Abstract
BACKGROUND
The first recommended step when an older woman is diagnosed with breast cancer is to determine life expectancy, but existing strategies to determine life expectancy are ill-suited for older women with breast cancer.
DESIGN
Prospective longitudinal study with 10 years of follow-up data.
SETTING
Hospitals or collaborating tumor registries in four geographic regions (Los Angeles, California; Minnesota; North Carolina; Rhode Island)
PARTICIPANTS
Women at least age 65 at time of breast cancer diagnosis with stage I-IIIA disease with self-rated health (SRH) and walking ability at baseline (N=615)
MEASUREMENTS
Baseline self-rated health, baseline self-reported walking ability, all-cause and breast cancer-specific estimated probability of survival at 5 and 10 years
RESULTS
Six hundred fifteen women with breast cancer were studied (17% age 80+, 52% stage I, 58% with ≥2 comorbidities). At the time of breast cancer diagnosis, 39% of women reported poor SRH, and 28% reported limited ability to walk several blocks. The all-cause survival curves appear to separate after about 3 years, and the difference in survival probability between those with low SRH combined with limited walking ability compared to those with high SRH combined with no walking ability limitation was significant (0.708 vs. 0.855 at five years, p≤0.001; 0.300 vs. 0.648 at 10 years, p <0.001). There were no differences across groups in breast cancer-specific survival at 5 and 10 years (p=0.663 at 5 years, p=0.156 at 10 years).
CONCLUSION
The combination of low self-rated health and limited ability to walk several blocks at diagnosis is an important predictor of worse all-cause survival at 5 and 10 years. These easily assessed self-report measures in clinical practice may represent an effective strategy to improve treatment decision making in older adults with cancer.
Keywords: breast cancer, physical function, self-related health
INTRODUCTION
One of the first recommended steps when an older woman is diagnosed with breast cancer is to determine life expectancy.1 Knowing life expectancy can help balance risks and benefits of treatment and maximize quality of life.2, 3 However, few strategies are available to predict accurately future life expectancy to guide clinical decision-making. One strategy, prognostic indices, is based on general adult populations and do not distinguish past treated cancers from recently diagnosed cancers in their life expectancy estimates.4, 5 Another strategy, average breast cancer survival rates, is based on age and tumor characteristics,6 but older women with breast cancer are a heterogeneous population with respect to physical function and health. Therefore, measures other than chronologic age and tumor characteristics contribute importantly to predicting survival in this population.2
Two strong predictors of survival in general populations of older adults are self-rated health (SRH) and walking ability.4, 7, 8 SRH is often assessed by a single question that asks patients to rate their overall health on a scale from “excellent” to “poor”. Patients who rank their health as “poor” have 5-year mortality rates that are 7 times higher than the rates of patients who rank their health as “excellent”.9 In women with breast cancer, studies of SRH and mortality in women younger than 65 have shown results specific to breast cancer stages.10, 11 The other strong predictor of survival, walking ability, is essential to maintaining the independence of community-dwelling older adults. Older adults who report the inability to walk a quarter mile have one-year mortality rates eight times higher than the mortality rates of those who report no difficult walking the same distance.7 Despite the strong association between physical function and survival, there is limited information on self-reported walking ability and survival in patients with cancer.
We therefore sought to determine whether SRH and walking ability could predict 5- and 10-year survival in older women with early stage breast cancer. We analyzed data from a prospective longitudinal study to determine if these two measures could aid in clinical-decision making in this population.
METHODS
Study sample
The longitudinal study design and subject recruitment procedures have been previously reported.12 Six hundred and sixty women ≥65 years old with stage I tumor diameter ≥1 cm or stage II-IIIA disease and permission from attending physician to be contacted in four geographic regions (Los Angeles, California; Minnesota; North Carolina; Rhode Island) were identified through regular pathology report review at hospitals or collaborating tumor registries. Women could not have a prior primary breast cancer or simultaneously diagnosed or treated second primary tumor. Women signed a consent form approved by the institutional review board at each site.
For this secondary data analysis, subjects were excluded if they did not have data in the primary variables of interest: SRH or ability to walk several blocks at baseline.
