Abstract
Gold(III) compounds continue to be explored for their potential utility as anticancer agents. A recognized limitation is the reactivity of gold(III), which is typically reduced to the more labile gold(I) state under physiological conditions. The use of porphyrins can overcome this problem. However, to date the stabilization provided by the use a strongly chelating porphyrin is offset by the poor solubility of the resulting complex in aqueous media. In this work, we describe the synthesis and in vitro anti-cancer activity of a gold(III)porphyrin complex with relatively good aqueous solubility. As judged from standard antiproliferation assays, this complex displays an IC50 of 9 μM for the A2780 human ovarian cancer cell line. This is a higher level of potency than displayed by two related control systems.
Keywords: gold, porphyrin, anti-cancer, solubility
INTRODUCTION
As cancers continue to develop resistance to traditional chemotherapies, such as cisplatin and its derivatives, there is a corresponding need to develop novel drugs that operate by different mechanisms of action [1]. One approach involves testing other metal complexes. In this context, significant effort has been devoted towards use of gold(III) species for the treatment of cancer, as well as many other illnesses [2]. However, in most ligand environments gold(III) is unstable under physiological conditions, and is reduced quickly to the more labile gold(I). A number of stabilizing ligand have been explored in order to avoid reduction and retain the presumably more active Au(III) oxidation state, a number of stabilizing ligand have been explored [3]. A particular attractive complexing agent would be a porphyrin due to its known ability to stabilize otherwise labile metal centers, as well as the recognized cancer targeting capabilities demonstrated by many porphyrin derivatives [4]. One major drawback of most easy-to-prepare porphyrins is their poor solubility in aqueous media. Various synthetic strategies have been utilized to overcome this latter deficiency, including the use of methylated pyridine-, sulfate-, and polysaccharide-modified porphyrins. However, these modifications affect the biological properties of the core porphyrin species, including if or how it triggers cell death [5]. In this study, we report the synthesis of a water soluble, hydroxyl modified tetraphenyl gold porphyrin and show that it has antiproliferative activity against the human ovarian cancer cell line, A2780.
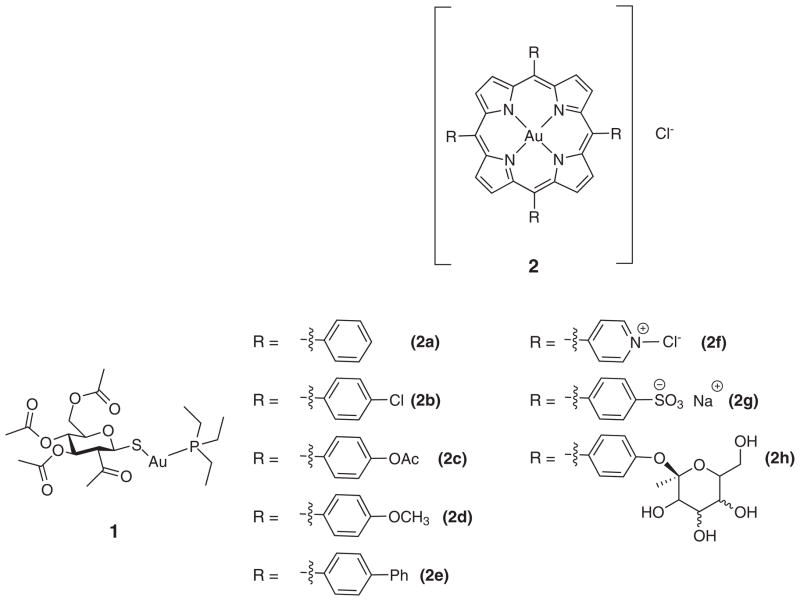
Gold compounds have been used since 2500 B.C. for the treatment of smallpox and measles [6]. Gold complexes were incorporated into the modern pharmacopeia in the 1920’s by Koch when he used them for the treatment of tuberculosis, with an eventual shift to gold(I) thiolates to mediate toxicity [2a and 2b]. Since then, gold-containing agents have been extensively studied for the treatment of rheumatoid arthritis, and recently HIV [2h–2j]. In 1965 Lorber and coworkers reported the anti-cancer activity of auranofin (1) against strains of cancer derived from the HeLa cell line Fig. 1 [7]. Berners–Price and Sadler studied the ligand exchange of gold(I) and gold(III) complexes in 1996 [8]. In that same year, Kelland and co-workers reported in vivo studies of 2-[(dimethylamino) methyl] phenylgold(III) against a panel of human cancer cell lines including colon, breast, ovarian and bladder. Unfortunately, this complex was characterized by limited activity, an effect ascribed to poor aqueous solubility [9]. Since that time, a number of groups have developed a wide range of gold complexes and have reported varying degrees of efficacy against a variety of cancer cell lines, both in vivo and in vitro [2, 10].
