care | 0.6 | 0.3 | 0.1 |
APNCU, Adequacy of Prenatal Care Utilization; GED, General Educational Development high school equivalency examination; HS, high school. Column percentages may not add up to 100% due to missing values.
Congenital anomalies included anencephaly, spina bifida, hydrocephalus, microcephalus, other central nervous system anomaly, heart malformations, other circulatory/respiratory disorder, rectal atresia/stenosis, tracheo-esophageal fistula, omphalocele, gastroschisis, other gastrointestinal anomalies, malformed genitalia, renal agenesis, other urogenital anomalies, cleft lip/pal ate, polydactyly, syndactyly, adactyly, clubfoot, diaphragmatic hernia, other musculoskeletal anomaly, Down syndrome, other chromosomal anomaly, and other diagnosis without category.
The prevalence of enrollment in the EI program increased with each decreasing week of gestation before 41 weeks, ranging from 11.1% at 41 weeks’ gestation to 34.9% at 34 weeks’ gestation (Fig 2). By gestational age grouping, the prevalence of enrollment was 23.5% among late preterm infants, 14.9% among early term infants, and 11.9% among term infants (data not shown). However, the absolute number of term births enrolled (n = 49 084) was 7 times higher than late preterm births (n = 6423) and nearly 3 times higher than early term births (n = 17 240).
FIGURE 2.
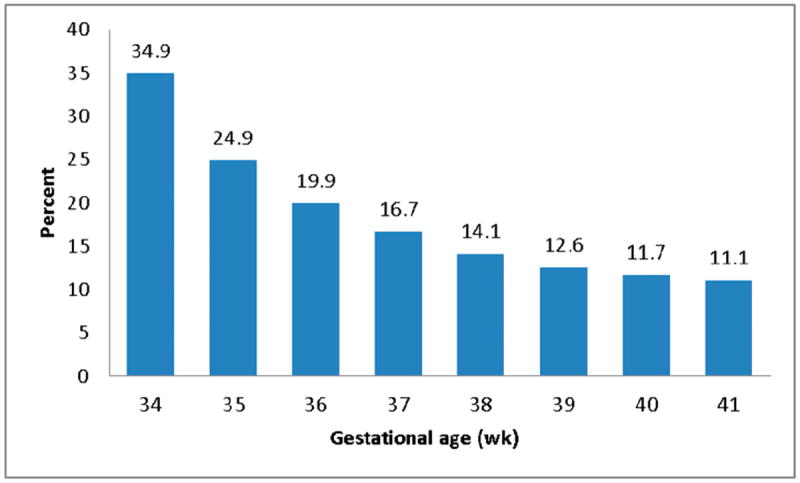
Percent of children enrolled in EI program services by gestational age, MA, 1998–2008.
For most characteristics, the prevalence of enrollment in EI program services by gestational age group was highest for late preterm births, followed by early term and term births (Table 2). Late preterm infants born to mothers with less than a high school education had the highest prevalence of enrollment for all gestational age groups (29.9%), whereas term infants who were girls or with Asian and Pacific Islander mothers had the lowest prevalence.
TABLE 2.
