SUMMARY
Objective
Depression in older adults is often associated with cognitive abnormalities and may predict later development of a primary cognitive disorder. This double-blind, randomized, placebo-controlled pilot study was designed to assess the safety and efficacy of galantamine augmentation of antidepressant treatment for depressive and cognitive symptoms in older adults with major depression.
Methods
Thirty-eight, non-demented older adults (age >50) with major depression were randomized to receive galantamine or placebo augmentation of standard antidepressant pharmacotherapy (venlafaxine XR or citalopram). Mood and cognitive status were monitored for 24 weeks using the 24-item Hamilton Rating Scale for Depression and the Repeatable Battery for the Assessment of Neuropsychological Status.
Results
Both groups showed significant improvements in mood and cognition over 24 weeks, but no significant difference was found in change over time between groups. An exploratory post-hoc analysis suggested that patients randomized to galantamine had lower depression scores compared to patients in the placebo group after 2 weeks of treatment. Dropout was high with more subjects randomized to antidepressant plus galantamine withdrawing early from the study.
Conclusions
This pilot study failed to demonstrate a benefit for galantamine augmentation of antidepressant medication in the treatment of depression in older adults. Future studies should explore strategies for reducing dropout in such longitudinal trials and more carefully assess time to response with cholinesterase inhibitor augmentation.
Keywords: late-life depression, galantamine, acetylcholinesterase inhibitors, venlafaxine, citalopram, major depression, antidepressant treatment
INTRODUCTION
Converging lines of evidence suggest a physiological connection between late-life depression (LLD) and Alzheimer’s disease (AD). LLD is often associated with cognitive impairment, which may be severe enough to mimic dementia (McAllister, 1983; Fisman, 1985), and cognitively impaired LLD patients are at a higher risk for dementia (Alexopoulos et al., 1993; Green et al., 2003). Clinico-epidemiological studies implicate lifetime major depression as a risk factor for AD (Green et al., 2003; Ownby et al., 2006), and for some patients depression may be a prodromal symptom of (AD) (Raskind, 1998). Further, AD patients with a history of depression have more hippocampal plaque and tangle pathology, and more rapid cognitive decline compared to AD patients without a depression history (Rapp et al., 2006). This appears to be true even among those AD patients not currently in a depressive episode.
Given this apparent link between LLD and AD, it is possible that AD therapies [e.g. acetylcholinesterase inhibitors (AchIs) (Kurz et al., 2003; Erkinjuntti et al., 2004; Birks, 2006)] may treat cognitive impairment associated with LLD. Use of these therapies might also improve depression by impacting symptom domains such as mood and apathy. Support for this has been mixed, with some studies suggesting that AchIs improve apathy associated with dementia (Edwards et al., 2004; Herrmann et al., 2005; Cummings et al., 2006), but another trial showing possible worsening of depression with rivastigmine treatment (Frankfort et al., 2006). Additionally, AchIs have been shown, in vitro, to increase dopamine release, which may be associated with improvements in mood and apathy (Zhang et al., 2004).
The aim of this pilot study was to test the efficacy of galantamine on cognition and depression severity when added to standard antidepressant therapy for LLD. We hypothesized that individuals receiving six months of galantamine (vs. placebo) would demonstrate greater improvement in global cognitive performance and depressive symptoms.
METHODS
Subjects
Participants were recruited from an outpatient geriatric psychiatry setting between February 2004 and August 2005. Inclusion criteria were: (1) age ≥50 years; (2) current Major Depressive Episode (documented by Structured Clinical Interview for the DSM-IV (SCID-I) (First et al., 1996) and confirmed by clinical interview); (3) able to taper off current antidepressant medications; (4) 17-item Hamilton Depression Rating Scale (HDRS-17) score >17; and (5) normal TSH, B12 and folate levels within the last 12 months or since the start of the depressive episode (whichever was most recent). Exclusion criteria were: (1) dementia or another neurodegenerative disorder (e.g. Parkinson’s disease) by clinical diagnosis or suggested by neuropsychological testing; (2) any major psychiatric disorder other than major depression as a primary diagnosis (e.g. bipolar disorder); (3) active psychotic symptoms; (4) unstable medical co-morbidity; (5) current substance abuse or dependence; (6) failure of an adequate trial of venlafaxine XR (defined as a dose ≥150 mg for at least 4 weeks); or (7) previous intolerance to venlafaxine XR.