Fig. 1.
Aurofin 1 [7] and several known gold(III) porphyrin systems [3, 4]
Many researchers have been attracted to gold(III) porphyrins as possible drugs due to the high stability and relative ease of synthesis typical of porphyrins [3]. A commonly studied system is meso-tetraphenylporphyin gold chloride (2a) [3, 4]. Among other benefits complex 2a binds non-covalently to human serum albumin (HSA) [4n]. Many metal-based drugs, including oxaliplaten and auranofin, bind strongly with plasma proteins, predominately HAS, which makes gold(III) porphyrins further attractive in the context of pharmaceutical lead development.
Compound 2a has shown significant cytotoxicity against numerous cell lines, including KB-3-1 and the multi-drug resistant variant, KB-V1 [4i]. The effectiveness against KB-V1 was taken as evidence that the P-glycoproteins, proteins that remove foreign substances including drugs from the cell, have little effect on 2a. These studies also revealed a ten-fold increase in the cytotoxicity towards tumor cells as compared to healthy cells. Additionally, it is worth noting that the zinc derivative (ZnTPP) proved at least 100 fold less cytotoxic than the corresponding gold porphyrin, 2a [4]. In vivo studies involving the human ovarian A2780 and A2780cis (cisplatin resistant) cell line-derived xenografts in nude mice revealed severely retarded tumor growth, and higher concentration of apoptotic cells [4o]. Similar results were seen with 2a in the case of other studies, as noted in several recent reviews [4a, 4b and 5].
Although 2a displays many attributes that make it attractive as a potential anti-cancer agent, it is severely limited by its poor solubility in aqueous media. To address this deficiency, a number of other gold porphyrins (e.g. 2b–2h) have been prepared [3, 4]. Among the most widely studied of these are those containing methylated pyridine-(2f), phenyl sulfate-(2g), and polysaccharide subunits. While showing greatly increased solubility, as a general rule, the cytotoxicity of these compounds is generally diminished relative to 2a [4n]. In fact, the cytotoxicity of the charged species is all but eliminated, a finding attributed to the decreased lipopholicity of the complexes in question [4n]. On the other hand, the polysaccharide functionalized system (2h) displays attractive anti-angiogenic properties [4n]. This leads us to suggest that new gold(III) systems with good aqueous solubility may show significant anticancer activity. We report here one such system, the gold(III) porphyrin complex 6 derived from the polyhydroxylated porphyrin 3.
