Abstract
Molecular technologies have produced diverse arrays of animal models for studying genetic diseases and potential therapeutics. Many have neonatal phenotypes. Spinal muscular atrophy (SMA) is a neuromuscular disorder primarily affecting children, and is of great interest in translational medicine. The most widely used SMA mouse models require all phenotyping to be performed in neonates since they do not survive much past weaning. Pre-clinical studies in neonate mice can be hindered by toxicity and a lack of quality phenotyping assays, since many assays are invalid in pups or require subjective scoring with poor inter-rater variability. We find, however, that passive electrocardiography (ECG) recording in conscious 11-day old SMA mice provides sensitive outcome measures, detecting large differences in heart rate, cardiac conduction, and autonomic control resulting from disease. We find significant drug benefits upon treatment with G418, an aminoglycoside targeting the underlying protein deficiency, even in the absence of overt effects on growth and survival. These findings provide several quantitative physiological biomarkers for SMA preclinical studies, and will be of utility to diverse disease models featuring neonatal cardiac arrhythmias.
Keywords: ECG, Arrhythmia, Preclinical Outcome Measure, Phenotypic Assay, Mouse, Neonate, Spinal Muscular Atrophy, Translational Readthrough, SMN, Survival Motor Neuron, Review
2. INTRODUCTION
Spinal muscular atrophy (SMA) results from a clearly defined genetic deficit ultimately leading to death in children affected by its most frequent form (Type I, or Werdnig-Hoffman disease). SMA can be modeled and treated in animals. From patient studies, SMA is caused by insufficient dosage of the survival motor neuron (SMN) gene product. This insufficiency typically results from homozygous deletion of the SMN1 gene (1, 2). In most organisms equivalent mutations are lethal; however humans possess a nearly identical SMN2 gene (3). This gene is capable of expressing the identical SMN protein and is present in every SMA patient, but produces lower levels of the protein because of alternative splicing (4). Copy number analyses have shown that SMN2 enables survival and that an increase in SMN2 copy number correlates with an improved prognosis (5). Transgenic animal models faithfully reproduce the monogenic biochemical defect causing SMA and confirm that increasing SMN dosage through transgenic crosses, gene therapy, or drug-based methods improves mouse phenotypes. Together, patient copy number and animal model studies show there is an exciting possibility to rationally develop therapies by targeting SMN2 gene induction in patients. Compounds being pursued to therapeutically increase SMN include HDAC inhibitors which increase SMN2 transcription (6), and aminoglycoside antibiotics which work through a translational readthrough drug mechanism to stabilize the SMNdelta7 protein (7–9).
Preclinical mouse studies have been important in drug discovery for disorders in which heart rhythms are affected by disease (10–12). Recently, we found multiple mouse models of SMA display cardiac arrhythmias (13–15). These models present relatively severe phenotypes, in which mice are grossly undersized and die within days or weeks after birth (13, 15, 16). Studies of these models require that phenotyping assays be performed in neonates. However, many functional outcome measures are invalid in neonates due to mouse size, assay invasiveness, equipment incompatibility, or the large number of mice required (17). For electrocardiography (ECG) in particular, the invasiveness, restraint or surgery required by many systems can interfere with the heart rate itself or endanger the life of a fragile, neonatal disease model mouse (18–22). Preclinical outcome measures used in place of established clinical tests in neonates typically have a subjective component that demands inter-rater reliability testing, and that can be a confounding factor in translating findings between laboratories. As drug discovery advances for SMA and other diseases requiring neonatal testing, additional assays that are quantitative and independent of the observer will become increasingly important.
Drug development can be hindered by toxicity or bioavailability issues. Compound toxicity that affects neonate lethargy, survival, growth rates, subjective appearance and/or performance in phenotyping assays can affect individual assays or complicate the interpretation of drug effect. As a result, we sought to determine if ECG can be used as an objective and sensitive biomarker for preclinical studies in neonate mice. More specifically, we wanted to explore the utility of ECG in instances where there may be confounding drug toxicity or a lack of dramatic size and survival benefits. Towards these ends, we evaluated the effects of G418 treatment on SMA mice and ECG parameters. G418 is an aminoglycoside antibiotic that operates through a drug mechanism known as translational readthrough. We have previously reported that G418 successfully increases SMN levels both in vitro and in vivo by causing readthrough of the SMNdelta7 stop codon to further lengthen and stabilize the SMN isoform produced from this transcript. In vivo, this produces an increase in motor function of SMNdelta7 mice (7). However, G418 treatment ultimately results in toxicity in healthy mice and dogs (23, 24). Regardless of this toxicity, the clear efficacy of G418 at causing translational readthrough establishes it as a powerful proof-of-concept compound in basic science studies (7, 25–27). Here in blinded proof-of-concept studies, we find G418 treatment significantly improves bradycardia, heart block, cardiac conduction, and heart rate variability in SMA mice. We also find heart rate and PR intervals are altered during end stage toxicity in unaffected carriers. These results indicate passive recording of ECG in conscious neonate mice can be used as an objective and sensitive quantitative biomarker in preclinical and proof-of-principle mechanism studies.