Procedures
Depression ratings included the 24-item Hamilton Depression Rating Scale (HDRS-24) (Williams, 1988) at baseline and the HDRS-24, Clinical Global Impression-Improvement (CGI-I) and Patient Global Impression-Improvement (PGI-I) scales every 2 weeks for a total of 24 weeks. If the subject demonstrated a therapeutic response (defined as a decrease in the HDRS-24 by ≥50% from baseline on two consecutive ratings), depression scales were subsequently obtained monthly. Treatment-related adverse effects were assessed at each study visit and neuropsychological evaluation (described in detail below) was performed at baseline, 12 weeks and 24 weeks.
At study entry, subjects were tapered off current antidepressant medications. One week (five weeks for fluoxetine) after antidepressant discontinuation, participants completed baseline evaluations. All subjects received venlafaxine XR and were randomized to receive concomitant treatment with either placebo or galantamine using block randomization with two blocks of size 20. Subjects initially received 37.5 mg/day of venlafaxine XR, with an increase to 75 mg/day after 1 week, as tolerated. Participants who showed treatment response (as previously defined) within 3 weeks on 75 mg/day remained at this dose; otherwise, the dose was increased to 150 mg/day after the third week of non-response. After 6 additional weeks on 150 mg/day without therapeutic response, the venlafaxine XR dose was further increased to 225 mg/day. If subjects were not able to tolerate venlafaxine XR, or if they failed to show a positive response after 12 weeks, then venlafaxine XR was tapered, and the subject was started on citalopram. Citalopram was dosed as follows (if tolerated): 10 mg/day in week 1, 20 mg/day in weeks 2–4, 40 mg/day in weeks 5–6, and 60 mg/day in weeks 7–12. As with venlafaxine, the subject’s current dose was maintained and not increased if the subject demonstrated an antidepressant response.
Galantamine or placebo was dosed as follows: galantamine 4mg or identical placebo twice daily (8 mg per day) for 1 month, and then galantamine or identical placebo 8mg twice daily (16 mg per day) for the remainder of the study. Subjects, clinicians and raters were blinded to treatment allocation.
Neuropsychological evaluation
At baseline, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph et al., 1998) and Mini Mental State Examination (MMSE) (Folstein et al., 1975) were administered. The RBANS is a 40-min neuropsychological battery that assesses multiple domains of cognitive function, including memory, attention, language, and visuospatial abilities, and has two forms to allow repeated administration over time such that practice effects are minimized with limited impact of practice effects.
Data analyses
This study was designed as a pilot, proof-of-concept study. To determine the effectiveness of randomization, we compared baseline measures between the two groups (placebo vs. galantamine augmentation) using χ2 and t-tests as appropriate. For analysis of change in depression scores over time, we performed a repeated measures growth curve analysis (an intentto- treat approach) (Pothoff and Roy, 1964) using all available data (baseline, every 2 weeks for 8 weeks, and every 4 weeks for 16 weeks for a total of nine repeated measures over 24 weeks). A similar analysis was used for the RBANS total and subscales, except these included only three repeated measures: baseline, 12 weeks and 24 weeks. These analyses focused on the group by time interaction terms.
RESULTS
All study procedures were approved by the Emory University Institutional Review Board and written informed consent was received from all subjects prior to enrollment in the study. Forty subjects were enrolled. After randomization, but before taking the first dose of study medication, two subjects (one each in the placebo and galantamine groups) were identified as ineligible because of baseline neuropsychological testing consistent with dementia; data from these subjects were not included at any time point. One subject (randomized to placebo) dropped out of the study before taking any study medication and before the baseline assessment. Data from this subject were included for demographic comparisons only. Two additional subjects took at least one dose of the study medication but withdrew from the study prior to any post-baseline assessment. For these subjects, data were included for baseline and safety analyses, but not for efficacy.
Sample characteristics for the 38 included subjects are shown in Table 1; baseline comparisons of HDRS-24 and RBANS are available only for the 37 subjects that completed this testing. All subjects were free of antidepressant medication at time of randomization. Subjects randomized to placebo were, on average, older, less educated and more depressed (HDRS-24) than subjects randomized to galantamine. In addition, placebo subjects had poorer cognitive functioning (RBANS total) at baseline; however, when group comparisons were controlled for age, this difference was no longer statistically significant (adjusted F = 0.86, df = 1,34, p = 0.359). Subsequent analyses of RBANS scores were adjusted for age, and HDRS-24 analyses were adjusted for baseline depression severity.