EXPERIMENTAL
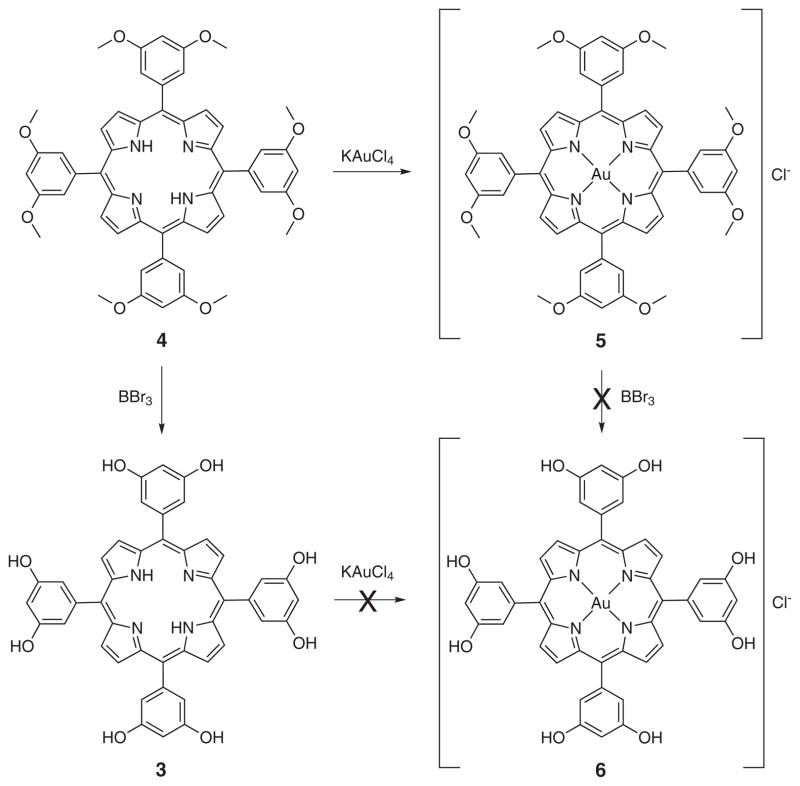
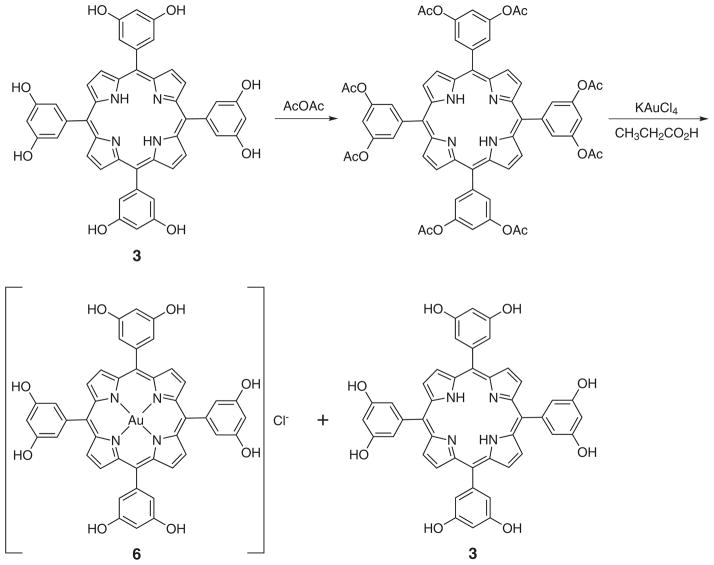
Octahydroxy tetraphenylporphyrin (3) was synthesized from octamethoxytetraphenyl porphyrin (4) by subjecting the methoxy group to boron tribromide-mediated cleavage [11]. Carrying out this bond scission using a pre-formed gold(III) octamethoxy tetraphenylporphyrin resulted in demetalation. Equally unsuccessful was a strategy wherein the cleavage step was carried out prior to metalation and then metalation was attempted using with potassium tetrachloride aurate. Under these conditions, only starting material was recovered (Scheme 1). However, the desired gold(III) complex could be obtained by protecting the hydroxyl groups by treating with acetic anhydride prior to metalation. This protection was found to proceed in near quantitative yield. Metalation, concomitant with deprotection, was then accomplished by heating the porphyrin with potassium tetrachloroaurate at reflux in propionic acid [12]. The desired, deprotected product was isolated in 20% yields, along with the octahydroxy freebase porphyrin (3) (Scheme 2). No evidence of the corresponding acetoxy protected metalated porphyrin was observed after the reaction.
Scheme 1.
Attempted synthesis of octahydroxy gold(III) tetraphenylporphyrin 6
Scheme 2.
Synthesis of Au(III) octahydroxytetraphenylporphyrin. Also shown is the demetalated freebase porphyrin (3) obtained as a side product during the reaction sequence
Gold porphyrin complexes were characterized by high-resolution mass spectrometretry, in addition to UV/Vis and NMR spectroscopy. Compared to the metal-free form 3, the Soret band of the gold complex 6 is hypsochromically shifted by 6 nm compared in ethyl acetate solution. This shift has been explained by Jamin and Iwamoto in terms of a stabilization of the HOMO orbitals as the result of strong Au-N interactions [13]. The 1H NMR spectrum of the Au(III) complex 6 recorded in CD3OD showed all the expected CH signals. However, the hydroxyl protons proved difficult to identify; presumably, this reflects –OH to –OD exchange. Analysis by HPLC shows one peak with a slight shoulder. This peak is thought to be due to ligand exchange with acetate under the conditions of HPLC analysis.