3. MATERIALS AND METHODS
3.1. Animal care and drug dosing
Experiments were conducted under approved Institutional Animal Care and Use Committee (IACUC) protocols. SMNdelta7 model mice were obtained from Jackson Laboratories (strain # 605025) (16). All mice were housed in a controlled animal facility, fed ad libitum with a 12 h light:12 h dark photoperiod and monitored daily for health. SMA mice and unaffected control littermates were produced by breeding heterozygous SMN2+/+;SMNdelta7+/+;Smn+/− mice. Individual mice were genotyped by polymerase chain reaction (PCR) (7).
Study mice (n of 10 mice per group) were administered 14 mg/kg G418 sulfate (GIBCO) or an equivalent volume of sterile saline vehicle. These were prepared and vials coded to blind the study. Treatments consisted of daily AM intraperitoneal injections beginning at postnatal day (PND) 5 and ending at PND20, or when mice reached death endpoints. SMA mice that died prior to the day of ECG recording were replaced to maintain a sample size of 10 per group for ECG. Mice meeting functional death endpoints (20 percent loss of weight, inability to right, obvious state of distress) were euthanized with CO2 followed by cervical dislocation as a secondary measure.
3.2. Motor function
Motor function was assayed at PND10 using a negative geotaxis test developed by Psychogenics, Inc. Mice were placed on a 30 degree incline in an orientation at which they stood upright facing the bottom of the incline. If the mouse was successfully able to re-orient itself 180 degrees to stand facing the top of the ramp, the mouse successfully passed the test. A mouse failed the test if it could not reorient itself within 30 s.
3.3. Electrocardiography
Electrocardiograms were recorded on PND11 using the ECGenie system (Mouse Specifics). All recordings were made non-invasively in conscious mice, as previously described (28, 29). A 30 degree Celsius temperature-controlled thermal cup was used to maintain body temperature. Mice were acclimated for 10 minutes prior to ECG. Data were acquired using LabChart 6 (ADInstruments) and analyzed using e-MOUSE ECG Analysis (Mouse Specifics).
3.4. Statistical analyses
Data are presented as mean plus or minus standard deviation unless otherwise noted. All data were obtained from 10 mice per group randomly assigned to receive drug or vehicle after genotyping. ECGs of PND11 mice were analyzed by ANOVA with post-hoc analyses of drug and genotype effects. ECGs of end-stage G418 toxicity were analyzed by Student’s t-test. Survival curves were compared using log-rank test.
4. RESULTS
4.1. G418 improves motor function of SMA mice without weight or survival increase
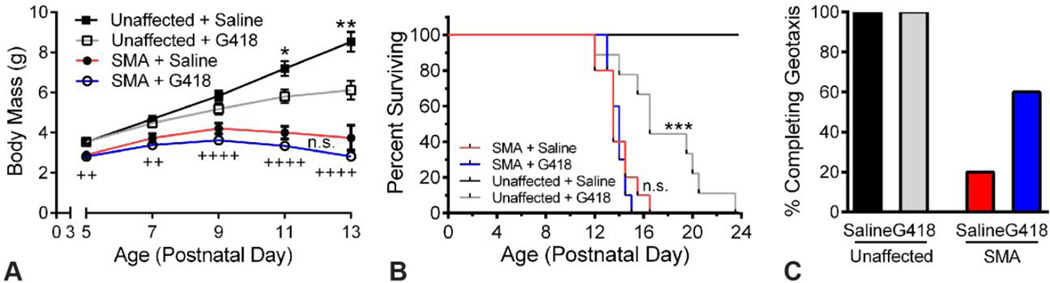
SMA model mice (16) and unaffected littermates received either G418 (14 mg/kg) or vehicle beginning at PND5, were assayed for motor function at PND10, and ECGs were recorded at PND11. We found no significant effect of G418 on body mass of SMA mice at any age (Figure 1 A). In unaffected littermates, however, G418 significantly decreased body mass from PND11 onward. Consistent with previous studies, SMA mice were significantly smaller than control littermates at all ages assayed (P less than 0.0.1), and showed an average life span of approximately 14 days (Figure 1 B). No difference was observed between the lifespan of SMA mice treated with G418 (13.9. +/− 0.7. days) or vehicle (13.9. +/− 1.4. days). However, G418 was found to be toxic in unaffected littermates. This manifested in lethality, with an average lifespan of 17.6. +/− 3.6. days for G418 compared to an absence of death events for vehicle (P less than 0.0.001). In contrast to the lack of effect on SMA mouse body mass and survival, we found G418 increased SMA mouse motor function. At PND10, only 20 percent of vehicle SMA mice were able to complete negative geotaxis (Figure 1C). G418 treatment increased this number to 60 percent of SMA mice. All unaffected littermates from both treatment groups were able to complete geotaxis. These data, in agreement with our original report (7) indicates G418 is able to increase mouse motor function, despite toxicity that ultimately prevents it from increasing survival.