Prevalence of Enrollment in EI Program Services by 3 Years Old by Selected Characteristics, MA, 1998–2008
| Prevalence of Enrollment in EI Program Services, %
|
Prevalence Risk Ratioa
|
|||||
|---|---|---|---|---|---|---|
| Late Preterm (N = 6423) | Early Term (N = 17 240) | Term (N = 49 084) | Late Preterm (N = 6423) | Early Term (N = 17 240) | Term (N = 49 084) | |
| Infant characteristics | ||||||
| Infant gender | ||||||
| Boy | 27.0 | 18.3 | 15.5 | 1.39 (1.32–1.45) | 1.67 (1.61–1.72) | 1.85 (1.82–1.89) |
| Girl | 19.3 | 11.0 | 8.3 | Reference | Reference | Reference |
| Congenital anomaliesb | ||||||
| Yes | 35.6 | 23.2 | 17.0 | 1.55 (1.46–1.65) | 1.49 (1.43–1.56) | 1.31 (1.27–1.35) |
| No | 22.3 | 14.3 | 11.6 | Reference | Reference | Reference |
| Maternal demographics | ||||||
| Maternal age, y | ||||||
| <20 | 27.1 | 19.5 | 16.1 | 0.91 (0.83–1.00) | 1.04 (0.97–1.11) | 1.02 (0.98–1.06) |
| 20–24 | 25.8 | 17.1 | 13.4 | 0.99 (0.92–1.07) | 1.04 (0.99–1.09) | 0.98 (0.96–1.01) |
| 25–29 | 22.4 | 13.7 | 11.0 | Reference | Reference | Reference |
| 30–34 | 21.5 | 13.6 | 11.1 | 1.02 (0.96–1.09) | 1.07 (1.03–1.12) | 1.10 (1.07–1.12) |
| 35–39 | 24.2 | 14.9 | 11.8 | 1.16 (1.08–1.24) | 1.18 (1.13–1.24) | 1.16 (1.13–1.20) |
| 40–44 | 24.9 | 15.4 | 13.1 | 1.20 (1.08–1.34) | 1.21 (1.13–1.30) | 1.29 (1.23–1.35) |
| 45+ | 29.2 | 15.0 | 14.3 | 1.51 (0.98–2.35) | 1.24 (0.79–1.93) | 1.47 (1.07–2.00) |
| Maternal education | ||||||
| No HS or GED | 29.9 | 21.1 | 17.7 | 1.20 (1.12–1.28) | 1.24 (1.19–1.30) | 1.29 (1.25–1.32) |
| HS or GED | 24.1 | 15.3 | 12.4 | Reference | Reference | Reference |
| Any post HS | 21.1 | 13.1 | 10.4 | 0.96 (0.91–1.01) | 0.97 (0.94–1.01) | 0.94 (0.92–0.96) |
| Maternal race/ethnicity | ||||||
| Non-Hispanic white | 23.4 | 14.5 | 11.4 | Reference | Reference | Reference |
| African American, non-Hispanic | 22.9 | 15.3 | 13.1 | 0.83 (0.77–0.89) | 0.89 (0.84–0.94) | 1.00 (0.97–1.04) |
| Hispanic | 26.9 | 19.3 | 16.2 | 1.09 (1.01–1.17) | 1.21 (1.16–1.27) | 1.28 (1.24–1.32) |
| Asian/Pacific Islander | 17.6 | 9.9 | 8.3 | 0.88 (0.79–0.99) | 0.81 (0.75–0.87) | 0.87 (0.83–0.91) |
| Other | 23.7 | 13.9 | 11.6 | 1.00 (0.86–1.15) | 0.92 (0.83–1.03) | 0.97 (0.91–1.03) |
| Delivery payer source | ||||||
| Private | 20.8 | 12.9 | 10.4 | Reference | Reference | Reference |
| Public | 28.0 | 18.9 | 15.4 | 1.34 (1.26–1.42) | 1.41 (1.36–1.47) | 1.41 (1.38–1.44) |
| Other | 15.5 | 9.5 | 8.3 | 0.75 (0.52–1.08) | 0.73 (0.58–0.92) | 0.83 (0.73–0.95) |
| Mother’s language preference | ||||||
| English | 23.9 | 14.9 | 11.9 | Reference | Reference | Reference |
| Not English | 20.1 | 14.2 | 12.2 | 0.78 (0.71–0.86) | 0.86 (0.82–0.92) | 0.89 (0.86–0.93) |
| Maternal nativity | ||||||
| US-born | 24.4 | 15.5 | 12.2 | Reference | Reference | Reference |
| Non US-born | 20.8 | 13.2 | 11.1 | 0.85 (0.79–0.91) | 0.79 (0.75–0.83) | 0.78 (0.76–0.81) |
| Reproductive history | ||||||
| Parity | ||||||
| 1 | 22.8 | 13.9 | 11.1 | Reference | Reference | Reference |
| 2 | 23.0 | 15.0 | 12.2 | 0.99 (0.94–1.04) | 1.08 (1.04–1.11) | 1.10 (1.08–1.12) |
| 3 | 24.6 | 15.7 | 12.8 | 1.02 (0.95–1.09) | 1.07 (1.02–1.11) | 1.08 (1.05–1.11) |
| 4 or more | 27.4 | 17.5 | 14.3 | 1.04 (0.96–1.13) | 1.09 (1.03–1.15) | 1.10 (1.06–1.13) |
GED, General Educational Development high school equivalency examination; HS, high school.
