Abstract
TDP-43 pathology is a disease hallmark that characterizes amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD-TDP). Although a critical role for TDP-43 as an RNA-binding protein has emerged, the regulation of TDP-43 function is poorly understood. Here we identify lysine acetylation as a novel post-translational modification controlling TDP-43 function and aggregation. We provide evidence that TDP-43 acetylation impairs RNA-binding and promotes accumulation of insoluble, hyper-phosphorylated TDP-43 species that largely resemble pathological inclusions in ALS and FTLD-TDP. Moreover, biochemical and cell-based assays identify oxidative stress as a signaling cue that promotes acetylated TDP-43 aggregates that are readily engaged by the cellular defense machinery. Importantly, acetylated TDP-43 lesions are found in ALS patient spinal cord, indicating that aberrant TDP-43 acetylation and loss of RNA binding are linked to TDP-43 proteinopathy. Thus, modulating TDP-43 acetylation represents a plausible strategy to fine-tune TDP-43 activity, which could provide new therapeutic avenues for TDP-43 proteinopathies.
TDP-43 is a highly conserved and ubiquitously expressed nuclear protein that contains two RNA-recognition motifs (RRMs) involved in RNA and DNA binding, as well as a glycine-rich C-terminal sequence, which harbors the majority of the ALS-linked mutations1, 2. TDP-43 has diverse cellular roles in regulating RNA splicing and RNA stability as well as other gene regulatory functions3-5. High throughput sequencing approaches have shown that TDP-43 binds ~6000 genes and regulates target RNAs that are essential for proper neuronal development and synaptic function4-6. In addition to RNA targets, TDP-43 binds to proximal gene promoters and regulates gene expression of SP-10 (acrosomal vesicle protein 1) and cdk6 (cyclin-dependent kinase 6)7-9, suggesting a distinct role in gene transcription. Recent studies have also implicated TDP-43 as a stress-responsive RNA-associated factor required for local translation in the cytoplasm10. Supporting this finding, TDP-43 is a major component of neuronal RNA granules and cytoplasmic stress granules (SGs)11-14, which are active sites of RNA regulation and sorting during exposure to stress. Thus, the normal physiological functions of TDP-43 could involve the response to environmental stress via regulation of downstream genes and RNAs. While the identification of TDP-43 target RNAs has been a major focus recently, the mechanisms that regulate TDP-43 function remain poorly understood.
TDP-43 is predominantly nuclear localized; however, pathological TDP-43 found in diseased brain and spinal cord becomes abnormally aggregated primarily in the cytoplasm, which has been linked to onset and/or progression of TDP-43 proteinopathy by several pathogenic mechanisms15, 16. The physical presence of cytoplasmic aggregates could exert a toxic gain of function via impaired vesicle trafficking as well as cytoskeletal abnormalities17, 18. In addition, substantial evidence indicates that TDP-43 aggregates induce loss of normal nuclear TDP-43 functions, as nuclear depletion of normally soluble TDP-43 due to TDP-43 aggregates led to loss of splicing and transcription activities in cultured cells and transgenic mice19-23. Pathological TDP-43 is abnormally phosphorylated on C-terminal serine residues (Ser-403/404 and Ser-409/410) by multiple kinases24-27 and has emerged as a disease-specific marker of human TDP-43 proteinopathy16, 25, 28. Although phospho-TDP-43 immunoreactivity is valuable as a postmortem diagnostic tool, the significance of phosphorylation as it relates to TDP-43 biology is not clear. Indeed, several studies have indicated that phosphorylation actually prevents rather than promotes TDP-43 aggregation29, 30, suggesting that additional signaling mechanisms likely exist to modulate TDP-43 functions and aggregate formation in diseased individuals.
Lysine acetylation has emerged as a major covalent modification controlling diverse cellular processes and has been implicated in Alzheimer’s disease (AD) and other neurodegenerative disorders31-35. For example, we demonstrated that acetylation of misfolded tau proteins marks mature neurofibrillary tangles (NFTs) in AD and related tauopathies and represents a disease-specific marker of AD pathology31, 33, 34. In addition to tau, a global proteomics approach identified ~1750 proteins that are subject to lysine acetylation, including a distinct subset of RNA-binding proteins and associated factors36. Since TDP-43 is an RNA binding protein implicated in ALS, we asked if TDP-43 is subject to acetylation, a modification that could regulate cellular processes linked to ALS pathogenesis. Here, we show that acetylation occurs on TDP-43 lysine residues within the RNA-binding domains (RRMs), which functionally abrogates RNA-binding and promotes the accumulation of insoluble TDP-43 aggregates. Our identification of acetylated TDP-43 lesions present in ALS patient spinal cord highlights a novel pathological signature and provides a rationale to explore TDP-43 acetylation as a potential therapeutic target in ALS and related TDP-43 proteinopathies.
Results
TDP-43 is subject to acetylation at specific lysine residues
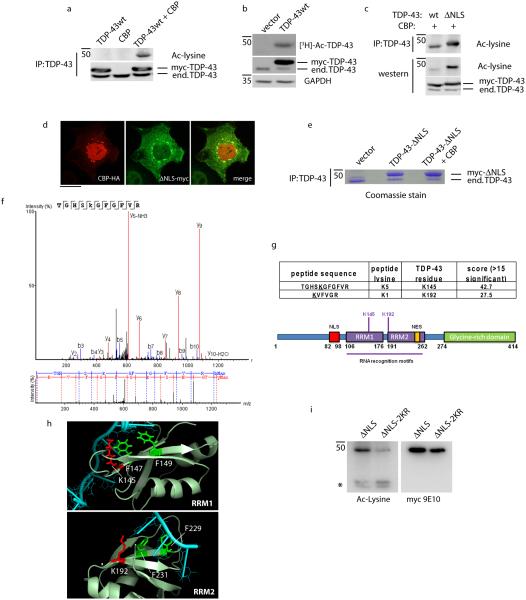
To determine whether TDP-43 is acetylated, co-transfection experiments were performed with TDP-43 and the acetyltransferase Creb-binding protein (CBP), followed by immunoprecipitation and immunoblotting using a pan-acetyl-lysine antibody. Ectopically expressed myc-tagged wild-type (WT) TDP-43 was prominently acetylated only in the presence of CBP (Fig. 1a and Supplementary Fig. 1). To further confirm TDP-43 acetylation, WT TDP-43 expressing cells were incubated with [3H]-acetate, and we observed the incorporation of [3H]-labeled acetyl groups onto TDP-43 by SDS-PAGE and autoradiography (Fig. 1b and Supplementary Fig. 1). Interestingly, mutating the TDP-43 nuclear localization sequence (TDP-43-ΔNLS) led to increased TDP-43 acetylation upon mislocalization to the cytoplasm (Fig. 1c and Supplementary Fig. 1), a compartment where TDP-43 forms inclusions in diseased human brain and spinal cord. Although CBP is predominantly nuclear localized, immunofluorescence analysis indicated that a sub-population of transfected CBP was recruited to cytoplasmic TDP-43-ΔNLS foci (Fig. 1d), thereby implicating direct CBP-mediated acetylation of TDP-43. There were no appreciable differences in acetylation among a panel of ALS-associated TARDBP mutations also directed to the cytoplasm (Supplementary Fig. 2), consistent with the mild aggregation phenotype observed for these mutations in cultured cell lines2.
Figure 1. TDP-43 is subject to lysine acetylation within the RNA-binding domain.
a) QBI-293 cells were transfected with TDP-43 in the presence or absence of the acetyltransferase CBP and acetylated TDP-43 was evaluated by immunoprecipitation (anti-TDP-43 clone 171) followed by immunoblotting using anti-acetylated lysine antibody. b) Cells transfected with wild-type TDP-43 were pulse-labeled with [3H]-acetate for 2 hr followed by immunoprecipitation and analysis by SDS-PAGE and autoradiography for 2 weeks. Total cellular lysates representing 5% total input were immunoblotted using anti-TDP-43 and GAPDH antibodies. c) TDP-43 acetylation assay was performed similar to (a) above using either wild-type TDP-43 or the cytoplasmic mutant TDP-43-ΔNLS in the presence of the acetyltransferase CBP. Samples were analyzed by immunoprecipitation (top panel) and direct immunoblotting (bottom panels) using acetylated lysine and total TDP-43 antibodies. d) Double-labeling immunofluorescence was performed on cells transfected with CBP and TDP-43-ΔNLS using anti-HA and anti-myc antibodies, respectively. The merge indicates co-localization of CBP with cytoplasmic localized TDP-43. The scale bar represents 25 µm. e-f) Cells were transfected with vector alone or TDP-43-ΔNLS in the absence or presence of CBP and total TDP-43 was immunoprecipitated, separated by SDS-PAGE followed by gel excision, and analyzed by mass spectrometry, which identified the K145-acetylated peptide shown in f. g) CBP-mediated acetylation of two lysine residues clustered within the RNA-binding regions with significant ion scores and p-values are shown. h) Crystal structure analysis of RNA-binding domains RRM1 and RRM2 bound to DNA (Protein Data Bank entries 3D2W and 4IUF) illustrates Lys-145 and Lys-192 (shown in red) adjacent to critical Phe residues (shown in green) in the context of bound DNA (shown in cyan). i) Cytoplasmic TDP-43-ΔNLS or a comparable construct containing K→R mutations at Lys-145 and Lys-192 (TDP-43-ΔNLS-2KR) were analyzed by immunoblotting in the presence of CBP and HDAC inhibitors (nicotinamide/TSA) to promote full TDP-43 acetylation. The asterisk (*) indicates acetylated cross-reactive bands that are unaltered by TDP-43.