Figure 1.
G418 improves motor function but not survival of SMA mice. A) Body mass was assayed from PND5 to PND13 for SMA mice and unaffected littermates receiving G418 or vehicle (n of 10, n.s. is not significant, *P less than 0.0.5, **P less than 0.0.05 for G418 versus saline, ++P less than 0.0.1, ++++P less than 0.0.001 for unaffected saline versus SMA saline). B) Kaplan-Meier survival curve of mice receiving G418 or saline vehicle (n.s. is not significant, *** P less than 0.0.005). C) Percentage of mice able to complete the negative geotaxis motor function test at PND10.
4.2. ECG reveals benefits of G418 drug treatment on arrhythmias in SMA mice
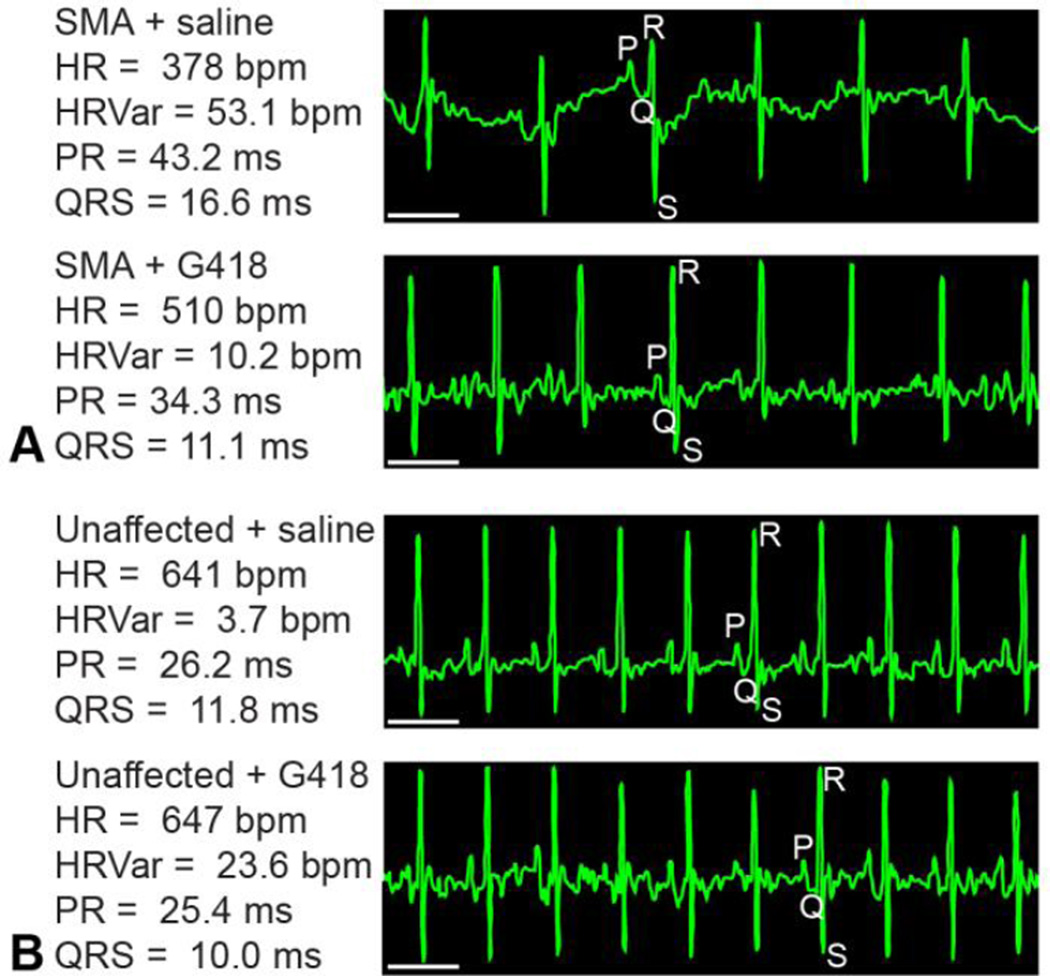
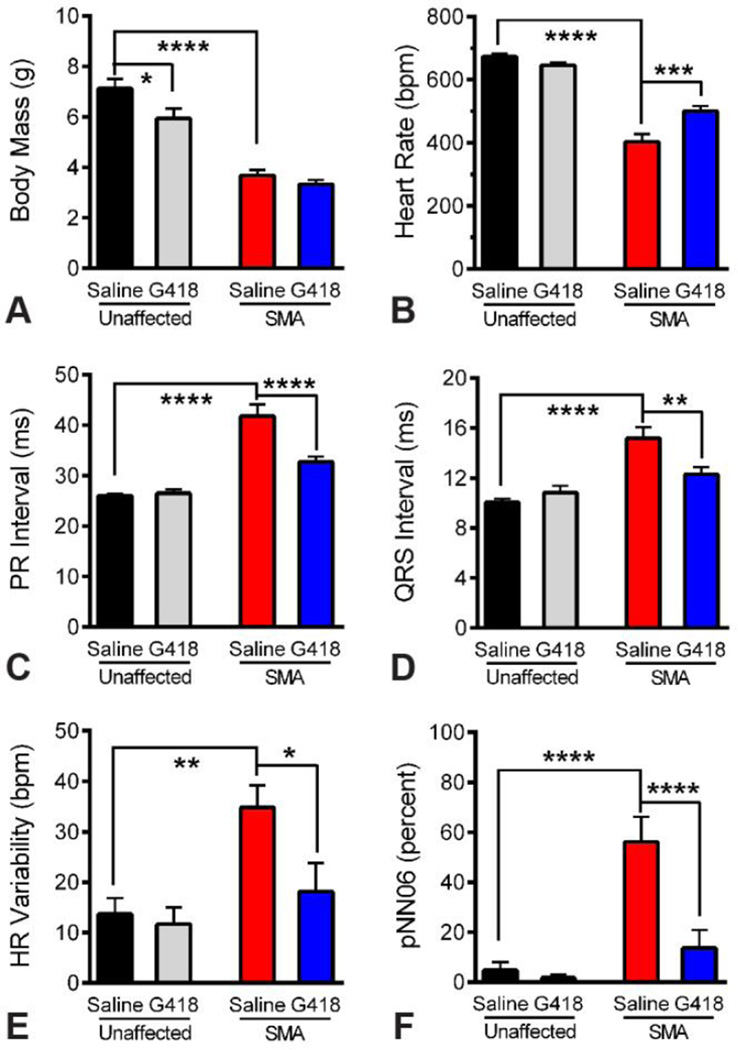
We recorded ECG waveforms in conscious mice at PND11 (Figure 2). SMA mice were significantly smaller than unaffected littermates at this age (P less than 0.0.001). No differences were detected in the body mass of SMA mice treated with G418 (3.3.3 +/− 0.5.2 g) or vehicle (3.6.8 +/− 0.7.2 g) at the time of ECG (Figure 3 A), although a significant decrease (P less than 0.0.5) was observed for unaffected littermates treated with G418 (5.9.4 +/− 1.2.4 g) versus saline (7.1.2 +/− 1.2.2 g). Examining the electrocardiogram, we found SMA mice exhibited a significantly lower heart rate than unaffected littermates (P less than 0.0.001). Drug treatment significantly improved (P less than 0.0.005) the SMA heart rate from 403 +/− 78 beats per minute (bpm) for vehicle to 500 +/− 52 bpm for G418 mice (Figure 3 B). In contrast, G418 treatment caused a slight decrease in the heart rate of unaffected littermates (from 674 +/− 29 bpm for vehicle to 646 +/− 26 bpm for G418). This indicates G418 improves heart rate specifically within SMN-deficient SMA mice, without effects to the overall body size of these mice.
Figure 2.
ECG of SMA and G418 treatment groups. Representative ECG waveform tracings obtained from conscious (A) SMA and (B) unaffected littermates at PND11 are presented, along with the mean waveform values for that mouse recording. Individual waveforms are denoted by P, Q, R or S. Scale bar represents 100 ms.
Figure 3.