In vitro cytotoxicity studies
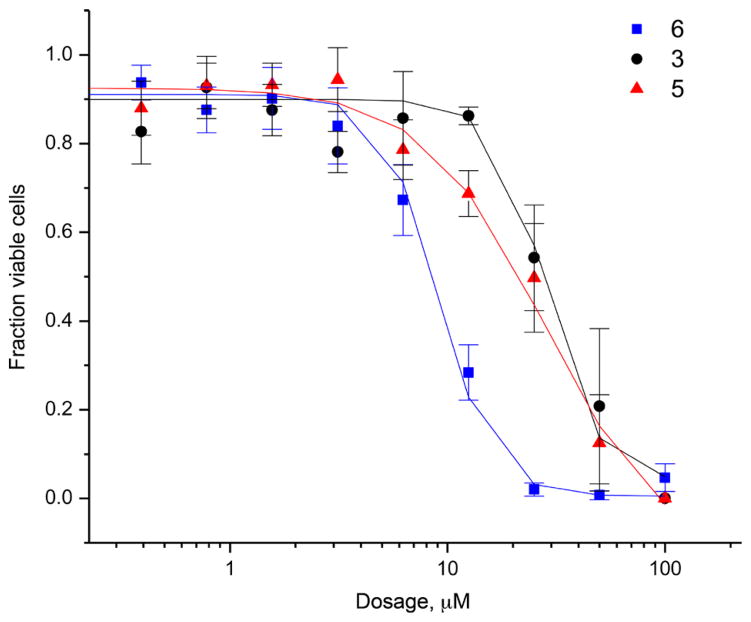
The cytotoxicity of octahydroxy gold(III) porphyrin (6), octamethoxy gold(III) porphyrin (5) and the metal-free octahydroxy porphyrin (3) were evaluated using the human ovarian cancer cell line A2780. Cells were incubated with varying dosages at 37 °C for five days before treatment with thianol blue for 4 h.
The above cell studies revealed an IC50 value of 9 μM in the A2780 cell line for the octahydroxy gold(III) porphyrin (6). Under analogous conditions, IC50 values of 29 μM and 28 μM were found for the octamethoxy (5) and freebase (3) derivatives respectively. These results are shown in Fig. 2, which includes the error bars reflecting reproducibility (standard deviation) across at least three separate measurements.
Fig. 2.
A2780 cells dosed with either compounds 3, 5, and 6 at 100 μM, followed by 50% serial dilutions. Antiproliferative activity was determined from MTT assays. Error bars are the standard deviations for measurements performed in triplicate
RESULTS AND DISCUSSION
The mechanism by which the gold porphyrins trigger cell death is not completely understood. It was initially thought to be similar to cisplatin in that it involves dative binding to DNA [3]. However, more recently it has been proposed that gold complexes interact with proteasomes and thioredoxin reductase [4a, 4d]. More generally, lipophilic planar cations have been proposed as mitochondria targeting agents [4n]. Gold(III) porphyrins bearing only neutral substituents (and thus bearing only a single cationic charge in the case of a poorly coordinating axial ligand) are thus of particular interest since they would allow the limits of this latter postulate to be tested in an operational sense within the context of anticancer drug discovery efforts. The system we report here, complex 6, was designed with such considerations in mind. It incorporates multiple hydroxyphenyl substitutents. Gratifyingly, it displays good solubility in aqueous media without the high net charge characteristic of many solubilized gold porphyrins. As part of the synthetic effort leading to 6, the free base form 3, as well as the gold complex of a protected version, 5, were obtained.
The A2780 cell line was used to test the antiproliferative activity of 6 and two related species. Standard MTT assays were used. These revealed an IC50 value of 9 μM for the octahydroxy gold(III) porphyrin (6). In contrast, IC50 values of 28 μM, and 29 μM were recorded for the octamethoxy (5) gold(III) complex and the freebase porphyrin (3), respectively. On this basis, we conclude that the complexation of a gold(III) center in a water solubilized porphyrin ligand can produce a system with more favorable cytotoxic features than the constituents from whence it is formally derived.