Table 1.
Demographic and baseline characteristics
| Measure | Placebo (n = 19) | Galantamine (n = 19) | Statistic | p |
|---|---|---|---|---|
| Age (mean [SD]) | 69.5 (10.3) | 62.5 (9.1) | t = 2.20 | 0.034* |
| Gender (% Male) | 31.6 | 36.8 | χ2 = 0.12 | 0.732 |
| Race/ethnicity (% White) | 84.2 | 78.9 | χ2 = 0.18 | 0.676 |
| Marital Status (% Married) | 31.6 | 22.2 | χ2 = 2.36 | 0.308 |
| Education (% with >12 years) | 42.1 | 88.9 | χ2 = 8.88 | 0.003* |
| Number of prior antidepressant medications (mean [SD]) | 1.9 (1.2) | 2.6 (2.0) | t = −1.34 | 0.189 |
| Baseline HDRS-24 (mean [SD])** | 30.3 (6.7) | 26.4 (4.5) | t = 2.06 | 0.047* |
| Baseline MMSE (mean [SD])** | 27.4 (1.5) | 28.0 (2.0) | t = −0.88 | 0.384 |
| Baseline RBANS total (mean [SD])** | 81.6 (14.6) | 90.9 (16.5) | t = −1.82 | 0.078 |
HDRS-24 = 24-item Hamilton Depression Rating Scale; MMSE = Mini-Mental Status Examination; RBANS = Repeatable Battery for Neuropsychological Status.
p < .05.
Does not include data from one subject (randomized to placebo) who did not have baseline testing; see text for details.
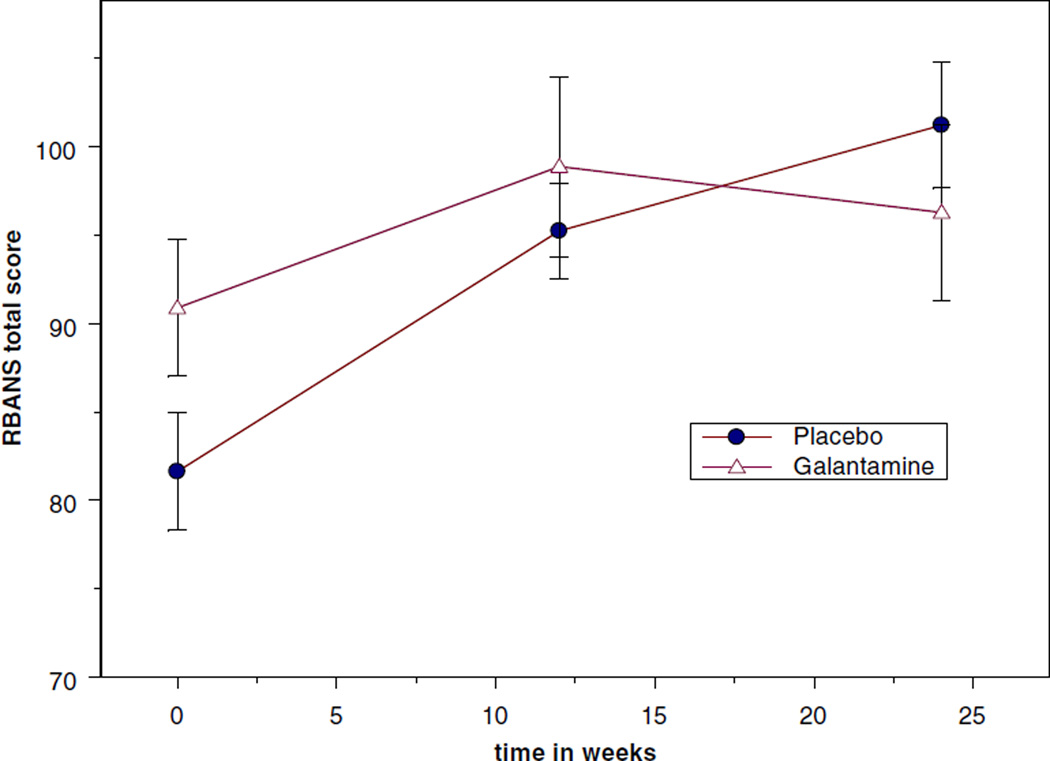
Group-specific analyses showed that both groups had significant improvement in depression over time (Table 2, time effect). However, change in depression severity did not significantly differ between subjects receiving galantamine or placebo (Table 2, interaction effect). Similarly, both groups showed improvement in cognitive functioning over time, but there was no statistically significant group by time interaction (Table 2; Figure 2).