To identify putative lysine acetylation sites, TDP-43-ΔNLS was co-transfected with CBP and subsequently immunoprecipitated from cell lysates using a TDP-43-specific monoclonal antibody (clone 171), resulting in enrichment of TDP-43-ΔNLS that was readily detected by standard SDS-PAGE and Coomassie stained gels (Fig. 1e and Supplementary Fig. 3). TDP-43 protein bands were gel excised, trypsin digested, and analyzed by mass spectrometry using nanoLC/nanospray/MS/MS. Based on ~75% sequence coverage of human TDP-43, we identified two major acetylated sites in the RNA-binding domains (Fig. 1f and Supplementary Fig. 4); Lys-145 in RRM1, which spans residues 105-169, and Lys-192 immediately adjacent to RRM2, which spans residues 194-257 (see Fig. 1g for schematic of TDP-43 acetylation sites). Amino acid mapping onto known crystal structures of either RRM1 or RRM2 bound to target DNA37, 38 showed that acetylation of the Lys-145 and Lys-192 side chains could potentially interact with and alter nucleic acid binding (Fig. 1h, Lys residues highlighted in red). Supporting this possibility, both lysines are adjacent to neighboring Phe residues in RRM1 (Phe-147/149) and RRM2 (Phe-229/231), previously shown to interact directly with nucleic acids39 (Fig. 1h, Phe residues highlighted in green). To determine whether Lys-145 and Lys-192 represent major acetylated residues in TDP-43, we generated a TDP-43-ΔNLS mutant containing lysine substitutions (TDP-43-ΔNLS-2KR). The acetylation-deficient mutant showed reduced but not completely abrogated acetylation (Fig. 1i and Supplementary Fig. 5), suggesting Lys-145 and Lys-192 are dominant sites of acetylation, although additional acetylated lysines may be present that are not detectable using this approach.
TDP-43 acetylation enhances aggregate formation
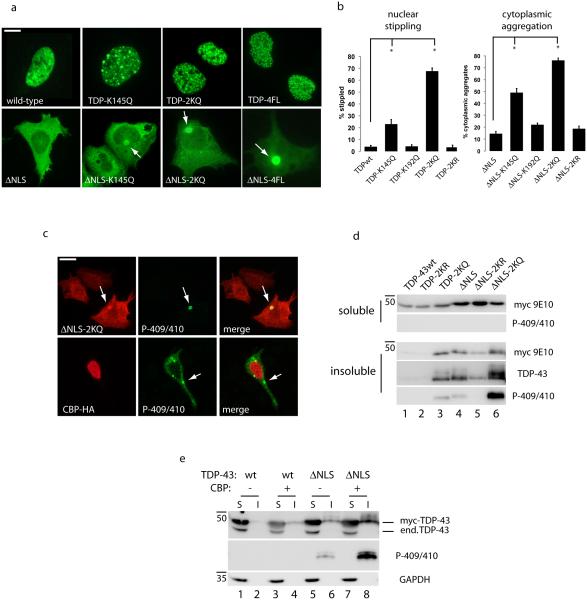
To determine the consequences of TDP-43 acetylation at these lysine residues, acetylation mimic (K→Q) and non-mimic (K→R) mutants were generated and analyzed by immunofluorescence microscopy. As shown in Fig. 2a, nuclear WT TDP-43 exhibits a diffuse nucleoplasmic pattern with punctate nuclear bodies as previously reported23, 40. However, a single acetylation mimic TDP-K145Q, and more prominently, the double acetylation mimic TDP-2KQ, displayed a severely stippled nuclear localization in ~65% of cells (see Fig. 2b for quantification), which closely resembled the TDP-4FL RNA-binding deficient Phe mutant41, strongly suggesting impaired RNA binding. Strikingly, targeting acetylation to the cytoplasm using TDP-43-ΔNLS containing single or double acetylation mimics showed a distinct phenotype characterized by cytoplasmic inclusions that resembled pathological inclusions seen in ALS and other TDP-43 proteinopathies16, 28, 42 (Fig. 2a-b, ~75% aggregates for TDP-43-ΔNLS-2KQ).
Figure 2. TDP-43 acetylation promotes pathological features associated with TDP-43 proteinopathies.
a) Double-labeling immunofluorescence was performed on cells transfected with myc-tagged wild-type TDP-43 or cytoplasmic localized TDP-43-ΔNLS containing the single acetylation mimic (K145Q), double mimic (TDP-2KQ), or 4FL RNA-binding deficient mutations (F147/149/229/231L). White arrows highlight cytoplasmic accumulation of TDP-43 aggregates. Scale bar represents 10 µm. b) Nuclear stippling and cytoplasmic aggregation phenotypes were quantified for all TDP-43 acetylation-mimic and non-mimic mutants at residues Lys-145 and Lys-192. Error bars indicate standard error of the mean (SEM), and the single asterisk indicates statistical significance with p-value < .05 as measured by two-tailed unpaired t-test with unequal variance from N=3 biological replicates. c) Cells expressing acetylation-mimic TDP-43-ΔNLS (TDP-43-ΔNLS-2KQ) or CBP-acetylated TDP-43-ΔNLS were analyzed by immunofluorescence microscopy using a phospho-TDP-43 antibody (P-409/410) to mark pathological TDP-43 aggregates. Scale bar represents 25 µm. d-e) Soluble (S) and insoluble (I) fractions from cells expressing acetylation-mimics as well as CBP-mediated acetylated TDP-43 were analyzed by immunoblotting using the indicated myc-9E10, TDP-43 (1038), phospho-TDP-43 (P409/410), or GAPDH antibodies.
Acetylation of cytoplasmic TDP-43 using either acetylation-mimic mutations or co-transfected CBP led to the formation of bona fide TDP-43 aggregates that are phosphorylated at Ser-409 and Ser-410 (P-409/410), a disease hallmark of TDP-43 pathology28, 43 (Fig. 2c). Similarly, solubility assays confirmed that targeting TDP-43 acetylation mimics to the cytoplasm increased P-409/410 levels in insoluble fractions (Fig. 2d, lane 6 and Supplementary Fig. 6). Importantly, acetylation-induced aggregation was not observed with non-mimic K→R substitutions (Fig. 2d, compare lanes 4-6). Indeed, we attribute the TDP-43 insolubility to acetylation, as co-transfection of CBP similarly enhanced the phosphorylation of TDP-43-ΔNLS that was recovered in the insoluble fraction (Fig. 2e, lanes 6 and 8 and Supplementary Fig. 6). Cytoplasmic TDP-43 acetylation similarly induced aggregation in differentiated Neuro2A cells precluding any cell-type specific differences in TDP-43 aggregation propensity (Supplementary Fig. 7). Thus, these results support lysine acetylation as a critical determinant of TDP-43 aggregate formation and highlight distinct aggregation pathologies resulting from TDP-43 subcellular localization.