Quantitative ECG benefits from G418 treatment of SMA mice. A) Body mass of treatment groups at time of ECG analysis on PND11. B) Heart rate of treatment groups, showing SMA bradycardia and effects of drug treatment. C) PR interval, showing heart block in SMA mice and effects of drug treatment. D) QRS interval, showing cardiac conduction deficits in SMA mice and effects of drug treatment. E–F) Measures of heart rate variability, showing alteration of HR Variability (E) and pNN06 (F) in SMA mice and the effects of drug treatment. (n of 10, * P less than 0.0.5, ** P less than 0.0.1, *** P less than 0.0.005, **** P less than 0.0.001)
Heart block was detected in SMA mice as a significant elongation of PR intervals (P less than 0.0.001). G418 treatment significantly decreased this interval (P less than 0.0.001), from 41.8. +/− 7.3. ms for vehicle to 32.7. +/− 3.4. ms for G418 (Figure 3 C). Impaired cardiac conduction was present in SMA mice as found by significantly slower QRS intervals than unaffected littermates (P less than 0.0.001). Drug treatment significantly improved this cardiac conduction parameter in SMA mice (P less than 0.0.1), decreasing the QRS interval from 15.2. +/− 2.8. ms with vehicle to 12.3. +/− 1.8. ms with G418 (Figure 3 D). In contrast, no effects of G418 were present on PR or QRS intervals in unaffected littermates at this age, which showed values of 26.1. +/− 1.0.3 ms and 10.0. +/− 0.9. ms, respectively, for vehicle. These data show that G418 significantly improves cardiac conduction in SMA mice without affecting these parameters in healthy mice.
We examined measures of heart rate variability, reflective of autonomic nerve control of the heart, by assaying HR Variability and pNN06, or the percentage of adjacent RR intervals differing by greater than 6 ms (Figure 3, E and F). SMA mice had irregular heartbeats with a clear increase in these parameters when compared to unaffected littermates (P less than 0.0.05 and 0.0.001, respectively). G418 treatment did not change either parameter in unaffected littermates, with vehicle injected control littermates showing values of 13.7. +/− 9.9. bpm for HR Variability and 4.8. +/− 10.2. percent for pNN06. Unaffected littermates treated with G418 showed a trend of decrease for pNN06 (to 1.7. +/− 3.9. percent) but this was not significant. SMA mice showed a significant decrease (P less than 0.0.5) in HR Variability upon G418 treatment (18.2. +/− 18.0. bpm) in comparison to vehicle (34.8. +/− 14.0. bpm). The pNN06 values for SMA mice also significantly decreased (P less than 0.0.001) upon G418 treatment (13.6. +/− 22.9. percent) in comparison to vehicle (56.1. +/− 31.6. percent). Together, these data suggest G418 improves autonomic control of the SMA mouse heart.
4.3. ECG of non-SMA mice at end stage of drug toxicity
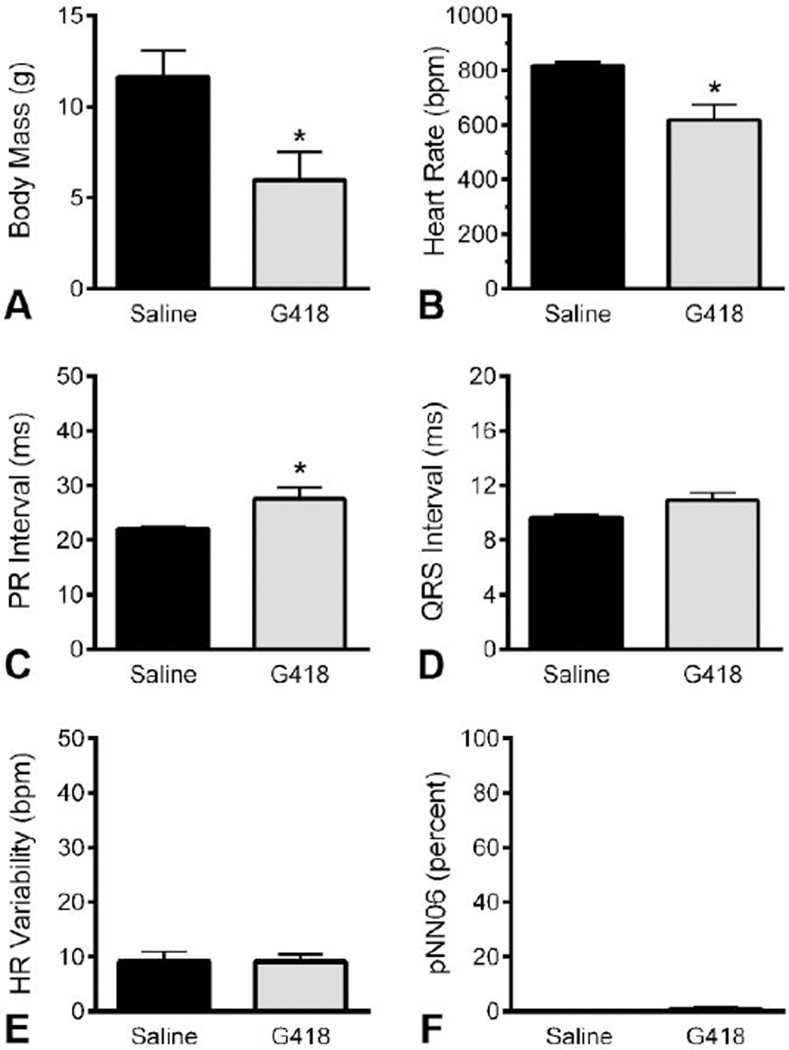
We assayed a subset of unaffected littermates at end stages of G418 toxicity to observe effects of drug toxicity on ECG waveforms. At this point, G418 significantly decreased the body mass (P less than 0.0.5) of treated mice (5.9.8 +/− 3.0.9 g) compared to vehicle (11.6.5 +/− 2.8.8 g) (Figure 4 A). ECG revealed a significant decrease (P less than 0.0.5) in mouse heart rates for G418 treated mice (619 +/− 108 bpm) compared to vehicle (817 +/− 27 bpm) injected mice (Figure 4 B). G418 treatment resulted in heart block at end stages of treatment (P less than 0.0.5), with longer PR intervals of 27.6. +/− 4.1. ms for G418 versus 22.1. +/− 0.7. ms for vehicle (Figure 4 C). No significant differences were detected for QRS intervals, HR Variability, or pNN06 measures, for which saline mice expressed values of 9.6. +/− 0.5. ms, 9.2. +/− 3.5. bpm, and 0 +/− 0 percent, respectively. These data indicate drug toxicity produced a reduction of heart rate in conjunction with heart block in unaffected genotypes. This also indicates G418 benefits to SMA arrhythmias are not a general result of drug effects on heart function, rather, the benefits of G418 to heart function are specific to the SMA genotype and are greater than any drug side effects.
Figure 4.