Table 2.
Outcome variables over time (by group)
| Measure | Placebo | Galantamine | p-value for effect | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 18) |
Week 12 (n = 16) |
Week 24 (n = 13) |
Baseline (n = 19) |
Week 12 (n = 9) |
Week 12 (n = 9) |
group effect |
time effect |
group *time effect |
|
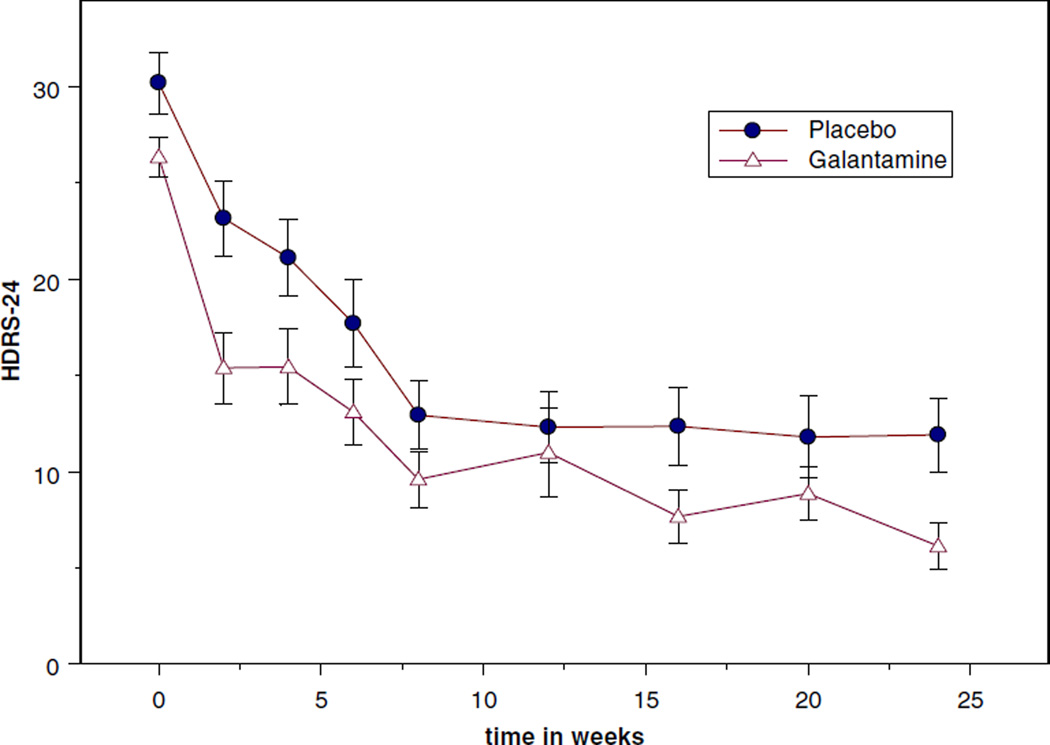
| HDRS-24 | 30.3 (6.7) | 12.3 (7.3) | 11.9 (6.9) | 26.4 (4.5) | 11.0 (6.9) | 6.1 (3.2) | 0.187 | <0.0001 | 0.575 |
| RBANS | |||||||||
| IM | 86.7 (16.2) | 97.9 (12.8) | 106.2 (10.8) | 90.6 (15.6) | 101.4 (11.0) | 103.1 (9.2) | 0.775 | 0.0002 | 0.453 |
| VIS | 83.7 (18.2) | 97.1 (17.2) | 98.8 (13.5) | 87.0 (19.6) | 97.1 (21.6) | 87.6 (18.5) | 0.966 | 0.019 | 0.219 |
| Language | 87.9 (13.1) | 94.6 (4.7) | 96.4 (6.8) | 95.1 (10.0) | 97.4 (7.0) | 96.0 (7.5) | 0.020 | 0.011 | 0.159 |
| Attention | 84.6 (20.5) | 95.3 (13.5) | 96.6 (15.5) | 94.3 (18.2) | 94.1 (17.8) | 94.3 (16.1) | 0.620 | 0.033 | 0.167 |
| DLmem | 86.7 (14.2) | 99.6 (13.8) | 107.5 (13.9) | 98.7 (10.4) | 104.4 (12.7) | 107.0 (12.2) | 0.028 | <0.0001 | 0.090 |
| Tsotal | 81.6 (14.6) | 95.3 (10.9) | 101.2 (12.9) | 90.9 (16.5) | 98.9 (15.3) | 96.3 (13.1) | 0.226 | 0.0001 | 0.084 |
Dlmem = Delayed Memory; HDRS-24 = 24-item Hamilton Depression Rating Scale; IM = Immediate Memory; RBANS = Repeatable Battery for Neuropsychological Status; VIS = Visuospatial/Construction.