Acetylation-mimics impair TDP-43 RNA-regulatory functions
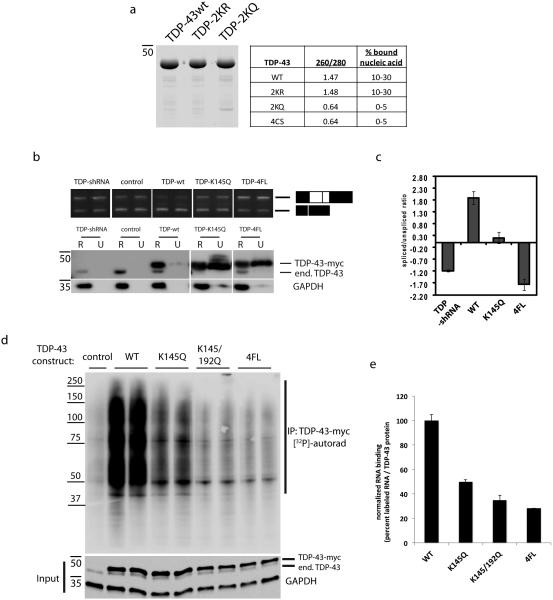
Given that the acetylated lysines are located within the RRM domains, we sought to determine whether TDP-43 acetylation functionally altered RNA binding. Recombinant full-length TDP-43 proteins containing acetylation mimic and non-mimic mutations were sequentially extracted from bacteria yielding highly pure TDP-43 proteins by Coomassie staining (Fig. 3a and Supplementary Fig. 8). Consistent with high affinity nucleic acid binding, absorbance readings (A260/280) of WT and TDP-2KR protein preparations indicated the presence of co-purifying nucleic acids that were not abundant in preparations of the TDP-2KQ acetylation-mimic, suggesting a conformational change induced by charge neutralization (i.e. Lys → Gln substitutions) that abrogated binding to nucleic acids. To assess the functional consequence of reduced RNA binding to TDP-43, we utilized a cell-based nuclear CFTR splicing assay in which TDP-43 expression facilitates exon 9 skipping of a CFTR minigene (Fig. 3b and Supplementary Fig. 8)39, 44, 45. As expected, TDP-43 shRNA led to accumulation of the exon 9 included (unspliced) transcript and a dramatic reduction in the spliced/unspliced ratio, while WT TDP-43 over-expression reversed this effect by enhancing the exon 9 excluded product (Fig. 3c). Consistent with a loss of TDP-43 function, the acetylation-mimic K145Q mutant displayed a partial reduction in the spliced/unspliced ratio relative to baseline, and a similar but more severe loss of function was observed with the RNA-binding deficient 4FL mutant, which completely abrogated RNA binding39 (Fig. 3c, compare WT to K145Q and 4FL).
Figure 3. TDP-43 acetylation impairs RNA-regulatory functions.
a) Recombinant full length wild-type TDP-43, non-mimic TDP-2KR and acetylation mimic TDP-2KQ mutants were purified by a sequential extraction according to the methods, and co-purifying nucleic acid was quantified by measuring absorbance at 260/280nm (A260/280). The percent co-purifying nucleic acid is estimated based on known A260/280 values, which are significantly reduced for TDP-2KQ and 4CS mutant proteins11, indicating reduced association of these proteins with total extracted nucleic acids. b) Cells expressing vector control, TDP-43shRNA, wild-type TDP-43, TDP-K145Q, or RNA-binding deficient 4FL plasmids were co-transfected with a CFTR minigene. TDP-43 protein levels were determined by immunoblotting, and spliced/unspliced CFTR transcripts were assessed by RT-PCR using specific primers flanking exon 9. c) RT-PCR products were quantified using the Agilent 2100 Bioanalyzer and relative values of CFTR transcripts are represented as a log ratio of spliced/unspliced products. All samples are normalized to the vector control. Splicing analysis was performed using three independent experiments (N=3), and error is displayed as standard error of the mean (SEM). d) Cross-linking immunoprecipitation (CLIP) assay was performed on cells expressing wild-type TDP-43, K145Q, 2KQ (K145/192Q), or TDP-4FL plasmids. TDP-43 bound RNAs were immunoprecipitated using anti-myc (9E10), end-labeled with [32P]-ATP, and visualized using bis-tris gel immunoblotting followed by phosphorimaging analysis. Shown is a representative image containing duplicate samples for each construct that was obtained from N=3 independent experiments. e) TDP-43-associated RNAs were quantified using TYPHOON software and normalized to total TDP-43 protein inputs, as determined by immunoblotting with an anti-myc antibody shown in d. All mutants analyzed (K145Q, K145Q/192Q, 4FL) showed significant reduction in RNA-binding compared to WT as assessed by student t-test (p-value ≤ .01) from N=3 independent experiments. Error bars indicate standard deviation (SD) of normalized intensity signals derived from experimental replicates.
Finally, we utilized a cross-linking immunoprecipitation assay (CLIP)4, 5 to directly assess the RNA-binding activity of acetylated TDP-43. As expected, WT TDP-43 contains functional RNA-binding properties5 and physically bound to total cellular RNAs as determined by TDP-43 gel migration as ~ 50-250 kD protein/RNA complexes (Fig. 3d, see TDP-43 bound target RNAs within the bracket, and Supplementary Fig. 8). However, a single acetylation-mimic mutant, and more prominently the double mutant (TDP-K145Q or TDP-2KQ), showed a ~ 50-65% decrease in RNA binding, a partial loss of function that approached that observed with the RNA-binding deficient 4FL mutant (~ 70% decrease), as determined by quantification and normalization of cross-linked high molecular weight [32P]-labeled RNAs (Fig. 3e). Thus, a single acetylation-mimic mutation at Lys-145 within RRM1 is sufficient to significantly impair TDP-43 binding to its cellular mRNA targets.
Arsenite-induced oxidative stress induces TDP-43 acetylation
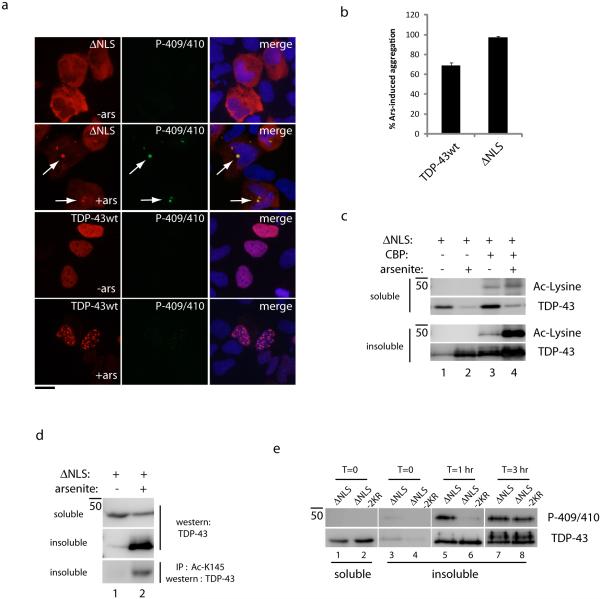
Given that TDP-43 has high-affinity RNA binding under normal conditions46, 47, we hypothesized that accessibility to target lysines would occur more readily following detachment of TDP-43 from RNAs. Therefore, we employed an oxidative stress paradigm in which sodium arsenite treatment was previously shown to impair TDP-43 binding to RNAs, leading to loss of function and insoluble TDP-43 accumulation11. Supporting stress-induced loss of TDP-43 function, acute arsenite exposure promoted rapid accumulation of TDP-43-ΔNLS into phosphorylated cytoplasmic inclusions, while WT TDP-43 formed insoluble nuclear foci that were not phosphorylated (Fig. 4a). Quantification of these results indicated that cytoplasmic TDP-43 showed a slightly increased propensity to undergo stress-induced re-localization and aggregation compared to WT nuclear TDP-43 (Fig. 4b). Therefore, either oxidative stress or TDP-43 acetylation is capable of promoting TDP-43 aggregation and loss of function (Fig. 2b and 4b), suggesting a common signaling pathway in which oxidative stress could mediate TDP-43 acetylation and subsequent aggregation.
Figure 4. Oxidative stress promotes TDP-43 acetylation and pathological TDP-43 aggregation.
(a) Double-labeling immunofluorescence microscopy of WT TDP-43 or cytoplasmic TDP-43 was performed using anti-myc and anti-phospho-TDP-43 (P-409/410) antibodies, which illustrate TDP-43 phosphorylation and aggregation upon acute exposure to 0.2 mM arsenite for 1 hr. The scale bar represents 25 µm. (b) Quantification of nuclear or cytoplasmic TDP-43 stippling and aggregation phenotypes for all lysine mutants was performed from N=3 independent experiments as detailed in the methods section. Error bars represent standard error of the mean (SEM). (c) Immunoblotting of soluble and insoluble fractions using anti-myc and anti-acetyl-lysine antibodies illustrates the accumulation of acetylated TDP-43 upon co-transfection of CBP and exposure to 0.2 mM arsenite for 1 hr. (d) Immunoprecipitation reaction using Ac-K145 followed by immunoblotting with a monoclonal anti-TDP-43 antibody allowed increased sensitivity for detection of acetylated TDP-43 in the absence of CBP (see also Supplementary Figure 11). (e) Immunoblotting analysis of TDP-43-ΔNLS and the double lysine mutant, TDP-43-ΔNLS-2KR, was evaluated in an arsenite-induced aggregation time-course, which showed delayed accumulation of phosphorylated TDP-43-ΔNLS-2KR aggregates compared to TDP-43-ΔNLS.