Measures
Data were collected by medical record review (definitive surgery date, surgery type and tumor characteristics) and telephone interview (socio-demographic, psychosocial, health and breast cancer therapy) between 3 and 5 months after surgery.
All-cause and breast cancer-specific survival
Decedents were identified by first and last name, middle initial, social security number, date of birth, sex, race, marital status, and state of residence matched against National Death Index (NDI) and Social Security Death Index (SSDI) records. All-cause survival time was in years from date of definitive surgery until date of death. Subjects not found in the NDI or SSDI were censored at the last follow-up. Breast cancer-specific survival time was censored on DOD from another cause or at end of follow-up, whichever came first.
Self-rated health (SRH)
SRH before diagnosis was assessed by a single item from the Medical Outcomes Study-Short Form-36 (MOS SF-36).13 The item categorized SRH on a 5-point scale: “In general, would you say your health right before your breast cancer was diagnosed was excellent, very good, good, fair, or poor?” Five-and 10-year survival were compared across the 5 levels of SRH, and based on a step-off in 5- and 10-year survival between “very good” and “good”, this measure was dichotomized to high SRH (excellent/very good) and low SRH (good/fair/poor) to improve efficiency in the analysis.
Walking ability
Walking ability before diagnosis was assessed by a single questionfrom the Physical Function Index (PFI)-10, a subset of the MOS SF-36.14Subjects were asked at baseline interview, “Right before your breast cancer was diagnosed, did your health limit you in walking several blocks?” Subjects could answer “Yes, I was limited a lot”; “Yes, I was limited a little”; or “No, I was not limited at all.” The first two answer groups were combined given the small number of responses of “Yes, I was a limited a little” and a previous study showing subjects with cancer tend to be optimistic when answering questions about pre-cancer diagnosis function.15 Thus, baseline walking ability was a two-level variable: “limited” or “not limited”.
Combination measure
In order to examine all possible combinations of the individual dichotomous variables,SRH and walking ability at baseline were combined into a 4-level variable: high SRH and walking ability not limited, high SRH and walking ability limited, low SRH and walking ability not limited, or low SRH and walking ability limited.
Socio-demographic characteristics
We classified patient age as 65-69, 70-79, ≥80 years; race as white and nonwhite; education as <12 years, 12 years, >12 years; marital status as married (yes/no); and having adequate finances to meet needs (yes/no). Social support was measured using a reduced set of eight-items derived from the 19-item Medical Outcomes Study Social Support Scale (MOS-SSS) scored from 0 to 100 with higher scores indicating more support and ≥80 considered good social support.16, 17
Health status characteristics
Comorbid conditions were defined from the 14 medical conditions in the Index of Co-existent Disease (ICED)18, 19; comorbid conditions were divided into three groups based on the number of conditions (0 to 1, 2 to 3, or ≥4). Body mass index (BMI) was divided into obese (>30 kg/m2) and not obese (<30 kg/m2).
Emotional health was assessed by the 5-question Mental Health Inventory (MHI5), a general measure of emotional health from the MOS SF-36.14The MHI5 is a measure of emotional health that correlates strongly with standardized measures of anxiety and depression. MHI5 score was based on answers for each of the five items and then scaled from 0 to 100. An MHI5 score ≥ 80 was considered good emotional health.13
Cancer-specific characteristics
Tumor stage was categorized using the TNM classification,20 and ER status was classified as positive or negative. Initial therapy was classified as “definitive” [either mastectomy with axillary node dissection (AND) or breast-conserving surgery with AND followed by radiation therapy] or “other” based on recommended breast cancer treatment guidelines at the time of study enrollment.21 The receipt of adjuvant chemotherapy as part of initial therapy was defined by “yes” or “no”.
Statistical Analysis
All statistical testing was performed with a significance value of 0.05 (α = 0.05) unless otherwise specified, and all statistical analysis was performed using SAS 9.1 (Cary, NC). We examined descriptive statistics on all study variables across SRH, walking ability, and the 4-level combination variable using chi-square test. Five- and 10-year survival was analyzed using the Kaplan-Meier survival functions stratified by the combination of SRH and walking ability. The five- and 10-year Kaplan-Meier estimated probabilities of survival are reported for each stratum. The overall model was tested using the log-rank test, and the likelihood ratio test was used to compare survival between strata.