CONCLUSION
We have developed a gold(III) porphyrin with increased water solubility relative to tetraphenyl gold porphyrin (1a). This increase in solubility, combined with the fundamental complexation features inherent in the use of gold porphyrins, produces a species, complex 6, that shows promising anticancer activity as judged from initial cellular antiproliferative studies. Importantly, complex 6 retains the stability and ease of synthesis of previously reported porphyrin systems [4]. In marked contrast to many of the previous strategies used to increase the aqueous solubility of gold porphyrins through use of ionic substituents, no increase in charge is associated with the use of 6 as compared to gold(III) tetraphenylporphyin. No reduction in the anticancer activity nor, presumably, a change in the mode of action was thus expected. In fact, the use of neutral substituents produces an increased in vitro anticancer activity relative to the corresponding methylated gold porphyrin system B5. Moreover, the higher antiproliferative activity seen for the gold(III) porphyrin complex 6 relative to the corresponding freebase system 3, is consistent with the overall cytotoxicity being the result of both the gold center as well as the choice hydrophilic porphyrin. The fortuitous combination of both enhanced aqueous solubility and antiproliferative activity seen in 6 leads us to suggest that this class of complex may have a role to play in the treatment of neoplastic diseases. Currently, complex 6 is being tested against a number of cancer cells lines with the goal of commencing near-term in vivo efficacy and toxicity studies.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant CA 68682 to J.L.S.).
Footnotes
Dedicated to Professor Shunichi Fukuzumi on the occasion of his retirement
Supplementary material is available free of charge via the Internet at http://www.worldscinet.com/jpp/jpp.shtml.
References
- 1.(a) Stordal B, Pavlakis N, Davey R. Cancer Treat Rev. 2007;33:688. doi: 10.1016/j.ctrv.2007.07.013. [DOI] [PubMed] [Google Scholar]; (b) Siddik ZH. Oncogene. 2003;22:7165. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 2.(a) Huanzi Z, Yuantao N. Gold Bull. 2001;34:24. [Google Scholar]; (b) Orvig C, Abrams MJ. Chem Rev. 1999;99:2201. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]; (c) Koch R. Dtsch Med Wochenschr. 1890;16:756. [Google Scholar]; (d) Forestier JJ, Lab J. Clin Med. 1935;20:827. [Google Scholar]; (e) Sneader W. Drug Discovery: A History. John Wiley & Sons Ltd; Chichester, England: pp. 59–61. [Google Scholar]; (f) Shaw CF., III Chem Rev. 1999;99:2589. [Google Scholar]; (g) Abrams MJ, Murrer BA. Science. 1993;261:725. doi: 10.1126/science.8102010. [DOI] [PubMed] [Google Scholar]; (h) Brown CH, Smith WE. Chem Soc Rev. 1980;9:217. [Google Scholar]; (i) Fonteh PN, Keter FK, Meyer D. BioMetals. 2010;23:185. doi: 10.1007/s10534-010-9293-5. [DOI] [PubMed] [Google Scholar]; (j) Sun RW-Y, Yu W-Y, Sun H, Che C-M. Chem Bio Chem. 2004;5:1293. doi: 10.1002/cbic.200300773. [DOI] [PubMed] [Google Scholar]; (k) Che C-M, Sun W-Y. Chem Commun. 2011;47:9554. doi: 10.1039/c1cc10860c. [DOI] [PubMed] [Google Scholar]