ECG at end stages of G418 toxicity. ECGs were recorded in mice of unaffected genotypes at advanced stages of G418 toxicity, along with littermate saline controls. A) Body mass of G418 and saline injected mice. B) Heart rates and (C) PR intervals were affected by G418 toxicity. D) QRS interval was not significantly affected by toxicity. E–F) Measures of heart rate variability, reflecting autonomic nerve control of the heart, were not affected by toxicity. (n of 4, ages range from PND19 to PND23, * P less than 0.0.5)
5. DISCUSSION
Specific ECG parameters can provide insight into different aspects of cardiac and neurologic pathophysiology. Here we show SMA mice have a decrease in heart rate, together with increases in heart rate variability measures that indicate autonomic nervous system involvement. Since heart rate variability is driven by autonomic control of the heart (30–32), we initially hypothesized from these ECG findings that autonomic nerves were being affected by the SMA genotype, and that an imbalance of vagal tone was driving the heart into a state of bradycardia. Indeed, we have found that SMA mouse hearts show reduced levels of sympathetic innervation upon whole-mount immunostaining (33). Here, improvements in heart rate variability measures upon G418 treatment are consistent with the idea that SMN-inducing compounds can improve neurological defects in SMA mice. Recent reports provide further evidence suggesting the involvement of autonomic dysfunction in SMA mice and/or patients. Cardiac function studies in adeno-associated virus gene therapy (scAAV9-SMN) rescued mice show a failure of gene therapy to fully rescue arrhythmias (34), and suggest autonomic involvement. Necrosis in intermediate or rescued SMA mice is thought to result from decreased vascularization and innervation (6, 35, 36). Intestinal problems have been found to be associated with decreased innervation in SMA patients and mice (37, 38). Together, these findings support the idea that autonomic deficits are a feature of SMA mice, support the application of ECG as a preclinical therapeutic biomarker, and suggest autonomic dysfunction may be of significance in clinical SMA.
Generally, models of chronic genetic disorders such as pediatric arrhythmias, or neuromuscular disorders such as SMA and Duchenne muscular dystrophy, frequently exhibit neonatal phenotypes that can benefit from neonatal dosing or phenotyping methods (6, 7, 10–12, 14, 15, 39–42). Dosing of neonates can be complicated by drug toxicity or bioavailability issues that are either inherent to the proof-of-concept compound itself as is the case for G418 (7, 23, 24), or arise due to developmental differences of neonate mice (43–45). Our data show HR, PR, QRS, and heart rate variability measures are sensitive biomarkers that can be passively monitored in awake neonate mice to provide quantitative insight into drug and genotype effects. By comparing effects in disease versus unaffected genotypes, we can discern 1) whether a drug is having primary benefits to phenotypes specifically resulting from that genetic mutation, 2), if there is a general effect of that compound on cardiac rhythms, and 3) if there are drug toxicities that affect the heart. Here we find G418 effects on ECG deficits are specific to the SMA genotype, and treatment moved those values towards those found in unaffected controls. We observed no initial effects of G418 on ECG of unaffected mice, indicating G418 does not generally affect the cardiac conduction system within this time frame in vivo. Further, we were able to resolve significant heart block in a small number of mice at end stages of G418 toxicity, indicating ECG and the PR interval can be used to detect drug toxicities in young mice. Previously, G418 has been found to display general toxicity in mice and dogs with damage to the liver, kidney and immune systems (23, 24). Side effects of primary concern for clinical aminoglycoside antibiotics include kidney damage and hearing loss, both associated with disrupted ion homeostasis. Here, the atrioventricular block caused by prolonged G418 treatment of unaffected genotypes in our study is consistent with heart toxicity resulting as either a primary effect or as a consequence of disrupted ion homeostasis, kidney function, and/or liver function. Together, our data show ECG can be used as a sensitive biomarker, even in proof-of-principle studies in which an investigational compound is ultimately toxic.