To investigate this possibility, biochemical analysis showed that insoluble TDP-43-ΔNLS aggregates became increasingly acetylated upon co-transfection of CBP and exposure to acute arsenite stress (Fig. 4c, lanes 3-4 and Supplementary Fig. 9). To assess whether stress-induced TDP-43 acetylation could occur in the absence of ectopically expressed CBP acetyltransferase, we generated a site-specific acetylated TDP-43 antibody against Lys-145 to increase the specificity and detection sensitivity for acetylated TDP-43 (referred to as Ac-K145, see Supplementary Fig. 10 for antibody characterization). Ac-K145 did not reliably detect acetylated TDP-43 by direct immunoblot analysis of insoluble lysates, however, we found that Ac-K145 immunoprecipitated acetylated TDP-43 from insoluble fractions of arsenite-treated cells, and was then detected by immunoblotting with total TDP-43 antibodies (Fig. 4d, lane 2 and Supplementary Fig. 11). To assess the aggregation propensity of a non-acetylated mutant at Lys-145 and Lys-192 (TDP-43-ΔNLS-2KR) in response to arsenite stress, a time-course analysis of TDP-43 aggregation was performed, which showed a delay in the progressive accumulation of phosphorylated TDP-43 species (P-409/410) ~1 hr after arsenite exposure (Fig. 4e, lanes 5-6, and Supplementary Fig. 11), suggesting acetylation at these specific lysines could contribute to stress-induced maturation of TDP-43 aggregates. Thus, potent oxidative stressors can act as environmental signals to induce acetylation, aggregation, and loss of critical TDP-43 functions.
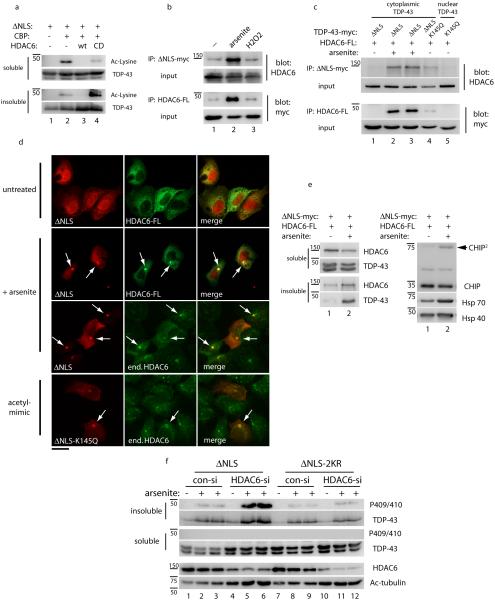
HDAC6 interacts with and deacetylates TDP-43
Since our data suggest that acetylation promotes TDP-43 aggregation, we sought to identify a TDP-43 deacetylase capable of regulating this process. One potential candidate is the cytoplasmic deacetylase HDAC6, previously implicated in the clearance of misfolded protein aggregates in several neurodegenerative diseases48-57. A more recent study demonstrated a direct physical interaction between HDAC6 and TDP-43 in vivo58. Therefore, we evaluated whether HDAC6 regulates TDP-43 acetylation and aggregation. Co-expression of WT HDAC6, but not a catalytically inactive H803A mutant, together with CBP was sufficient to deacetylate cytoplasmic TDP-43 under normal cellular conditions (Fig. 5a, lanes 2-4 and Supplementary Fig. 12). Moreover, co-immunoprecipitation experiments revealed an interaction between cytoplasmic TDP-43 and HDAC6 that was enhanced upon exposure to arsenite stress, but not a less potent oxidative stress induced by hydrogen peroxide (Fig. 5b and Supplementary Fig. 13). The arsenite-induced TDP-43-HDAC6 interaction was rather unexpected since HDAC6 recruitment during arsenite exposure was not sufficient to promote TDP-43 deacetylation (see Fig. 4c, d), suggesting an inability of cytoplasmic TDP-43 aggregates to become efficiently deacetylated by HDAC6. Confirming HDAC6 recruitment to TDP-43 aggregates, both acetylation-mimic (TDP-43-ΔNLS-K145Q) and arsenite-induced acetylated TDP-43 aggregates physically interacted with HDAC6 by co-immunoprecipitation assays (Fig. 5c, lanes 2-4 and Supplementary Fig. 13) and also co-localized with ectopically expressed or endogenous HDAC6 protein (Fig. 5d). Notably, cytoplasmic TDP-43 aggregates also recruited and activated the CHIP/Hsp70 complex (Fig. 5e and Supplementary Fig. 14 and 15), previously implicated together with HDAC6 in the targeted clearance of the microtubule-associated tau protein48, 59, 60.
Figure 5. The deacetylase HDAC6 binds and deacetylates TDP-43 in a stress-regulated manner.
a) Cells were co-transfected with TDP-43-ΔNLS, CBP, and either wild-type HDAC6 or the catalytically dead HDAC6-H611A mutant and TDP-43 acetylation was determined by immunoblotting with acetyl-lysine antibodies similar to Figure 1c. b) Cells transfected with myc-tagged TDP-43-ΔNLS and FLAG-tagged HDAC6 were analyzed by co-immunoprecipitation reactions under basal or stimulated conditions in the presence of 0.1 mM arsenite or 1 mM peroxide stress for 2 hr. Input, representing 5% of total lysate volume, was analyzed by immunoblotting using the indicated antibodies. c) Co-immunoprecipitation analysis using either TDP-43-ΔNLS or acetylation mimic mutants (TDP-43-K145Q or TDP-43-ΔNLS-K145Q) and wild-type HDAC6 were performed in the absence or presence of arsenite, and immunoprecipitated proteins were analyzed by western blotting with the indicated antibodies similar to b above. d) Immunofluorescence microscopy was performed on cells expressing myc-tagged TDP-43-ΔNLS, acetylation mimic TDP-43-ΔNLS-K145Q, and HDAC6-FLAG where indicated, using anti-FLAG or endogenous HDAC6 antibodies. TDP-43 acetylation-mimic mutations or acute treatment with arsenite led to the formation of cytoplasmic aggregates that co-localized with HDAC6. The scale bar represents 25 µm. e) Protein levels of the HDAC6/CHIP/Hsp70 complex were determined by western analysis of soluble and insoluble fractions using HDAC6, CHIP, Hsp70, and Hsp40 specific antibodies. Note, CHIP dimers (arrowhead) suggest activation of CHIP E3 ligase function, as previously reported68. f) Depletion of HDAC6 was performed using a specific HDAC6 siRNA followed by arsenite treatment, and solubility of TDP-43-ΔNLS and TDP-43-ΔNLS-2KR proteins were analyzed by immunoblotting with total and phosphorylated TDP-43 antibodies. HDAC6 siRNA knock-down efficiency was validated by assessing total levels of HDAC6 and its major substrate acetylated tubulin.
These results suggested that HDAC6-mediated deacetylation of TDP-43 may represent a homeostatic response to alleviate the burden of TDP-43 aggregates. To address this possibility, we analyzed arsenite-induced aggregation of cytoplasmic TDP-43 in the absence of HDAC6 using siRNA-mediated gene silencing. HDAC6 siRNA led to an increase in arsenite-stimulated TDP-43-ΔNLS aggregation, which was reflected by an increase in both total TDP-43 as well as phosphorylated TDP-43 recovered in insoluble fractions (Fig. 5f, compare lanes 2-3 to lanes 5-6 and Supplementary Fig. 16). Importantly, the effect of HDAC6 on insoluble TDP-43 accumulation was dependent on Lys-145 and Lys-192 acetylation sites, as loss of HDAC6 did not promote comparable aggregation of a TDP-43-ΔNLS-2KR mutant lacking those critical lysine residues (Fig. 5f, compare lanes 8-9 to lanes 11-12). Taken together, these data suggest that although HDAC6 can deacetylate TDP-43 under basal conditions, prolonged exposure to oxidative stress promotes the formation of TDP-43 aggregates that are not effectively deacetylated and thereby accumulate as mature aggregated TDP-43 species.