- 3.(a) McKeage MJ, Maharaj L, Berners-Price BJ. Coord Chem Rev. 2002;232:127. [Google Scholar]; (b) Calamai P, Carotti S, Guerri A, Mazzei A, Messori L, Mini E, Orioli P, Speroni GP. Anticancer Drug Des. 1998;13:67. [PubMed] [Google Scholar]; (c) Fricker SP. Met Based Drugs. 1999;6:291. doi: 10.1155/MBD.1999.291. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ronconi L, Marzano C, Zanello P, Corsini M, Miolo G, Macca C, Trevisan A, Fregona D. J Med Chem. 2006;49:1648. doi: 10.1021/jm0509288. [DOI] [PubMed] [Google Scholar]; (e) Messori L, Marcon G, Orioli P. Bioinorg Chem Appl. 2003;1:177. doi: 10.1155/S1565363303000141. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Messori L, Abbate F, Marcon G, Orioli P, Fontani M, Mini E, Mazzei T, Carotti S, O’Connell T, Zanello P. J Med Chem. 2000;43:3541. doi: 10.1021/jm990492u. [DOI] [PubMed] [Google Scholar]; (g) Marcon G, Carotti S, Coronnello M, Messori L, Mini E, Orioli P, Mazzei T, Cinellu MA, Minghetti G. J Med Chem. 2002;45:1672. doi: 10.1021/jm010997w. [DOI] [PubMed] [Google Scholar]; (h) Bruni B, Guerri A, Marcon G, Messoriv L, Orioli P. Croat Chem Acta. 1999;72:221. [Google Scholar]; (i) Aldinucci D, Lorenzon D, Stefani L, Giovagnini L, Colombatti A, Fregona D. Anticancer Drugs. 2007;18:323. doi: 10.1097/CAD.0b013e328011ae98. [DOI] [PubMed] [Google Scholar]; (j) Casas JS, Castano MV, Cifuentes MC, García-Monteagudo JC, Sánchez A, Sordo J, Abram U. J Inorg Biochem. 2004;98:1009. doi: 10.1016/j.jinorgbio.2004.02.019. [DOI] [PubMed] [Google Scholar]; (k) Buckley RG, Elsome AM, Fricker SP, Henderson GR, Theobald BR, Parish RV, Howe BP, Kelland LR. J Med Chem. 1996;39:5208. doi: 10.1021/jm9601563. [DOI] [PubMed] [Google Scholar]; (l) Rigobello MP, Scutari G, Folda A, Bindoli A. Biochem Pharmacol. 2004;67:689. doi: 10.1016/j.bcp.2003.09.038. [DOI] [PubMed] [Google Scholar]; (m) Kim NH, Lee MY, Park SJ, Choi JS, Oh MK, Kim IS. Immunol. 2007;122:607. doi: 10.1111/j.1365-2567.2007.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Hirano T. Int Rev Immunol. 1998;16:249. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]; (o) Kisselev AF, Goldberg AL. Chem Biol. 2001;8:739. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]; (n) Goldberg AL. Science. 1995;268:522. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]; (p) Adams J. Drug Discov Today. 2003;8:307. doi: 10.1016/s1359-6446(03)02647-3. [DOI] [PubMed] [Google Scholar]; (q) Almond JB, Cohen GM. Leukemia. 2002;16:433. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]; (r) Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Cancer Res. 1999;59:2615. [PubMed] [Google Scholar]; (s) Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Clin Chem. 2000;46:67. [PubMed] [Google Scholar]; (t) Tan C, Waldmann TA. Cancer Res. 2002;62:1083. [PubMed] [Google Scholar]
- 4.(a) Milacic V, Dou QP. Coord Chem Rev. 2009;253:1649. doi: 10.1016/j.ccr.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ott I. Coord Chem Rev. 2009;253:1670. doi: 10.1016/j.ccr.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Milacic V, Fregona D, Dou QP. Histol Histopathol. 2008;23:101. doi: 10.14670/HH-23.101. [DOI] [PubMed] [Google Scholar]; (d) Wang X, Guo Z. Dalton Trans. 2008;1521 doi: 10.1039/b715903j. [DOI] [PubMed] [Google Scholar]; (e) Kostova I. Anti-Cancer Agents Med Chem. 2006;6:19. doi: 10.2174/187152006774755500. [DOI] [PubMed] [Google Scholar]; (f) Tiekink ERT. Crit Rev Oncol Hematol. 