Adjusted for all variables included in the table.
Congenital anomalies included anencephaly, spina bifida, hydrocephalus, microcephalus, other central nervous system anomaly, heart malformations, other circulatory/respiratory disorder, rectal atresia/stenosis, tracheo-esophageal fistula, omphalocele, gastroschisis, other gastrointestinal anomalies, malformed genitalia, renal agenesis, other urogenital anomalies, cleft lip/pal ate, polydactyly, syndactyly, adactyly, clubfoot, diaphragmatic hernia, other musculoskeletal anomaly, Down syndrome, other chromosomal anomaly, and other diagnosis without category.
Adjusted analyses of factors associated with EI program service enrollment for each gestational age group are shown in Table 2. For all gestational age groups, the strongest predictors of enrollment (aRR ≥1.20) were male gender and presence of congenital anomalies at birth as well as infants of mothers who were ≥40 years old, not high school graduates, and publicly insured.
Earlier gestational age was associated with an increased risk of EI enrollment after adjusting for gender, congenital anomalies, maternal age, education, race/ethnicity, delivery payer source, preferred language, nativity, and parity (Table 3). A linear trend of increasing risk was observed with nonoverlapping CIs for each gestational week from41 to 34 weeks. Also, when analyses were adjusted for pregnancy complications, labor and delivery complications, and prenatal care use, similar magnitudes of risk were observed.
TABLE 3.
Association Between Gestational Age and EI Program Enrollment, MA, 1998–2008
| Gestational Age, wk | No. of Children Enrolled | Prevalence of Enrollment in EI Program | Crude Risk Ratio | aRRa (95% CI) |
|---|---|---|---|---|
| 34 | 4070 | 34.9 | 2.97 | 2.48 (2.39–2.57) |
| 35 | 7487 | 24.9 | 2.13 | 1.93 (1.85–2.01) |
| 36 | 15 788 | 19.9 | 1.69 | 1.57 (1.52–1.62) |
| 37 | 33 821 | 16.7 | 1.42 | 1.36 (1.32–1.39) |
| 38 | 82 214 | 14.1 | 1.20 | 1.16 (1.14–1.19) |
| 39 | 154 179 | 12.6 | 1.08 | 1.06 (1.04–1.08) |
| 40 | 163 077 | 11.7 | Reference | Reference |
| 41 | 94 311 | 11.1 | 0.95 | 0.95 (0.93–0.97) |
Adjusted for gender, congenital anomalies, maternal age, education, race/ethnicity, delivery payer source, preferred language, nativity, and parity.
EI Program Service Usage
The most frequently used services among EI program enrollees were developmental specialists, occupational therapists, and speech and language pathologists (Table 4). We did not observe major differences in the types of services children received once enrolled among the different gestational age groups. Some minor differences include higher proportions of children using speech language pathologists if born early term (69.3%) and term (70.3%) compared with those born late preterm (62.9%), and higher proportions of children using physical therapy and nursing services if born late preterm (56.4% and 37.8%) compared with early term (48.8% and 33.1%) and term (47.8% and 31.8%) counterparts. On average, each child used 4 program services regardless of gestational age group. Calendar year data revealed that infants born late preterm were enrolled in EI program services slightly longer (~1 month) than early term and term counterparts.
TABLE 4.