Acetylated TDP-43 inclusions are linked to ALS
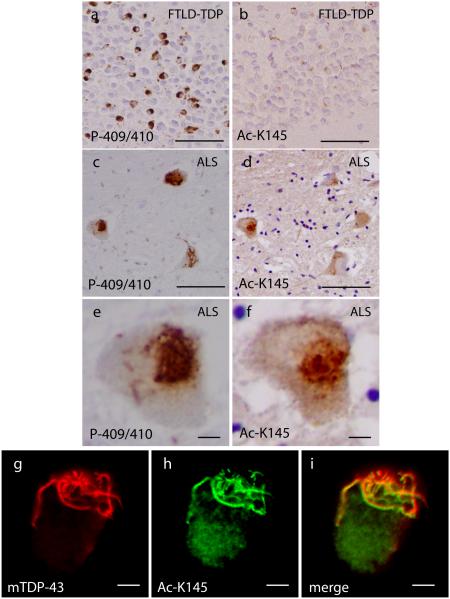
To investigate TDP-43 acetylation in human TDP-43 proteinopathies, immunohistochemistry (IHC) was performed on human brain and spinal cord tissues from subjects with frontotemporal dementia with TDP-43 pathology (FTLD-TDP) and ALS (see Table 1 for case demographics), both of which are characterized by robust TDP-43 pathology. As expected, FTLD-TDP brain contained strongly P-409/410-immunoreactive TDP-43 inclusions (Fig. 6a), which were not readily detectable with Ac-K145 antibody (Fig. 6b and Table 1), indicating a lack of TDP-43 acetylation at Lys-145 in FTLD-TDP. However, given that pathological TDP-43 species in FTLD-TDP were shown to consist predominantly of C-terminal truncated fragments beginning with residue Arg-20840, we speculated that TDP-43 fragmentation in FTLD-TDP brain would preclude the detection of TDP-43 acetylation on Lys-145 since this residue is not present in the cleaved pathological TDP-43 fragment.
| case # | diagnosis | Sex | Age at Death | PMI (hr) | P-409/410 pathology | Ac-K145 pathology |
|---|---|---|---|---|---|---|
| 1 | Normal | Female | 51 | 16 | − | − |
| 2 | Normal | Male | 90 | 6 | − | − |
| 3 | ALS | Male | 39 | 5 | +++ | ++ |
| 4 | ALS | Male | 56 | 11.5 | ++ | + |
| 5 | ALS | Male | 57 | 4 | +++ | ++ |
| 6 | ALS | Female | 55 | 6 | +++ | ++ |
| 7 | ALS | Female | 53 | 24 | +++ | ++ |
| 8 | ALS | Female | 81 | 10 | ++ | + |
| 9 | FTLD-TDP | Male | 54 | 18 | ++ | − |
| 10 | FTLD-TDP | Female | 63 | 17 | ++ | − |
| 11 | FTLD-TDP | Female | 75 | 13.5 | ++ | − |
Cases listed here were used for IHC analyses. Abbreviations: PMI = postmortem intervals. (−) no pathology; (+) rare occurrence of Ac-K145 or P-409/410 pathology; (++) moderate occurrence of Ac-K145 or P-409/410 pathology; (+++) more frequent occurrence of Ac-K145 or P-409/410 pathology
Figure 6. TDP-43 acetylation is a pathological feature of ALS but not FTLD-TDP.
a-f) Immunohistochemistry (IHC) was performed on cortical brain and spinal cord sections from frontotemporal dementia (FTLD-TDP) and ALS cases that harbor abundant TDP-43 pathology. Shown are representative images of TDP-43 inclusions detected with phosphorylated (P-409/410) and acetylated (Ac-K145) TDP-43 antibodies. FTLD-TDP inclusions showed characteristic phosphorylated TDP-43 immunoreactivity with P-409/410 (a), which was not detected with Ac-K145 (b). Serial ALS spinal cord sections analyzed by IHC identified TDP-43 inclusions that were immunoreactive with both P409/410 (c, e) and Ac-K145 antibodies (d, f), indicating acetylation of TDP-43 in ALS spinal cord lesions. See Table 1 for a summary of P-409/410 and Ac-K145 immunoreactivity and details of ALS cases used in this study. g-i) Double labeling immunofluorescence was performed on ALS spinal cord sections using monoclonal anti-TDP-43 (panel g, red) and Ac-K145 (panel h, green) antibodies. The merged image (panel i) highlights the co-localization observed between total TDP-43 and acetylated TDP-43. Scale bar represents 50 µm (panels a-d) and 10 µm (panels e-f and g-i).
To further examine Lys-145 acetylation in TDP-43 proteinopathies, we analyzed ALS spinal cord, which in contrast to FTLD-TDP, harbors significant TDP-43 pathology that consists predominantly of full-length TDP-43, as both N- and C-terminal TDP-43 antibodies are capable of detecting TDP-43 inclusions in ALS motor neurons28. Therefore, pathological TDP-43 species in ALS spinal cord should contain the targeted Lys-145 acetylation site. Using serial spinal cord sections from ALS patients, we detected robust P-409/410 immunoreactive inclusions (Fig. 6, panels c and e), and most of the phosphorylated TDP-43 lesions also showed significant skein-like Ac-K145 immunoreactivity (Fig. 6, panels d and f, and Table 1). To confirm that Ac-K145 detected TDP-43-positive inclusions in ALS spinal cord, double-labeling immunofluorescence revealed substantial co-localization of acetylated TDP-43 (Ac-K145) with total TDP-43, as detected with a human-specific monoclonal TDP-43 antibody (Fig. 6, panels g-i). These results reveal that acetylated TDP-43 is a pathological feature associated with full-length, phosphorylated TDP-43 lesions in ALS spinal cord but not FTLD-TDP brain and support acetylation-induced loss of TDP-43 function and aggregation as a potential pathogenic mechanism in ALS and possibly other TDP-43 proteinopathies.
Discussion
In this study, we have identified TDP-43 acetylation as a novel post-translational modification regulating TDP-43 functional and biochemical properties. Although WT nuclear TDP-43 is also subject to acetylation, we found increased cytoplasmic TDP-43 acetylation, possibly due to enhanced lysine accessibility or conformational differences between nuclear and cytoplasmic TDP-43. Given that TDP-43 primarily forms cytoplasmic inclusions in diseased brain and spinal cord, we speculate that increased acetylation in the cytoplasm could be responsible, in part, for inclusion formation in TDP-43 proteinopathies. Indeed, using an acetylation-specific antibody (Ac-K145), we detected acetylated TDP-43 pathology in ALS spinal cord, suggesting TDP-43 acetylation is linked to ALS pathogenesis (Fig. 6). Supporting a pathological role, acetylation at Lys-145 was sufficient to increase TDP-43 pathogenicity in cells, as determined by both nuclear and cytoplasmic TDP-43 aggregation, increased C-terminal TDP-43 phosphorylation, and loss of normal nuclear TDP-43 function. Thus, we propose that aberrant TDP-43 acetylation can act in a pathogenic manner to trigger aggregation and neurodegeneration (see Fig. 7 model). Future studies are required to determine whether TDP-43 acetylation is limited to ALS or whether this modification occurs in a range of other TDP-43 proteinopathies, potentially expanding the clinical and therapeutic relevance of this finding.
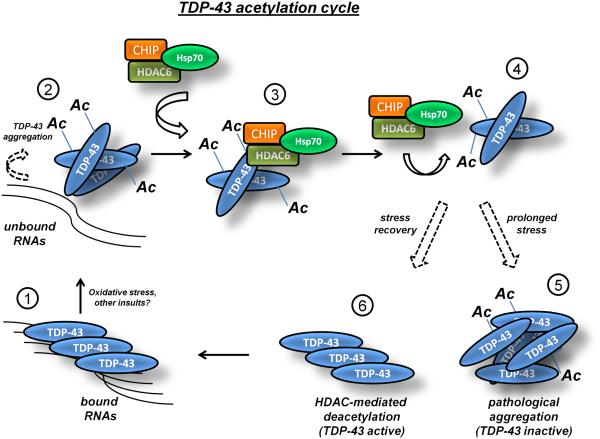
Figure 7. Model for reversible regulation of TDP-43 via lysine acetylation.
TDP-43 binds with high affinity to nucleic acids under normal cellular conditions (step 1). Oxidative stress and potentially other environmental or genetic insults promote cysteine disulfide cross-linking11 and lysine acetylation (this study), which abrogate binding to TDP-43 target RNAs leading to accumulation of unbound TDP-43 in the nucleus and cytoplasm (step 2). Free unbound TDP-43 is susceptible to lysine acetylation within the RNA-binding domain on Lys-145 and Lys-192, which prevents TDP-43 association with target RNAs and promotes TDP-43 aggregation most readily in the cytoplasmic compartment (Fig. 2). Acetylated TDP-43 aggregates are readily engaged by an HDAC6/CHIP/Hsp70 complex (step 3) that is capable of deacetylating TDP-43 under normal conditions (step 4), but not during sustained exposure to potent oxidative stimuli (step 5), the latter of which favors the formation of pathological full-length and acetylated TDP-43 lesions that are detectable in ALS spinal cord (Fig. 6). TDP-43 deacetylation conversely would result in engagement of TDP-43 mRNA targets, stabilization of TDP-43 conformation, and re-establishment of normal TDP-43 function as an active RNA regulatory protein (step 6, return to step 1).