2002;42:225. doi: 10.1016/s1040-8428(01)00216-5. [DOI] [PubMed] [Google Scholar]; (g) Shaw CF., III Chem Rev. 1999;99:2589. [Google Scholar]; (h) Sun RW-Y, Yu W-Y, Sun H, Che C-M. Chem Bio Chem. 2004;5:129. [Google Scholar]; (i) Che C-M, Sun RW-Y, Yu W-Y, Ko C-B, Zhu N, Sun H. Chem Commun. 2003:1718. doi: 10.1039/b303294a. [DOI] [PubMed] [Google Scholar]; (j) Chow KH-M, Sun RW-Y, Lam JBB, Li CK-L, Xu A, Ma D-L, Abagyan R, Wang Y, Che C-M. Cancer Res. 2010;70:329. doi: 10.1158/0008-5472.CAN-09-3324. [DOI] [PubMed] [Google Scholar]; (k) Wang D, Lippard SJ. Nat Rev Drug Discovery. 2005;4:307. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]; (l) Takahara PM, Rosenzweig AC, Frederick CA, Lippard JS. Nature. 1995;377:649. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]; (m) Chu G. J Biol Chem. 1994;269:787. [PubMed] [Google Scholar]; (n) Sun RW-Y, Li CK-L, Ma DL, Yan JJ, Lok CN, Leung CH, Zhu N, Che C-M. Chem Eur J. 2010;16:3097. doi: 10.1002/chem.200902741. [DOI] [PubMed] [Google Scholar]; (o) Lum CT, Sun RW-Y, Zou T, Che C-M. Chem Sci. 2014;5:1579. [Google Scholar]
- 5.Bindoli A, Rigobello MP, Scutari G, Gabbiani C, Casini A, Messori L. Coord Chem Rev. 2009;253:1692. [Google Scholar]
- 6.Fricker SP. Gold Bull. 1996;29:53. [Google Scholar]
- 7.Simon TM, Kunishima DH, Vibert GJ, Lorber A. Cancer. 1979;44:1965. doi: 10.1002/1097-0142(197912)44:6<1965::aid-cncr2820440602>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Berners-Price SJ, Sadler SJ. Coord Chem Rev. 1996;151:1. [Google Scholar]
- 9.Buckley RG, Elsome AM, Fricker SP, Henderson GR, Theobald BRC, Parish RV, Howe BP, Kelland LR. J Med Chem. 1996;39:5208. doi: 10.1021/jm9601563. [DOI] [PubMed] [Google Scholar]
- 10.(a) Cattaruzza L, Fregona D, Mongiat M, Ronconi L, Fassina A, Colombatti A, Aldinucci D. Int J Cancer. 2011;128:206. doi: 10.1002/ijc.25311. [DOI] [PubMed] [Google Scholar]; (b) Fregona D, Ronconi L, Aldinucci D. Drug Discovery Today. 2009;14:1075. [Google Scholar]; (c) Ronconi L, Fregona D. Dalton Trans. 2009:10670. doi: 10.1039/b913597a. [DOI] [PubMed] [Google Scholar]; (d) Saggioro D, Rigobello MP, Paloschi L, Folda A, Moggach SA, Parsons S, Ronconi L, Fregona D, Bindoli A. Chem Biol. 2007;14:1128. doi: 10.1016/j.chembiol.2007.08.016. [DOI] [PubMed] [Google Scholar]; (e) Milacic V, Chen D, Ronconi L, Landis-Piwowar KR, Fregona D, Dou QP. Cancer Res. 2006;66:10478. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]; (f) Ronconi L, Giovagnini L, Marzano C, Bettio F, Graziani R, Pilloni G, Fregona D. Inorg Chem. 2005;44:1867. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]; (g) Giovagnini L, Ronconi L, Aldinucci D, Lorenzon D, Sitran S, Fregona D. J Med Chem. 2005;48:1588. doi: 10.1021/jm049191x. [DOI] [PubMed] [Google Scholar]
- 11.Oar MA, Dichtel WR, Serin JM, Fréchet JMJ, Rogers JE, Slagle JE, Fleitz PA, Tan L-S, Ohulchansky TY, Prasad PN. Chem Mater. 2006;18:3682. [Google Scholar]
- 12.Sun L, Chen H, Zhang Z, Yang Q, Tong H, Xu A, Wang C. J Inorg Biochem. 2012;108:47. doi: 10.1016/j.jinorgbio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Jamin ME, Iwamoto RT. Inorg Chim Acta. 1978;27:135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.