Figure 2.
Change in total Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores over time.
Visual inspection of the time course of change in depression scores (Figure 1) suggested a difference between the galantamine and placebo groups at week 2 of treatment. In a post-hoc, exploratory analysis, an ANOVA was performed to test the difference in HDRS-24 score at week 2 between the groups, controlling for baseline HDRS-24 score. Patients receiving galantamine were found to have a significantly lower HDRS-24 score at week 2 compared to patients receiving placebo (F = 4.02, df = 1, 30, p = 0.05); the groups did not differ significantly in HDRS-24 score at any other time point.
Figure 1.
Change in 24-item Hamilton Depression Rating Scale (HDRS-24) scores over time.
Venlafaxine XR dose was lower in subjects randomized to galantamine (mean [SD] for galantamine group = 128.6 [68.5]; mean [SD] for placebo group = 182.1 [56.7]; t = 2.25, df = 26, p = 0.03). Seven patients (four randomized to placebo, three randomized to galantamine) did not tolerate venlafaxine XR and switched to citalopram; citalopram was tolerated in these subjects with a median dose of 20 mg per day for placebo subjects and 40 mg per day for galantamine subjects (Mann–Whitney U = 4.50, p = 0.55). Efficacy results did not change when controlling for antidepressant medication.
Seventeen of 18 subjects randomized to placebo and eleven of 19 subjects randomized to galantamine experienced at least one adverse event (Table 3). Common adverse events included gastrointestinal distress, headaches, pulse rate changes, sweating and dizziness. Serious adverse events (SAEs) were defined as adverse events that led to hospitalization, permanent disability and/or death. Six subjects experienced SAEs (four placebo, two galantamine). SAEs included hospitalization for Raynaud’s phenomenon (one subject randomized to placebo), emphysema (one subject randomized to placebo), chest pain or cardiac abnormality (two subjects, one each randomized to placebo and galantamine), or dehydration (two subjects, one each randomized to placebo and galantamine). No subjects died or acquired permanent disability during the study.
Table 3.
Adverse events (by group)
| Adverse Event | Galantamine (n = 19) | Placebo (n = 18) |
|---|---|---|
| Headache | 2 | 2 |
| Gastrointestinal distress | 4 | 6 |
| Dizziness | 2 | 3 |
| Cardiac abnormality | 1 | 3 |
| Sweating | 4 | 5 |
| Other | 4 | 8 |
| Total | 11 (57%) | 17 (94%) |
Discontinuation rates were 16% in the placebo group and 53% in the galantamine group at 12 weeks (χ2 = 7.27, df = 1, p = 0.01), and 32% in the placebo group and 63% in the galantamine group at 24 weeks (χ2 = 4.66, df = 1, p = 0.03). However, reason for discontinuation (adverse event, inability to adhere to study protocol or lost to follow-up) did not differ between the groups. The primary reason reported for discontinuation was inability to adhere to the study protocol (specifically the follow-up assessment schedule).