EI Program Service Type, Frequency of Service, and Number of Years Enrolled by Gestational Age Group, MA, 1998–2008
| Late Preterm
|
Early Term
|
Term
|
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total infants | 6423 | 100 | 17 240 | 100 | 49 084 | 100 |
| Type of service useda | ||||||
| Developmental specialist | 5590 | 87.0 | 15 193 | 88.1 | 42 858 | 87.3 |
| Occupational therapist | 4211 | 65.6 | 10 791 | 62.6 | 30 463 | 62.1 |
| Speech language pathologist | 4037 | 62.9 | 11 945 | 69.3 | 34 501 | 70.3 |
| Social worker | 3796 | 59.1 | 10 022 | 58.1 | 28 481 | 58.0 |
| Physical therapist | 3621 | 56.4 | 8416 | 48.8 | 23 466 | 47.8 |
| Nurse | 2431 | 37.8 | 5698 | 33.1 | 15 621 | 31.8 |
| Psychologist counselor | 1828 | 28.5 | 4851 | 28.1 | 13 440 | 27.4 |
| Specialty provider | 295 | 4.6 | 799 | 4.6 | 2199 | 4.5 |
| Mean | SD | Mean | SD | Mean | SD | |
| Number of EI services used | 4.1 | 1.4 | 4.0 | 1.4 | 3.9 | 1.3 |
| Number of calendar yearsb enrolled in EI services | 2.2 | 0.9 | 2.0 | 0.8 | 2.0 | 0.8 |
Does not total to 100% because each a child may have used more than 1 service.
Calendar years maybe greater than 3. For example, a child who was born in October 1998 may still be enrolled in March 2002. The child is 3 years old but has been enrolled for 4 calendar years.
DISCUSSION
Our study revealed that earlier gestational age is associated with increased prevalence of EI program service enrollment among singleton, neonatal survivors born 340/7 through 416/7 completed weeks’ gestation. Enrollment increased with each week of gestation before 41 completed weeks with approximately one-third of children born at 34 completed weeks’ gestation and one-fourth of children born at 35 completed weeks’ gestation enrolled in the MA EI program. Male children and those with mothers who were ≥40 years old, not high school graduates, and publicly insured had the highest prevalence of enrollment; Asian children had the lowest compared with other race-ethnicity groups. Developmental specialists, occupational therapists, and speech and language pathologists were the most frequently used EI program services regardless of gestational age group.
Our findings are consistent with 2 other studies examining developmental delay and disability by using EI program services data from less educated and more racially and ethnically diverse populations. In 1 study, a 1996–1997 Florida birth cohort was used,14 and in the other, a 1999–2001 New York City birth cohort was used.26 In the Florida study, infants born late preterm had a higher risk of participation in EI than infants born at term (aRR, 1.36 [95% CI, 1.29–1.43]). We also observed a decline in prevalence of EI enrollment as gestational age increased, with a less apparent decline after 39 weeks’ gestational age. In the New York City study, the authors reported 21% of infants born late preterm and 14% of infants born early term were referred for EI program services, compared with 12% of infants born at term. These studies and ours contribute to growing evidence of increased risk for long-term adverse developmental outcomes such as behavioral problems, cerebral palsy, poor academic performance, and special education needs among children born late preterm and early term, compared with their term counterparts.12-19 However, most studies had not differentiated early term from term infants.