RESULTS
A total of 660 women were enrolled in the original study. For this secondary analysis, 45 subjects were excluded because they did not have baseline measures of SRH or walking ability. Women who were excluded, compared to those included, had a higher proportion who were older; in addition, those excluded had a higher proportion of women with more comorbidities, higher stage cancer, and poorer emotional health compared to those included. There was no difference between those excluded and those included on race, marital status, adequate finances, ER receptor status, type of surgery, receipt of chemotherapy, or social support.
Six hundred fifteen women were followed for up to 10 years after their initial surgery. Approximately one-fourth of the population came from each of the four study sites. Most were age 70 years or older (73%), were white (94%), had a high school education or greater (84%), and had adequate finances to meet their needs (91%). The majority of subjects (58%) had at least two comorbid conditions. About half of the women had stage I disease, and the majority (82%) received definitive initial therapy (Table 1).
Table 1.
Baseline characteristics for all subjects and across four-level combinations of SRH and walking ability
| Baseline Characteristic | All subjects (N=615) n (%) |
High SRH, walking ability not limited (N=316) |
Low SRH, walking ability not limited (N=127) |
High SRH, walking ability limited (N=58) |
Low SRH, walking ability limited (N=114) |
p value | |
|---|---|---|---|---|---|---|---|
| Socio-demographic characteristics | |||||||
| Age, years | 65-69 | 167 (27) | 98 (31) | 37 (29) | 13 (22) | 19 (17) | 0.027 |
| 70-79 | 343 (56) | 175 (55) | 67 (53) | 30 (52) | 71 (62) | ||
| 80+ | 105 (17) | 43 (14) | 23 (18) | 15 (26) | 24 (21) | ||
| Race | White | 579 (94) | 306 (97) | 114 (90) | 55 (95) | 104 (91) | 0.016 |
| Nonwhite | 36 (6) | 10 (3) | 13 (10) | 3 (5) | 10 (9) | ||
| Marital status | Married | 290 (47) | 159 (50) | 59 (46) | 26 (45) | 46 (40) | 0.316 |
| Not married |
325 (53) | 157 (50) | 68 (54) | 32 (55) | 68 (60) | ||
| Education level |
< 12 years |
101 (16) | 43 (14) | 26 (21) | 8 (14) | 24 (21) | 0.195 |
| 12 years | 211 (34) | 104 (33) | 48 (38) | 20 (34) | 39 (34) | ||
| >12 years | 302 (49) | 169 (54) | 52 (41) | 30 (52) | 51 (45) | ||
| Adequate Finances |
Yes | 551 (91) | 299 (95) | 106 (84) | 55 (95) | 91 (83) | <0.001 |
| No | 57 (9) | 15 (5) | 20 (16) | 3 (5) | 19 (17) | ||
| Social support score |
Good | 314 (51) | 190 (61) | 46 (36) | 35 (60) | 43 (38) | <0.001 |
| Poor | 297 (49) | 123 (39) | 81 (64) | 23 (40) | 70 (62) | ||
| Health status characteristics | |||||||
| Comorbid conditions |
0-1 | 256 (42) | 167 (53) | 47 (37) | 22 (39) | 20 (18) | <0.001 |
| 2-3 | 248 (41) | 113 (36) | 58 (46) | 22 (39) | 55 (49) | ||
| ≥ 4 | 106 (17) | 34 (11) | 21(17) | 13 (23) | 38 (34) | ||
| BMI | ≥30 kg/m2 |
129 (21) | 268 (85) | 102 (80) | 41 (71) | 75 (66) | <0.001 |