To elucidate possible physiological consequences of acetylation as it relates to normal TDP-43 biology, we investigated nucleo-cytoplasmic shuttling, TDP-43 protein stability, and TDP-43 association with target RNAs. Immunofluorescence experiments demonstrated that acetylation did not significantly alter TDP-43 nucleo-cytoplasmic shuttling, as acetylated TDP-43 was retained in its respective subcellular compartments, albeit with altered morphology as discrete nuclear foci and cytoplasmic aggregates (Fig. 2a). Acetylation also did not significantly alter steady-state TDP-43 protein levels in our cell-based system, suggesting a minimal effect of acetylation on TDP-43 half-life. This is in contrast to acetylation-induced protein degradation observed with the Huntingtin (Htt) protein61. Finally, we evaluated whether acetylation altered association with target mRNAs since our mass spectrometry data identified acetylation on Lys-145 and Lys-192 within the RRM domains of TDP-43 (Fig. 1g and 1h). Indeed, our results suggest that a single acetylation event is sufficient to impair RNA binding and splicing function (Fig. 3), implicating Lys-145 as a critical determinant of TDP-43 function. These data are consistent with a recent study showing that non-specific chemical acetylation of Lys-145 in vitro using acetic anhydride occurred most efficiently in the absence of RNA, suggesting that RNA binding protects Lys-145 from acetylation46. Taken together, we conclude that TDP-43 acetylation within the RRMs is sufficient to impair normal RNA binding (Fig. 7, step 2), thereby providing a rapid and efficient regulatory switch to modulate TDP-43 association with downstream target RNAs.
Our previous study demonstrated that TDP-43 is subject to redox regulation, in which oxidative stress promotes TDP-43 cysteine oxidation and disulfide cross-linking, which prevents RNA binding and splicing function11. Since our current study showed that oxidative stress also promotes TDP-43 acetylation, it is conceivable that cysteine oxidation and lysine acetylation represent two critical modifications that coordinately regulate TDP-43 function under stress conditions, although the temporal sequence of these modifications still remains unclear. However, since redox sensing is generally a rapidly transduced signal, cysteine oxidation and partial loss of RNA binding could initially provide increased accessibility for subsequent lysine acetylation within the RRMs and would support a “multiple hit” model previously suggested for TDP-43 pathogenesis2, 11. Once disengaged from its targets, TDP-43 likely assumes an inherently unstable conformation that is highly aggregation-prone11, 62, 63, and therefore we hypothesized that acetylation alone would be sufficient to trigger loss of RNA binding and increased aggregation propensity. Indeed, acetylation conferred by the acetyltransferase CBP or acetylation-mimic mutations was sufficient to induce loss of RNA binding and the accumulation of insoluble and phosphorylated TDP-43 (Fig. 2). Future studies are required to elucidate the cross-talk among multiple TDP-43 modifications (cysteine oxidation, acetylation, phosphorylation, ubiquitination, and C-terminal cleavage) and their individual contributions to TDP-43 function and aggregation. Regardless of the detailed sequence of events, our results reveal that a complex post-translational signaling cascade is likely involved in TDP-43 pathogenesis.
Cellular defense mechanisms exist to re-fold or clear potentially toxic aggregated species, which, if defective, may contribute to the pathogenesis of ALS64, 65. We speculate that elevated deacetylase activity may act in this regard to reduce TDP-43 aggregation, and thereby maintain active TDP-43 that is essential for cell survival. Since a recent study identified a physical interaction between TDP-43, HDAC6, and Parkin in vivo that may regulate TDP-43 cytoplasmic accumulation58, we focused on HDAC6 as one potential regulator of TDP-43 acetylation and aggregation. Interestingly, while HDAC6 was sufficient to deacetylate soluble cytoplasmic TDP-43, we observed that cytoplasmic TDP-43 aggregates were unable to be efficiently deacetylated, possibly due to limited accessibility to lysines that are buried within the RRM-containing aggregates. Thus, an inability of HDAC6 and related quality control machinery to effectively deacetylate TDP-43 could lead to increased TDP-43 aggregation and contribute, in part, to disease pathogenesis similar to that proposed for abnormal tau acetylation in Alzheimer’s disease31, 33-35. In support of this pathogenic mechanism, loss of HDAC6 exacerbated TDP-43 aggregation and led to an increase in insoluble and phosphorylated TDP-43 (Fig. 5f). Conversely, we predict that future approaches to reduce levels of acetylated TDP-43, for example by pharmacologically targeting putative TDP-43 acetyltransferase(s), might allow increased RNA binding and enhancement of critical TDP-43 nuclear functions (Fig. 7, step 6). Overall, the identification of a reversible TDP-43 acetylation switch provides a novel framework to further understand both physiological and pathological TDP-43 functions, which could lead to targeted therapies against ALS and related TDP-43 proteinopathies.
Methods
Plasmids and Cell Culture
Human TDP-43 was cloned into pCDNA5/TO vector (Invitrogen) and site-directed mutagenesis (Quikchange kit; Stratagene) was used to create K→Q or K→R mutations at residues K145 and K192 as indicated. Creb-binding protein (CBP) plasmids as well as WT-HDAC6 and HDAC6-H803A enzyme-inactive mutant expression plasmids were kindly provided by Dr. Tso-Pang Yao (Duke University). Plasmids were transfected into QBI-293 cells (MP Biomedicals) using Fugene 6 (Roche) per manufacturers instructions. HDAC6 siRNA (Invitrogen) sequence was as follows: AAAGUUGGAACUCUCACGGUGCAGC and was transfected using RNAi Max reagent (Invitrogen) following manufacturer protocols. For oxidative stress treatments, confluent QBI-293 cells grown in 6-well or 10cm dishes were exposed to 50 µM sodium arsenite or 1 mM hydrogen peroxide for 1-12 hr, as indicated in text. Cells were harvested and analyzed by western blotting using the indicated antibodies, as detailed below. Mouse Neuro2A neuroblastoma cells (ATCC) were transfected using Fugene 6 with ΔNLS, ΔNLS-K145Q and ΔNLS-2KQ plasmids and differentiated by 24 hr withdrawal of fetal bovine serum (FBS), which promotes extension of neuritic processes, followed by subsequent western blotting and immunofluorescence studies.
Splicing Assay
TDP-43 functional activity was assessed using a tandem transfection CFTR splicing assay. Either control (pcDNA-5TO vector), TDP-43 shRNA construct (Origene), wild-type (WT) TDP-43, TDP-K145Q, or TDP-4FL mutants were transiently transfected into QBI-293 cells using Lipofectamine 2000 reagent (Invitrogen) following the standard manufacturer's protocols. After 48 h, a hybrid minigene construct TG(13)T(5) (a generous gift from Dr F Baralle, International Centre for Genetic Engineering and Biotechnology, Trieste, Italy), which monitors CFTR exon 9 splicing was transiently transfected into the same cells. Twenty-four hours after minigene transfection, cells were harvested, and CFTR exon 9 inclusion versus exclusion in the presence of the indicated TDP-43 plasmids was then evaluated by RT–PCR from isolated total RNA prepared from cells 72 h after transfection of TDP-43 constructs and 24 h after transfection of the TG(13)T(5) CFTR minigene reporter construct. The RT-PCR primers were as follows: Bra2, TAGGATCCGGTCACCAGGAAGTTGGTTAAATCA; a2–3, CAACTTCAAGCTCCTAAGCCACTGC. PCR conditions were as follows: 95°C for 10 min (hot start), followed by 30 cycles of denaturing at 95°C for 30 s, annealing at 57°C for 30 s, and elongation at 72°C for 60 s. PCR products were visualized on a 1.5% agarose gel and Exon 9 splice products were quantified using the Agilent 2100 Bioanalyzer on a DNA 1000 chip and values were calculated as log ratios of spliced/unspliced products.
Cross-Linking Immunoprecipitation (CLIP) Assay
Cell-based cross-linking immunoprecipitation (CLIP) assay was performed similar to that described66, 67 with minor variations. Transfected QBI-293 cells grown in a 10-cm dish were UV irradiated twice consectively in 5ml cold PBS at 400 mJ/cm2. Cells were pelleted and stored at −80 until use. Cell pellets were lysed in NP-40 lysis buffer (50 mM Tris-HCL, pH 7.5; 150 mM NaCl, 0.5% NP-40, 50 mM NaF containing RNAsin (Promega) and incubated 10 min on ice followed by addition of RQ1 DNase (Promega, M6101; 30 U) to each tube and incubated at 37 °C for 5 min. RNase A (USB #70194Y) was added at 1:5000 dilution and incubated at 37° C for 5 min, followed by ultra-centrifugation with polycarbonate tubes at 30,000 rpm for 20 min at 4° C. The supernatant containing TDP-43 bound RNAs was mixed with protein A/G beads (Santa Cruz) and 5 µg anti-myc 9E10 antibody (Santa Cruz) and rotated 3-4 hr 4° C. Beads were washed as follows: two times with ice-cold NP-40 lysis buffer, two times buffer B [5× PBS (no Mg2+, no Ca2+), 0.5% NP-40] and two times buffer C (50 mM Tris–Cl, pH 7.4, 10 mM MgCl2, 0.5% NP-40). TDP-43 bound A/G beads were then treated with Calf-Intestinal Alkaline Phosphatase (NEB) in the presence of RNAsin according to the manufacturer’s protocol. Samples were washed once with Buffer C (see above), once with Buffer D (50 mM Tris-HCL, pH 7.5; 20 mM EGTA; 0.5% NP-40) and then twice with Buffer C. RNAs were end-labeled using [32P]-γ- ATP (Perkin Elmer, BLU002A) and T4 PNK enzyme (NEB, T4 polynucleotide kinase, M0201L) followed by bead washes: once NP-40 lysis buffer, twice Buffer B, and five times buffer C. Samples were loaded onto 10% Bis-Tris gels (Bio-Rad) using MOPS running buffer system, transferred onto nitrocellulose membranes and signals were quantified as pixel intensities using a TYPHOON phosphorimager system and normalized to WT TDP-43 bound RNAs.