Although late preterm infants may not have the same degree of risk for neurologic insult as their more preterm counterparts, their developing brains are still only 65% the weight of full-term brains and have not completed important glial and white matter interconnections. 27-29 And among early term infants, neuronal maturation continues. Both late preterm and early term infants are vulnerable to predelivery (eg, hypoxia, circulatory perturbations) and postdelivery events (eg, respiratory distress, apnea, hypoglycemia, and hyperbilirubinemia) that might adversely affect neurodevelopment.27,28,30-33
Understanding the magnitude of the effect of gestational age at birth on developmental delay on the full spectrum of preterm infants has implications for EI program planning, resource allocation, and physician practices for monitoring neurodevelopment. Early educational intervention services can have long-lasting favorable effects on cognitive and social outcomes, and children born late preterm may derive the most benefit. The Infant Health and Development Program34 revealed that preterm infants weighing 2001 to 2500 g at birth (corresponding mostly to infants born late preterm) and exposed to a comprehensive EI program had improved behavioral, reading, and mathematics outcomes when measured at 3, 8, and 16 years compared with those who had received follow-up services only. Infants born at lower birth weights, or very preterm, did not derive a measurable benefit from EI services. Also, mean expenditures for EI services for children aged 0 through 3 years decline with increasing gestational age: <28 weeks: $7182; 28 to 30 weeks: $5254; 31 to 33 weeks: $2654; 34 to 36 weeks: $1321; and 37 to 39 weeks: $69717; however, more infants born in the later gestational age groups results in larger absolute numbers.
Our study has several strengths including population-based data derived from a longitudinally linked data system and a large sample size. Also, our study may be less prone to misclassification and overestimation of preterm births (ie, misclassifying term infants as late preterm) infants because we estimated gestational age by using a combination of LMP and clinical estimate.22,35,36 This reduction in bias may provide more accurate estimated relative risks compared with earlier studies that estimated gestational age without using clinical estimates.
Despite these important strengths, our study does have some limitations. First, our findings likely undercount the true number of MA children with a developmental delay or disability, because we could only observe those who enrolled in the EI program. However, our findings are consistent with previous work revealing lower EI participation among MA children born to non-English speaking and/or foreign-born mothers. 23 This population may be less likely to know what services are available and may not have the skills or resources needed to receive appropriate services.37 It could also be that parents whose primary language is not English may choose not to refer their child or follow through with the referral, perhaps owing to cultural beliefs and lack of perceived need. Additional strategies may be needed to engage these populations. Second, although we had a high linkage rate of birth certificates to hospital discharge and death certificate records, 17% of EI records could not be linked to birth certificate records. We could not determine if nonlinked EI data were biased in relation to the presence or absence of certain birth characteristics because of the unavailability of birth certificate and birth hospital discharge information on children enrolled in EI who were born out of state or were adopted. Third, like all other studies that rely on vital statistics and administrative data, we were constrained to routinely collected data, and therefore, unmeasured differences may have led to residual confounding. Finally, the generalizability of this study to other states with more or less aggressive EI recruitment and eligibility and higher rates of late preterm and early term birth is unknown.
CONCLUSIONS
MA infants born late preterm and early term have higher prevalence of EI program services enrollment than infants born at term, and risk increases with each week of gestation before 41 weeks. Because enrollment in EI program services is a good proxy for concern of developmental delays, this study suggests that gestational age at birth may have independent long-term developmental impacts. From the pediatrician’s perspective, infants born late preterm and early term may benefit from more frequent monitoring for developmental delays or disabilities than the general population. Delivering care in the context of a family-centered medical home may facilitate early identification and appropriate management of developmental issues. From obstetrician’s perspective, caregivers should be counseled about potential developmental delay or disability. Because of increased risk of morbidity and mortality of infants born before 39 weeks’ gestation, the American Congress of Obstetricians and Gynecologists38,39 and the March of Dimes40 “Healthy Babies Are Worth the Wait” education campaign advise against nonmedically indicated deliveries before 39 weeks’ gestational age. Given increasing rates of late preterm and early term infants, this study has important implications for MA EI program planning, and for informing developmental screening decisions and anticipating developmental service delivery needs.
WHAT’S KNOWN ON THIS SUBJECT
Infants born late preterm and early term are at increased risk for short-term morbidities compared with term infants. Longer-term morbidity and disability in this group of infants is not well established.
WHAT THIS STUDY ADDS
Massachusetts infants born late preterm and early term are at increased risk of early intervention program enrollment than term infants. Boys and children whose mothers were less educated, older, and with public insurance were most affected.
Acknowledgments
FUNDING: Supported by the Centers for Disease Control and Prevention contract 200-2009-31671, “Pregnancy to Early Life Longitudinal Data System (PELL).”