| <30 kg/m2 |
486 (79) | 48 (15) | 25 (20) | 17 (29) | 39 (34) | ||
| Emotional health |
Good | 434 (71) | 251 (80) | 79 (62) | 45 (78) | 59 (52) | <0.001 |
| Poor | 180 (29) | 64 (20) | 48 (38) | 13 (22) | 55 (48) | ||
| Cancer-specific characteristics | |||||||
| Tumor Stage | Stage I | 321 (52) | 156 (49) | 73 (58) | 30 (52) | 62 (54) | 0.407 |
| Stage II- IIIa |
293 (48) | 160 (51) | 53 (42) | 28 (48) | 52 (46) | ||
| ER status | Positive | 457 (75) | 228 (73) | 98 (77) | 45 (80) | 86 (76) | 0.635 |
| Negative | 150 (25) | 83 (27) | 29 (23) | 11 (20) | 27 (24) | ||
| Adjuvant chemotherapy |
Yes | 137 (22) | 83 (26) | 30 (24) | 10 (17) | 14 (12) | 0.015 |
| No | 478 | 233 (74) | 97 (76) | 48 (83) | 100 (88) | ||
| Initial therapy | Definitive | 495 (82) | 265 (85) | 102 (83) | 44 (76) | 84 (74) | 0.011 |
| Other | 111 (18) | 46 (15) | 21 (17) | 14 (24) | 30 (26) | ||
Socio-demographic, health status, and cancer-specific characteristics across the four-level measure containing SRH and walking ability are in Table 1. The two groups containing women with limited walking ability had higher proportions of women aged 80+ compared to groups without limited walking ability. The two groups with low SRH had higher proportions of participants who were nonwhite, lacking adequate finances, had poor social support, and had poor emotional health compared to the groups with high SRH. The group with high SRH and not limited walking ability had the lowest proportion of women with at least 4 comorbid conditions (11%), while the group with low SRH and limited walking ability had the highest proportion (34%). Tumor stage and ER status both did not differ across groups, but the groups with limited walking ability had both lower rates of receiving adjuvant chemotherapy and lower rates of receiving definitive initial therapy compared to the groups without limited walking ability.
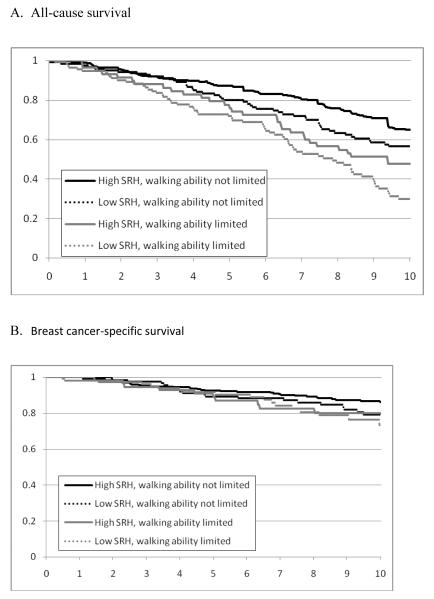
Figure 1 displays the Kaplan-Meier curves for both all-cause and breast cancer-specific survival over 5 and 10 years by the combination of SRH and walking ability, and Table 2 shows the survival at 5 and 10 years for all groups. Both groups with high SRH had higher all-cause survival at 5 and 10 years compared to the groups with low SRH, regardless of walking ability. Overall, the four-level variable was associated with both five-year (p=0.001) and ten-year all-cause survival (p<0.001). The survival curves appear to separate after about 3 years, and the difference in all-cause survival between those with low SRH and with limited walking ability compared to those with high SRH and no walking ability limitation was significant (0.708 vs. 0.855 at five years, p<0.001; 0.300 vs. 0.648 at 10 years, p <0.001). Breast cancer-specific survival did not differ among the groups (p=0.156).
Figure 1.
All-cause and breast cancer-specific estimated probability of survival over 10 years by self-rated health (SRH) and walking ability
Table 2.