DISCUSSION
Acetylcholinesterase inhibitors (AchIs) such as galantamine are well-established for the treatment of Alzheimer’s disease (Wilkinson and Murray, 2001). In patients with dementia, AchIs may have beneficial effects on certain behavioral symptoms (Edwards et al., 2004; Herrmann et al., 2005; Cummings et al., 2006), though not all studies have been positive (Frankfort et al., 2006). The use of AchIs in nondementing psychiatric conditions has been suggested (Burt, 2000), but few studies have been conducted. Donepezil has shown little benefit in improving mood or cognitive symptoms of schizophrenia (Stryjer et al., 2004; Tugal et al., 2004; Risch et al., 2006) or treatment-resistant mania (Eden Evins et al., 2006). However, the potential use of AchIs in depression—especially late-life depression, which may share a common pathophysiology with AD—has not been investigated.
This pilot study of an AchI in the treatment of depression in non-demented older patients failed to demonstrate a benefit for galantamine augmentation of a standard antidepressant medication in the treatment of either cognitive or depressive symptoms. These data do suggest galantamine augmentation may hasten antidepressant response in LLD patients. Additionally, these data suggest that galantamine augmentation of venlafaxine XR may lead to antidepressant benefit at a lower dose of venlafaxine XR. Clearly, these findings must be interpreted carefully given the exploratory nature of these post-hoc analyses. Dropout from the study was greater in the galantamine augmentation group, though number of adverse events (including serious adverse events) did not differ significantly between the groups. The reason for this differential discontinuation rate is unclear since there was no reason for discontinuation that differed significantly between the groups. Although the inability to assess for adverse events in patients who discontinued and were lost to follow-up may have underestimated the adverse event rate, most patients that discontinued were contacted and the reason for discontinuation was documented. The differential discontinuation rate may have influenced the results of the repeated measures analyses; however, this is unlikely since dropout was random within each group (Little and Rubin, 2002).
Limitations of this study include small sample size, high drop-out rate (with significantly more subjects in the galantamine augmentation group exiting the study prematurely) and the failure of randomization to result in similar baseline groups. Since both groups received active antidepressant treatment, it is also likely that only a very large effect for galantamine augmentation could have been identified with this design. Additionally, longer follow-up (beyond 6 months) might be needed to identify potential delayed beneficial and/or protective effects of galantamine augmentation on cognition or mood.
Future studies of AchIs in the treatment of depression should consider strategies for limiting dropout (e.g. fewer evaluations) and employ rigorous monitoring of side effects [e.g. using a structured instrument such as the SAFTEE (Levine and Schooler, 1986)]. Additional studies are needed to more carefully evaluate whether AchI augmentation of antidepressants accelerates antidepressant treatment response. These studies might also include other relevant clinical measures (such as quality of life measures and measures of functional impairment [e.g. performance of IADLs]). Finally, the antidepressant benefits of AchI monotherapy or augmentation could be explored in patients not responding or tolerating standard antidepressant therapy.
KEY POINTS.
Galantamine was not more effective than placebo in augmentation of venlafaxine or citalopram in the treatment of late-life depression.
Galantamine augmentation was safe and relatively well-tolerated.
Dropout over this 24 week study was high with significantly greater dropout in the galantamine augmentation group; however, no reason for this differential discontinuation rate could be identified.
Galantamine augmentation may be associated with a more rapid antidepressant effect in patients with late-life depression.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from Janssen Pharmaceuticals (RY), the Emory Mentored Clinical Research Scholars Program (PEH; K12 RR 017643), K23 MH 077869 (PEH) and the American Federation for Aging Research (PEH).
WMM has received grant and research support from the National Alliance for Research in Schizophrenia and Depression; American Foundation for Suicide Prevention; Fuqua Foundation; National Institute of Mental Health; National Institute of Neurological Disease and Stroke; Neuronetics, Inc.; and Janssen Pharmaceuticals, Inc. He is a consultant for Neuronetics, Inc.; Janssen Pharmaceuticals; Forest; Bristol-Meyers Squibb; Cyberonics; and Solvay Pharmaceuticals. He is on speakers bureaus for Janssen Pharmaceuticals; Forest; and Bristol-Meyers Squibb. He owns stock in Amgen; Teva; Pfizer; and Abbott. WMM assisted in the design of the study, writing and editing the final manuscript; however, he did not participate in patient recruitment, evaluation or data analysis for this study. PEH has received grants or honoraria from the American Federation for Aging Research (AFAR); Abbott Laboratories; Cyberonics, Inc.; DANA Foundation; Neuronetics, Inc.; National Alliance for Research in Schizophrenia and Depression; National Center for Research Resources; National Institute of Mental Health; National Institutes of Health Loan Repayment Program; Stanley Medical Research Institute; Woodruff Foundation. PEH serves as a consultant for Advanced Neuromodulation Systems, Inc.