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- 95% CI
95% confidence interval
- aRR
adjusted risk ratio
- EI
early intervention
- LMP
last menstrual period
- MA
Massachusetts
- PELL
Pregnancy to Early Life Longitudinal Data System
Footnotes
Dr Shapiro-Mendoza conceptualized and designed the study; interpreted the findings; drafted, revised, and wrote the final version of the article; and oversaw the project in general. Drs Kotelchuck and Barfield participated in planning the study, contributed to data interpretation, critically reviewed the multiple article drafts, and approved the final article as submitted. Ms Davin participated in planning the study, had full access to all of the data, performed the statistical programming and carried out the initial analyses, contributed to data interpretation, critically reviewed the multiple article drafts, and approved the final article as submitted. Drs Diop and Manning participated in planning the study, had full access to all of the data, contributed to data interpretation, critically reviewed the multiple article drafts, and approved the final article as submitted. Mr Silver participated in planning the study, had full access to all of the data, performed the statistical programming and analysis, contributed to data interpretation, critically reviewed the multiple article drafts, and approved the final article as submitted.
This work was presented in abstract form at the 23rd Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER); June 22–23, 2010; Seattle, WA.
FINANCIAL DISCLOSURE: Dr Kotelchuck was the original PI and funded by the Centers for Disease Control and Prevention contract 200-2009-31671, “Pregnancy to Early Life Longitudinal Data System (PELL)”; the other authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Behrman RE, Butler AS Institute of Medicine; US Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30(1):8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114(2):372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 4.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151(5):450–456. doi: 10.1016/j.jpeds.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics. 2008;121(2) doi: 10.1542/peds.2006-3629. Available at: www.pediatrics.org/cgi/content/full/121/2/e223. [DOI] [PubMed] [Google Scholar]
- 6.Escobar GJ, McCormick MC, Zupancic JA, et al. Unstudied infants: outcomes of moderately premature infants in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2006;91(4):F238–F244. doi: 10.1136/adc.2005.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90(2):125–131. doi: 10.1136/adc.2003.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JA, Kirmeyer S, Osterman M, Shepherd RA. Born a bit too early: recent trends in late preterm births. NCHS Data Brief. 2009;24:1–8. [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Vital Statistics. [February 11, 2013];Natality public use data 2007–2010 on CDC WONDER online database. 2012 Dec; Available at: http://wonder.cdc.gov/natalitycurrent.html.
- 10.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117(6):1279–1287. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Kramer MS. Variations in mortality and morbidity by gestational age among infants born at term. J Pediatr. 2009;154(3):358–362. doi: 10.1016/j.jpeds.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126(6):1124–1131. doi: 10.1542/peds.2010-1536. [DOI] [PubMed] [Google Scholar]
- 13.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154(2):169–176. doi: 10.1016/j.jpeds.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123(4) doi: 10.1542/peds.2008-1405. Available at: www.pediatrics.org/cgi/content/full/123/4/e622. [DOI] [PubMed] [Google Scholar]
- 15.Gurka MJ, LoCasale-Crouch J, Blackman JA. Long-term cognition, achievement, socioemotional, and behavioral development of healthy late-preterm infants. Arch Pediatr Adolesc Med. 2010;164(6):525–532. doi: 10.1001/archpediatrics.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL. School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. J Pediatr. 2008;153(1):25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3) doi: 10.1542/peds.2009-3598. Available at: www.pediatrics.org/cgi/content/full/127/3/e622. [DOI] [PubMed] [Google Scholar]