All-cause and breast cancer-specific estimated probability of survival across the combination of self-rated health and walking ability
| Group | N | All-cause | Breast cancer-specific | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year survival* |
10-year survival** |
5-year survival† |
10-year survival†† |
||||||
|
Estimated
probability |
p-
value |
Estimated
probability |
p-
value |
Estimated
probability |
p-
value |
Estimated
probability |
p-
value |
||
| High SRH, walking ability not limited |
316 | 0.855 | Ref | 0.648 | Ref | 0.925 | Ref | 0.862 | Ref |
| Low SRH, walking ability not limited |
127 | 0.800 | 0.073 | 0.566 | 0.074 | 0.893 | 0.411 | 0.792 | 0.158 |
| High SRH, walking ability limited |
58 | 0.741 | 0.111 | 0.477 | 0.006 | 0.868 | 0.243 | 0.801 | 0.208 |
| Low SRH, walking ability limited |
114 | 0.708 | <0.001 | 0.300 | <0.001 | 0.904 | 0.580 | 0.732 | 0.039 |
5-year breast cancer-specific survival model p-value = 0.663
10-year breast cancer-specific survival model p-value = 0.164
5-year all-cause survival model p-value = 0.002
10-year all-cause survival model p-value <0.001
DISCUSSION
We examined the relations between two measures—SRH and walking ability—to survival in older women with early stage breast cancer. Low SRH and limited walking ability in combination were associated with lower five- and 10-year all-cause survival but not with breast cancer-specific survival. These findings have important implications for the creation of a parsimonious measure that predicts all-cause survival for the aging population with cancer.
Currently there are few resources to help clinicians determine life expectancy in older adults with cancer, which is more difficult than in younger adults with cancer due to higher competing risks. Determining life expectancy is the first recommended step for clinicians when deciding on treatment of older adults with cancer. Ascertaining an individual’s SRH and self-reported walking ability represents a simple method for clinicians to estimate all-cause survival in older women with breast cancer. SRH could capture aspects of health not measured by traditional variables, such as age and comorbidity. It could capture factors that are difficult to measure, including other disease in a prodromal or preclinical stage, a person’s health trajectory, and internal and external resources available.8Walking ability captures an essential physical function needed to maintain independence. This study supports the use of parsimonious tools that incorporate SRH and walking ability, such as the Vulnerable Elders Survey (VES-13).22-24
Other novel approaches such as comprehensive geriatric assessment or frailty indices also recognize the heterogeneity among older adults; however, neither approach has been widely implemented into clinical oncology practices. Comprehensive geriatric assessment (CGA) includes a thorough evaluation of functional status, comorbidties, cognition, nutritional status, psychological state, and social support. While CGA has been shown to predict mortality in older women with breast cancer, the feasibility of implementing this time- and resource-intensive evaluation is a major barrier to its dissemination into clinical practice.25 The term “frailty” characterizes a group of individuals who are at increased susceptibility to stress and poor outcomes, such as institutionalization or mortality. Multiple research criteria have been put forth to characterize “frailty”,26, 27 including one promising set of criteria specifically for oncology practice;28 however, no one definition has been widely accepted into clinical practice.29
The development of ePrognosis, an online tool that gathers multiple prognostic indices for older adults, is an important recent step in translating research into a useful and practical resource to help clinicians estimate patient prognosis.30 While ePrognosis can assist with estimating prognosis for general patient populations, more studies are needed to develop and validate a prediction tool for older adults with cancer. Research should focus on a prediction tool that has enough items to offer precision and reproducibility in older women with breast cancer, but few enough items to be acceptable for clinical practice.
While the study lacked the power to examine all four levels of the combination measure with respect to survival over 10 years, the association between the combination of SRH and walking ability and all-cause survival appeared to strengthen over time. Estimated probability of survival is not adjusted for other characteristics in this study becauseit better reflects a strategy that could be used in clinical oncology practice.A similar analysis in a larger group of older women with cancer would more definitively test the utility of this combination in predicting survival. Other future areas of study include comparing the prognostic ability of SRH and walking ability across age groups and comparing the ease of use and prognostic ability of SRH and walking ability with other predictors, such as comorbidity scales, frailty measures, or performance status.
The strength of this study is its focus on individual health status and physical function in a population of older women with early stage breast cancer. These measures are rarely collected in either clinical trials or observational studies involving older adults. Moreover, our study had 17 percent of women over age 80. Thus, this study is unique in its focus on health status and physical function in women who are truly older.