Recombinant TDP-43 methods
TDP-43 recombinant proteins (WT, K145/192Q, K145/192R) were purified from BL-21 cells using the pCOLD vector expression system (Takara Bio Inc.). After protein induction, all TDP-43 proteins were present predominantly in insoluble fractions of bacterial lysates. To generate soluble purified TDP-43 protein, bacterial pellets were washed extensively 5X in RIPA buffer to remove soluble proteins, and insoluble pellets containing TDP-43 proteins were subsequently extracted repeatedly in 0.1% SDS, 0.1M MOPS pH 7.5 and TDP-43 solubility was monitored until total protein was solubilized. Re-solubilized TDP-43 proteins were estimated to be ~95% pure based on visualization by Coomassie staining. Protein stocks were maintained at high concentration (10 mg/ml) in 0.1% SDS, 0.1 M MOPS pH 7.5 to prevent loss of TDP-43 solubility. We note that TDP-2KQ mutant protein required repetitive extractions in 0.1% SDS, 0.1 M MOPS pH 7.5 to re-solubilize the protein, likely indicating that impaired binding to nucleic acids reduces TDP-43 solubility.
Peptide/Antibody generation
Polyclonal anti-acetyl TDP-43 (K145) antibodies were generated using the acetylated TDP-43 peptide GHSKGFG-C containing K145 acetylation to immunize rabbits (Pocono Rabbit Farm and Laboratory Inc., Canadensis, PA). Double affinity purification was performed using native and acetylated peptides sequentially using Sulfolink columns (Pierce Biotechnology). Site specificity of Ac-K145 was confirmed in vitro by western blot analysis of purified WT and lysine mutant proteins (see above) and by peptide ELISA assays using acetylated and unmodified peptides (Supplementary Figure 10).
Biochemical methods and co-immunoprecipitation analysis
Fractionation of all cell lysates was performed by sequential extraction using buffers of increasing strength. Cells from 6-well culture dishes were scraped into 300 ml RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1%NP-40, 5 mM EDTA, 0.5% sodium deoxycholate, 0.1%SDS) containing 1 mM phenylmethylsulfonyl fluoride, a mixture of protease inhibitors (1 mg/ml pepstatin, leuptin, N-p-Tosyl-l-phenylalanine chloromethyl ketone, Nα-Tosyl-l-lysine chloromethyl ketone hydrochloride, trypsin inhibitor; Sigma), and a mixture of phosphatase inhibitors (2 mm imidazole, 1 mm NaF, 1 mm sodium orthovanadate; Sigma). Samples were sonicated and centrifuged at 100,000xg for 30 min at 4°C, and then re-extracted in RIPA buffer to ensure complete removal of soluble proteins. Resultant insoluble pellets were extracted in urea buffer (7 M urea, 2 M Thiourea, 4%CHAPS, 30 mM Tris, pH 8.5), sonicated and centrifuged at 100,000xg for 30 min at room temperature. Soluble and insoluble fractions were analyzed by western blotting using the indicated antibodies. Antibodies for western analysis were as follows: rabbit polyclonal anti-TDP-43 (Proteintech) 1:1000, mouse monoclonal antibody anti-TDP-43 (Proteintech) 1:1000, rabbit polyclonal anti-TDP-43 C-terminal 1038 1:10,000, rabbit polyclonal anti-TDP-43 N-terminal 1065 1:10,000, anti-GAPDH (6C5 mouse mAb; Advanced ImmunoChemical Inc.) 1:3000, anti-acetylated tubulin (Sigma) 1:1000, anti-α-tubulin (Sigma) 1:1000, anti-HDAC6 (H-300; Santa Cruz) 1:1000, anti-acetyl-lysine (1:1000, Cell Signaling), polyclonal anti-CHIP (1:1000, Sigma). For insoluble Ac-K145 immunoprecipitation reactions, urea solubilized fractions were diluted 10-fold in Triton buffer (1% Triton-X-100, 40 mM Tris pH 8.0) prior to immunopreciptation reactions using 10 µg Ac-K145 antibody conjugated to protein A/G beads, followed by extensive washes and western blotting using monoclonal TDP-43 antibody (Proteintech). For co-immunoprecipitation studies, QBI-293 cells were lysed in NP-40 lysis buffer referred to as NETN buffer (50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM PMSF, 0.2 mM leupeptin, protease inhibitor cocktail). Soluble supernatants (1mg total protein) were cleared by centrifugation, incubated overnight with protein A/G beads (Santa Cruz) complexed to the indicated antibodies described in the figure legends, and subsequently analyzed by SDS-PAGE and immunoblotting.
Immunoprecipitation and mass spectrometry analysis
For cell-based mass spectrometry (MS) analysis, 10-cm dishes of QBI-293 cells were transiently transfected with TDP-43-ΔNLS and CBP plasmids. Total lysates were prepared and TDP-43 was immunoprecipitated from these fractions using anti-TDP-43 (clone 171) complexed to protein A/G beads (Sigma). Immunoprecipitated samples were analyzed by SDS-PAGE electrophoresis in the presence of reducing agents and analyzed by Coomassie staining and gel excision followed by nanoLC/nanospray/MS/MS. The analysis was performed using the University of Pennsylvania proteomics core facility and data were acquired with Xcalibur 2.0 (ThermoFisher) and analyzed with PEAKS 6.0 (Bioinformatics Solutions Inc.) and Scaffold 3 (Proteome Software) software packages. Cutoffs: Peptide p-value: <95%; Protein p-value <99.0%.
Immunocytochemistry and quantification
Cultured cells were fixed in 4% paraformaldehyde (PFA) for 10 min, rinsed 3X in PBS, and permeabilized with 0.2% Triton X-100 (Sigma) in PBS for 10 min. Cells were then blocked in 5% milk for 1 hr and subsequently incubated with primary antibodies of interest overnight at 4°C following standard immunofluorescence techniques. Cell-based nuclear stippling and cytoplasmic aggregation of lysine mutants was performed by immunofluorescence microscopy (40X magnification) using Olympus BX 51 microscope. Stippling and aggregation phenotypes were quantified as a percent of transfected cells using >10 fields and sampling error was calculated using the standard error of the mean (SEM). Statistical analysis was determined using a two-tailed unpaired t-test with unequal variance (significance set at p-values < .05). All quantitative fluorescence was independently validated with a minimum of N=3 biological replicates. Primary antibodies used for immunofluorescence were as follows: polyclonal anti-TDP-43 (Proteintech, 1:500), polyclonal anti-myc (1:4000, Sigma), monoclonal anti-myc 9E10 (Sigma, 1:1000), monoclonal anti-Hsp70 (Stressgen, 1:500).
Immunohistochemistry and double-labeling
For human studies, fixed, paraffin-embedded tissue blocks were obtained from the Center for Neurodegenerative Disease Research Brain Bank at the University of Pennsylvania. Consent for autopsy was obtained from legal representatives for all subjects in accordance with local institutional review board requirements. Briefly, fresh tissues from each brain were fixed with 70% ethanol in 150 mM NaCl, infiltrated with paraffin, and cut into 6-µm serial sections. Immunohistochemistry (IHC) was performed using the avidin-biotin complex (ABC) detection system (Vector Laboratories, Burlingame, CA) and 3,3'-diaminobenzidine. Briefly, sections were deparaffinized and rehydrated, antigen retrieval was done by incubating section in 88% formic acid, endogenous peroxidases were quenched with 5% H2O2 in methanol for 30 minutes, and sections were blocked in 0.1 mol/L Tris with 2% fetal bovine serum for 5 min. Primary antibodies were incubated for either 1 or 2 hrs at room temperature or overnight at 4°C. The following primary antibodies were used for IHC analysis: AcK145 (this study, 1:500), monoclonal TDP-43 (proteintech, 1:1000), P-409/410 (1:500). After washing, sections were sequentially incubated with biotinylated secondary antibodies for 1 hr and avidin-biotin complex for 1 hr. Bound antibody complexes were visualized by incubating sections in a solution containing 100 mM TrisHCl, pH 7.6, 0.1% Triton X-100, 1.4 mM diaminobenzidine, 10 mM imidazole, and 8.8 mM H2O2. Sections were then lightly counterstained with hematoxylin, dehydrated, and coverslipped. We note that IHC analysis of human tissue using Ac-K145 requires antigen retrieval (formic acid) for maximal detection sensitivity of TDP-43 inclusions and displays subtle cross-reactivity with nuclear proteins. Double-labeling Immunofluorescence- Double-labeling immunofluorescence (IF) analyses were performed using Alexa Fluor 488- and 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR). The sections were then treated to remove autofluorescence with Sudan Black solution for 5 min, and coverslipped with Vectashield mounting medium (Vector Laboratories). Digital images were obtained using an Olympus BX 51 microscope (Tokyo, Japan) equipped with bright-field and fluorescence light sources using a ProgRes C14 digital camera (Jenoptik AG, Jena, Germany) and Adobe Photoshop, version 9.0 (Adobe Systems, San Jose, CA) or digital camera DP71 (Olympus) and DP manager (Olympus).