Footnotes
CONFLICT OF INTEREST
No other affiliations or disclosures are reported for any of the other authors.
REFERENCES
- Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with ‘reversible dementia’: a controlled study. Am J Psychiatry. 1993;150:1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005593. CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt T. Donepezil and related cholinesterase inhibitors as mood and behavioral controlling agents. Curr Psychiatry Rep. 2000;2:473–478. doi: 10.1007/s11920-000-0005-7. [DOI] [PubMed] [Google Scholar]
- Cummings JL, McRae T, Zhang R. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry. 2006;14:605–612. doi: 10.1097/01.JGP.0000221293.91312.d3. [DOI] [PubMed] [Google Scholar]
- Eden Evins A, Demopulos C, Nierenberg A, et al. A double-blind, placebo-controlled trial of adjunctive donepezil in treatment-resistant mania. Bipolar Disord. 2006;8:75–80. doi: 10.1111/j.1399-5618.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Edwards KR, Hershey L, Wray L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 12-week interim analysis. Dement Geriatr Cogn Disord. 2004;17(Suppl 1):40–48. doi: 10.1159/000074681. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Roman G, Gauthier S. Treatment of vascular dementia-evidence from clinical trials with cholinesterase inhibitors. Neurol Res. 2004;26:603–605. doi: 10.1179/01610425017631. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Disorders—Research Version (SCID-I, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Fisman M. Pseudodementia. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:481–484. doi: 10.1016/0278-5846(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frankfort SV, Appels BA, De Boer A, et al. Treatment effects of rivastigmine on cognition, performance of daily living activities and behaviour in Alzheimer’s disease in an outpatient geriatric setting. Int J Clin Pract. 2006;60:646–654. doi: 10.1111/j.1368-5031.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Rabheru K, Wang J, Binder C. Galantamine treatment of problematic behavior in Alzheimer disease: post-hoc analysis of pooled data from three large trials. Am J Geriatr Psychiatry. 2005;13:527–534. doi: 10.1176/appi.ajgp.13.6.527. [DOI] [PubMed] [Google Scholar]
- Kurz AF, Erkinjuntti T, Small GW, et al. Long-term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer’s disease with cerebrovascular disease. Eur J Neurol. 2003;10:633–640. doi: 10.1046/j.1468-1331.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Little RJ, Rubin DB. Statistical Analyses with Missing Data. 2nd edn. New York: John Wiley and Sons, Inc.; 2002. [Google Scholar]
- McAllister TW. Overview: pseudodementia. Am J Psychiatry. 1983;140:528–533. doi: 10.1176/ajp.140.5.528. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoff RF, Roy SN. A generalized multivariate analysis of variance model useful especially for growth curve problems. Biometrika. 1964;51:313–326. [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- Raskind MA. The clinical interface of depression and dementia. J Clin Psychiatry. 1998;59(Suppl 10):9–12. [PubMed] [Google Scholar]
- Risch SC, Horner MD, McGurk SR, et al. Donepezil effects on mood in patients with schizophrenia and schizoaffective disorder. Int J Neuropsychopharmacol. 2006;9:603–605. doi: 10.1017/S1461145705006115. [DOI] [PubMed] [Google Scholar]
- Stryjer R, Strous R, Bar F, et al. Donepezil augmentation of clozapine monotherapy in schizophrenia patients: a double blind cross-over study. Hum Psychopharmacol. 2004;19:343–346. doi: 10.1002/hup.595. [DOI] [PubMed] [Google Scholar]
- Tugal O, Yazici KM, Anil Yagcioglu AE, Gogus A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2004;7:117–123. doi: 10.1017/S1461145703004024. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Murray J. Galantamine: a randomized, double-blind, dose comparison in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16:852–857. doi: 10.1002/gps.409. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou FM, Dani JA. Cholinergic drugs for Alzheimer’s disease enhance in vitro dopamine release. Mol Pharmacol. 2004;66:538–544. doi: 10.1124/mol.104.000299. [DOI] [PubMed] [Google Scholar]