Nonetheless, this study had several limitations. Substantial selection occurred during recruitment for this study, resulting in a healthy, educated sample of primarily white women and limiting generalizability. It is possible that those with low SRH or poor physical function were less likely to be approved for participation by their physicians or less likely to agree to participate in the study. In addition, those with missing information excluded from this secondary analysis might have had lower SRH or poorer physical function compared to those included. This differential participation and exclusion would likely have biased the findings towards the null. Another limitation of this study is that the groups with limited walking ability had slightly lower rates of adjuvant chemotherapy or definitive initial therapy receipt compared to the groups without walking ability limitation; with these differences in treatment, one might expect differences in breast cancer-specific survival across groups, which we did not find. However, we did not have the power to detect small differences across groups for breast cancer-specific survival.
In conclusion, this study provides the first longitudinal evidence that the combination of SRH and walking limitation predict 5- and 10-year all-cause survival in older women with breast cancer. Further studies of the combination of SRH and physical function, perhaps with other strong predictors of mortality such as comorbid conditions, in diverse populations of older adults with cancer are warranted to find acceptable measures to aid clinicians in treatment decision-making.
ACKNOWLEDGMENTS
Early results from this paper were presented at the 2012 meeting of the American Geriatrics Society and the 2011 meeting of the International Society of Geriatric Oncology.
Funding sources: Data collection for the initial study was supported by the National Cancer Institute (R01 CA106979, R01 CA/AG 70818, R01 CA84506). Dr. Eng was supported by the John A. Hartford Foundation and the Department of Veterans Affairs Quality Scholars Program. Dr. Cabral was supported by the Boston University Clinical and Translational Science Institute (CTSI). Dr. Silliman was supported by the National Institutes for Health (K05 CA92395).
Sponsor’s Role: N/A
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors: conception, design, analyses, interpretation of data, drafting of the manuscript or revising it critically for important intellectual content.
REFERENCES
- 1.Hurria A, Browner IS, Cohen HJ, et al. Senior adult oncology. J Natl Compr Canc Netw. 2012;10:162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurria A. Communicating treatment options to older patients: Challenges and opportunities. J Natl Compr Canc Netw. 2012;10:1174–1176. doi: 10.6004/jnccn.2012.0121. [DOI] [PubMed] [Google Scholar]
- 3.Schonberg MA, Marcantonio ER, Ngo L, et al. Does life expectancy affect treatment of women aged 80 and older with early stage breast cancers? J Geriatr Oncol. 2012;3:8–16. doi: 10.1016/j.jgo.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 5.Schonberg MA, Davis RB, McCarthy EP, et al. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24:1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed May 5, 2013];SEER Cancer Statistics Review, 1975-2010. 2012 Nov; http://seer.cancer.gov/csr/1975_2010/
- 7.Hardy SE, Kang Y, Studenski SA, et al. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 9.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 10.Efficace F, Biganzoli L, Piccart M, et al. Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Cancer. 2004;40:1021–1030. doi: 10.1016/j.ejca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Efficace F, Therasse P, Piccart MJ, et al. Health-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: An international multicenter study. J Clin Oncol. 2004;22:3381–3388. doi: 10.1200/JCO.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Silliman RA. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20:2680–2688. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 14.McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Litwin MS, McGuigan KA. Accuracy of recall in health-related quality-of-life assessment among men treated for prostate cancer. J Clin Oncol. 1999;17:2882–2888. doi: 10.1200/JCO.1999.17.9.2882. [DOI] [PubMed] [Google Scholar]
- 16.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 17.Moser A, Stuck AE, Silliman RA, Ganz PA, et al. The eight-item modified Medical Outcomes Study Social Support Survey: Psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012;65:1107–1116. doi: 10.1016/j.jclinepi.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenfield S, Apolone G, McNeil BJ, et al. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31:141–154. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield S, Blanco DM, Elashoff RM, et al. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–2770. [PubMed] [Google Scholar]
- 20.Fleming ID, Cooper JS, Henson DE, et al. AJCC cancer staging manual. 5th ed Lippincott Williams and Wilkins; Philadelphia, PA: 1997. [Google Scholar]
- 21.Breast cancer Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2003;1:148–188. doi: 10.6004/jnccn.2003.0016. [DOI] [PubMed] [Google Scholar]
- 22.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: A tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109:802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 24.Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57:2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 28.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Smith AK, Widera E. [Accessed August 20, 2013];Eprognosis. http://www.eprognosis.org.