Supplementary Material
Acknowledgments
Support for this work was provided by grants from the NIH/NIA/NINDS including P30-AG10124, PO1-AG17586, PO1-AG032953, K99/R00-NS080985 (TJC), the Department of Defense (DOD), W81XWH-11-1-0757, and the Association for Frontotemporal Degeneration (AFTD) (TJC). The Proteomics Core Facility at University of Pennsylvania is supported by P30CA016520 (Abramson Cancer Center), and ES013508-04 (CEET). We thank Dr. Kurt Brunden for critical reading of this manuscript and Dr. Scott Pesiridis for assistance with TDP-43 structure analysis. We would also like to thank the members of the Center for Neurodegenerative Disease Research, University of Pennsylvania, Philadelphia, PA, who contributed to the work, and the many patients studied and their families for making the research reviewed here possible.
Footnotes
Competing Financial Interests:
The authors declare no competing financial interests.
References
- 1.Cohen TJ, Lee VM, Trojanowski JQ. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med. 2011;17:659–667. doi: 10.1016/j.molmed.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pesiridis GS, Lee VM, Trojanowski JQ. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Human molecular genetics. 2009;18:R156–162. doi: 10.1093/hmg/ddp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling SC, et al. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polymenidou M, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nature neuroscience. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tollervey JR, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nature neuroscience. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sephton CF, et al. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. The Journal of biological chemistry. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abhyankar MM, Urekar C, Reddi PP. A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues: role for TDP-43 in insulator function. The Journal of biological chemistry. 2007;282:36143–36154. doi: 10.1074/jbc.M705811200. [DOI] [PubMed] [Google Scholar]
- 8.Acharya KK, Govind CK, Shore AN, Stoler MH, Reddi PP. cis-requirement for the maintenance of round spermatid-specific transcription. Developmental biology. 2006;295:781–790. doi: 10.1016/j.ydbio.2006.04.443. [DOI] [PubMed] [Google Scholar]
- 9.Ayala YM, Misteli T, Baralle FE. TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3785–3789. doi: 10.1073/pnas.0800546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang IF, Wu LS, Chang HY, Shen CK. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. Journal of neurochemistry. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen TJ, Hwang AW, Unger T, Trojanowski JQ, Lee VM. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 2012;31:1241–1252. doi: 10.1038/emboj.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombrita C, et al. TDP-43 is recruited to stress granules in conditions of oxidative insult. Journal of neurochemistry. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 13.Freibaum BD, Chitta RK, High AA, Taylor JP. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. Journal of proteome research. 2010;9:1104–1120. doi: 10.1021/pr901076y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu-Yesucevitz L, et al. ALS-linked mutations enlarge TDP-43-enriched neuronal RNA granules in the dendritic arbor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:4167–4174. doi: 10.1523/JNEUROSCI.2350-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neuro-Signals. 2008;16:41–51. doi: 10.1159/000109758. [DOI] [PubMed] [Google Scholar]
- 16.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 17.Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–649. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabashi E, et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Human molecular genetics. 2009;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- 19.Igaz LM, et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. The Journal of clinical investigation. 2010;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai KJ, et al. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. The Journal of experimental medicine. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wils H, et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. The Journal of biological chemistry. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choksi DK, et al. TDP-43 Phosphorylation by casein kinase Iepsilon promotes oligomerization and enhances toxicity in vivo. Human molecular genetics. 2014;23:1025–1035. doi: 10.1093/hmg/ddt498. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa M, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Annals of neurology. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kametani F, et al. Identification of casein kinase-1 phosphorylation sites on TDP-43. Biochemical and biophysical research communications. 2009;382:405–409. doi: 10.1016/j.bbrc.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 27.Liachko NF, McMillan PJ, Guthrie CR, Bird TD, Leverenz JB, Kraemer BC. CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration. Annals of neurology. 2013;74:39–52. doi: 10.1002/ana.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann M, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta neuropathologica. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. Journal of neurochemistry. 2011;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- 30.Li HY, Yeh PA, Chiu HC, Tang CY, Tu BP. Hyperphosphorylation as a defense mechanism to reduce TDP-43 aggregation. PloS one. 2011;6:e23075. doi: 10.1371/journal.pone.0023075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen TJ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook C, et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Human molecular genetics. 2014;23:104–116. doi: 10.1093/hmg/ddt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin DJ, et al. Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am J Pathol. 2013;183:344–351. doi: 10.1016/j.ajpath.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin DJ, et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain. 2012;135:807–818. doi: 10.1093/brain/aws013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min SW, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 37.Kuo PH, Chiang CH, Wang YT, Doudeva LG, Yuan HS. The crystal structure of TDP-43 RRM1-DNA complex reveals the specific recognition for UG- and TG-rich nucleic acids. Nucleic acids research. 2014;42:4712–4722. doi: 10.1093/nar/gkt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo PH, Doudeva LG, Wang YT, Shen CK, Yuan HS. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic acids research. 2009;37:1799–1808. doi: 10.1093/nar/gkp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. The Journal of biological chemistry. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 40.Igaz LM, et al. Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies. The Journal of biological chemistry. 2009;284:8516–8524. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayala YM, et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie IR, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Annals of neurology. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 43.Brettschneider J, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of neurology. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhardwaj A, Myers MP, Buratti E, Baralle FE. Characterizing TDP-43 interaction with its RNA targets. Nucleic acids research. 2013;41:5062–5074. doi: 10.1093/nar/gkt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukavsky PJ, et al. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat Struct Mol Biol. 2013;20:1443–1449. doi: 10.1038/nsmb.2698. [DOI] [PubMed] [Google Scholar]
- 48.Cook C, et al. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Human molecular genetics. 2012;21:2936–2945. doi: 10.1093/hmg/dds125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dompierre JP, et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govindarajan N, et al. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease. EMBO Mol Med. 2013;5:52–63. doi: 10.1002/emmm.201201923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. The Journal of cell biology. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G, Jiang H, Chang M, Xie H, Hu L. HDAC6 alpha-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases. J Neurol Sci. 2011;304:1–8. doi: 10.1016/j.jns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Miki Y, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology. 2011;31:561–568. doi: 10.1111/j.1440-1789.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 55.Odagiri S, et al. Brain expression level and activity of HDAC6 protein in neurodegenerative dementia. Biochemical and biophysical research communications. 2013;430:394–399. doi: 10.1016/j.bbrc.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 56.Pandey UB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 57.Quinti L, et al. Evaluation of histone deacetylases as drug targets in Huntington's disease models. Study of HDACs in brain tissues from R6/2 and CAG140 knock-in HD mouse models and human patients and in a neuronal HD cell model. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hebron ML, et al. Parkin ubiquitinates Tar-DNA binding protein-43 (TDP-43) and promotes its cytosolic accumulation via interaction with histone deacetylase 6 (HDAC6) The Journal of biological chemistry. 2013;288:4103–4115. doi: 10.1074/jbc.M112.419945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. The Journal of clinical investigation. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrucelli L, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Human molecular genetics. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 61.Jeong H, et al. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang YC, et al. Inhibition of TDP-43 aggregation by nucleic acid binding. PloS one. 2013;8:e64002. doi: 10.1371/journal.pone.0064002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. The Journal of biological chemistry. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssens J, Van Broeckhoven C. Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Human molecular genetics. 2013;22:R77–87. doi: 10.1093/hmg/ddt349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Human molecular genetics. 2010;19:R46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daigle JG, et al. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Human molecular genetics. 2013;22:1193–1205. doi: 10.1093/hmg/dds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ule J, Jensen K, Mele A, Darnell RB. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37:376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 68.Nikolay R, Wiederkehr T, Rist W, Kramer G, Mayer MP, Bukau B. Dimerization of the human E3 ligase CHIP via a coiled-coil domain is essential for its activity. The Journal of biological chemistry. 2004;279:2673–2678. doi: 10.1074/jbc.M311112200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.