- 18.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130(2) doi: 10.1542/peds.2011-2157. Available at: www.pediatrics.org/cgi/content/full/130/2/e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkind HS, Slopen ME, Pfeiffer MR, McVeigh KH. School-age outcomes of late preterm infants in New York City. Am J Obstet Gynecol. 2012;206(3):222–226. doi: 10.1016/j.ajog.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Engle WA, Tomashek KM, Wallman C Committee on Fetus and Newborn, American Academy of Pediatrics. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 21.Sunderam S, Kissin DM, Flowers L, et al. Centers for Disease Control and Prevention (CDC) Assisted reproductive technology surveillance—United States, 2009. MMWR Surveill Summ. 2012;61(7):1–23. [PubMed] [Google Scholar]
- 22.Qin C, Dietz PM, England LJ, Martin JA, Callaghan WM. Effects of different dataediting methods on trends in race-specific preterm delivery rates, United States, 1990-2002. Paediatr Perinat Epidemiol. 2007;21(suppl 2):41–49. doi: 10.1111/j.1365-3016.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 23.Clements KM, Barfield WD, Kotelchuck M, Wilber N. Maternal socio-economic and race/ethnic characteristics associated with early intervention participation. Matern Child Health J. 2008;12(6):708–717. doi: 10.1007/s10995-007-0291-3. [DOI] [PubMed] [Google Scholar]
- 24.Kotelchuck M. Adequacy of prenatal care utilization. Epidemiology. 1997;8(5):602–604. [PubMed] [Google Scholar]
- 25.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Curry AE, Pfeiffer MR, Slopen ME, McVeigh KH. Rates of early intervention referral and significant developmental delay, by birthweight and gestational age. Matern Child Health J. 2012;16(5):989–996. doi: 10.1007/s10995-011-0820-y. [DOI] [PubMed] [Google Scholar]
- 27.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 28.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Hüppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 30.Adamkin DH Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127(3):575–579. doi: 10.1542/peds.2010-3851. [DOI] [PubMed] [Google Scholar]
- 31.Jain L. Morbidity and mortality in late-preterm infants: more than just transient tachypnea! J Pediatr. 2007;151(5):445–446. doi: 10.1016/j.jpeds.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamkin DH. Late preterm infants: severe hyperbilirubinemia and postnatal glucose homeostasis. J Perinatol. 2009;29(suppl 2):S12–S17. doi: 10.1038/jp.2009.41. [DOI] [PubMed] [Google Scholar]
- 33.Canadian Paediatric Society. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation)—Summary. Paediatr Child Health. 2007;12(5):401–407. doi: 10.1093/pch/12.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–780. doi: 10.1542/peds.2005-1316. [DOI] [PubMed] [Google Scholar]
- 35.Mustafa G, David RJ. Comparative accuracy of clinical estimate versus menstrual gestational age in computerized birth certificates. Public Health Rep. 2001;116(1):15–21. doi: 10.1093/phr/116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander GR, Tompkins ME, Petersen DJ, Hulsey TC, Mor J. Discordance between LMP-based and clinically estimated gestational age: implications for research, programs, and policy. Public Health Rep. 1995;110(4):395–402. [PMC free article] [PubMed] [Google Scholar]
- 37.Yu SM, Nyman RM, Kogan MD, Huang ZJ, Schwalberg RH. Parent’s language of interview and access to care for children with special health care needs. Ambul Pediatr. 2004;4(2):181–187. doi: 10.1367/A03-094R.1. [DOI] [PubMed] [Google Scholar]
- 38.Committee on Obstetric Practice. ACOG committee opinion No. 404 April 2008. Latepreterm infants. Obstet Gynecol. 2008;111(4):1029–1032. doi: 10.1097/AOG.0b013e31817327d0. [DOI] [PubMed] [Google Scholar]
- 39.ACOG Committee on Practice Bulletins – Obstetrics. ACOG Practice Bulletin No. 107: Induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 40.Main E, Oshiro B, Chagolla B, Bingham D, Dang-Kilduff L, Kowalewski L. California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care. Developed under contract #08-85012 with the California Department of Public Health; Maternal, Child, and Adolescent Health Division; [February 4, 2013]. Elimination of nonmedically indicated (elective) deliveries before 39 weeks’ gestational age. Published July 2010. Available at: www.cdph.ca.gov/programs/mcah/Documents/MCAH-EliminationOfNon-MedicallyIndicatedDeliveries.pdf. [Google Scholar]