Moving forward, advances in molecular biology are allowing us to zero in on the molecular etiology of pediatric arrhythmias with a wide array of causes, and to model these in mice. The ability to quantitatively resolve differences in neonate cardiac conduction through monitoring of HR, PR, and QRS intervals will be of utility to these models. For example, an autoimmune mouse model of lupus erythematosis features congenital heart block caused by maternal antibodies to nuclear antigen proteins, a mechanism consistent with placental transmission of such antibodies in humans (46). Models of sudden infant death syndrome (SIDS) have also been established; once a mysterious condition, insights are now being gained through channelopathy models including Connexin43 (Cx43), T-box 3 (Tbx3), and cardiac sodium channel gene (SCN5A) mutant mouse models, which feature impaired cardiac conduction, atrioventricular block and lethal arrhythmias at neonatal stages (39–42, 47). Our data establish that, as in these models of arrhythmias, in the SMNdelta7 model we find strong phenotypes in heart rate, QRS and PR intervals. This provides a large “window of efficacy”, or a strong phenotype within which to resolve significant drug effects. Indeed, we are able to detect significant drug benefits to each of these phenotypes, even in the absence of overt benefits to overall mouse health, size and survival. This indicates passive ECG recording in awake neonate mice is a powerful tool that can be used for neuromuscular mutants and for a diverse group of diseases in which cardiac conduction is impaired.
Growth in the number of transgenic mouse models has outpaced the development of phenotyping assays for neonatal mice in preclinical studies. Here we find non-invasive ECG monitoring can quantitatively measure arrhythmias as well as significant drug benefits in neonatal SMA mice with a proof-of-concept drug. With ECG waveforms obtained passively from conscious neonates, and results analyzed by waveform analysis software, this assay should provide minimal inter-rater and inter-lab differences to provide a sensitive, quantitative and objective assay. This will empower a broad user base to accurately measure cardiac rhythm changes arising from effects caused by genetic mutation, drug benefits, and drug toxicities in neonate mice.
ACKNOWLEDGEMENTS
This work was supported by grants from the Families of SMA (DID1214), Muscular Dystrophy Association (255785) and the National Institutes of Health (NINDS, R01 NS060926). CRH is supported by (5T32 AR056993). The authors acknowledge Peggy Murphy for editing assistance.
Abbreviations
- AAV
adeno-associated virus
- bpm
beats per minute
- ECG
electrocardiography
- HR
heart rate
- IACUC
institutional animal care and use committee
- PCR
polymerase chain reaction
- PND
postnatal day
- pNN06
percent of adjacent RR intervals differing by greater than 6 ms
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
REFERENCES
- 1.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frezal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum Mutat. 2000;15(3):228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Rochette CF, Gilbert N, Simard LR. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Genet. 2001;108(3):255–266. doi: 10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 4.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A. 1999;96(11):6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, Cizman Z, Di Prospero NA, Pellizzoni L, Fischbeck KH, Sumner CJ. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest. 2007;117(3):659–671. doi: 10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum Mol Genet. 2009;18(7):1310–1322. doi: 10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattis VB, Tom Chang CW, Lorson CL. Analysis of a read-through promoting compound in a severe mouse model of spinal muscular atrophy. Neurosci Lett. 2012;525(1):72–75. doi: 10.1016/j.neulet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolstencroft EC, Mattis V, Bajer AA, Young PJ, Lorson CL. A non-sequence-specific requirement for SMN protein activity: the role of aminoglycosides in inducing elevated SMN protein levels. Hum Mol Genet. 2005;14(9):1199–1210. doi: 10.1093/hmg/ddi131. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15(4):380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piatnitski Chekler EL, Butera JA, Di L, Swillo RE, Morgan GA, Rossman EI, Huselton C, Larsen BD, Hennan JK. Discovery of a class of potent gap-junction modifiers as novel antiarrhythmic agents. Bioorg Med Chem Lett. 2009;19(16):4551–4554. doi: 10.1016/j.bmcl.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304(5668):292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 13.Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, Didonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci. 2012;32(11):3818–3829. doi: 10.1523/JNEUROSCI.5775-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5(10):1569–1585. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogliotti RG, Cardona H, Singh J, Bail S, Emery C, Kuntz N, Jorgensen M, Durens M, Xia B, Barlow C, Heier CR, Plasterer HL, Jacques V, Kiledjian M, Jarecki J, Rusche J, DiDonato CJ. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Hum Mol Genet. 2013;22(20):4084–4101. doi: 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14(6):845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 17.Butchbach ME, Edwards JD, Burghes AH. Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy. Neurobiol Dis. 2007;27(2):207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Swirp S, Duff H. Age-dependent response of the electrocardiogram to K(+) channel blockers in mice. Am J Physiol Cell Physiol. 2000;278(1):C73–C80. doi: 10.1152/ajpcell.2000.278.1.C73. [DOI] [PubMed] [Google Scholar]
- 19.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279(2):H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- 20.Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods. 1993;30(4):209–215. doi: 10.1016/1056-8719(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274(3 Pt 2):H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 22.Stiedl O, Spiess J. Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6N mice. Behav Neurosci. 1997;111(4):703–711. doi: 10.1037//0735-7044.111.4.703. [DOI] [PubMed] [Google Scholar]
- 23.Aubrecht J, Goad ME, Czopik AK, Lerner CP, Johnson KA, Simpson EM, Schiestl RH. A high G418-resistant neo(R) transgenic mouse and mouse embryonic fibroblast (MEF) feeder layers for cytotoxicity and gene targeting in vivo and in vitro. Drug Chem Toxicol. 2011;34(4):433–439. doi: 10.3109/01480545.2010.544316. [DOI] [PubMed] [Google Scholar]
- 24.La Rocca PT, Baker F, Frantz JD, Szot RJ, Black HE, Schwartz E. Skin and mucous membrane ulceration in beagle dogs following oral dosing with an experimental aminoglycoside antibiotic. Fundam Appl Toxicol. 1985;5(5):986–990. [PubMed] [Google Scholar]
- 25.Auld DS, Thorne N, Maguire WF, Inglese J. Mechanism of PTC124 activity in cell-based luciferase assays of nonsense codon suppression. Proc Natl Acad Sci U S A. 2009;106(9):3585–3590. doi: 10.1073/pnas.0813345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McElroy SP, Nomura T, Torrie LS, Warbrick E, Gartner U, Wood G, McLean WH. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 2013;11(6):e1001593. doi: 10.1371/journal.pbio.1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangkuhl K, Schulz A, Rompler H, Yun J, Wess J, Schoneberg T. Aminoglycoside-mediated rescue of a disease-causing nonsense mutation in the V2 vasopressin receptor gene in vitro and in vivo. Hum Mol Genet. 2004;13(9):893–903. doi: 10.1093/hmg/ddh105. [DOI] [PubMed] [Google Scholar]
- 28.Heier CR, Hampton TG, Wang D, Didonato CJ. Development of electrocardiogram intervals during growth of FVB/N neonate mice. BMC Physiol. 2010;10:16. doi: 10.1186/1472-6793-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6. doi: 10.1186/1472-6793-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofer MA, Reiser MF. The development of cardiac rate regulation in preweanling rats. Psychosom Med. 1969;31(5):372–388. doi: 10.1097/00006842-196909000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Adolph EF. Ontogeny of heart-rate controls in hamster, rat, and guinea pig. Am J Physiol. 1971;220(6):1896–1902. doi: 10.1152/ajplegacy.1971.220.6.1896. [DOI] [PubMed] [Google Scholar]
- 32.Navaratnam V. The ontogenesis of cholinesterase activity within the heart and cardiac ganglia in man, rat, rabbit and guinea-pig. J Anat. 1965;99(Pt 3):459–467. [PMC free article] [PubMed] [Google Scholar]
- 33.Heier CR, Satta R, Lutz C, DiDonato CJ. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum Mol Genet. 2010;19(20):3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shababi M, Habibi J, Ma L, Glascock JJ, Sowers JR, Lorson CL. Partial restoration of cardio-vascular defects in a rescued severe model of spinal muscular atrophy. J Mol Cell Cardiol. 2012;52(5):1074–1082. doi: 10.1016/j.yjmcc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes AH. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet. 2008;17(8):1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O'Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest. 2010;120(4):1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galvis DA, Ang SM, Wells TR, Landing BH, Romansky SG. Microdissection study of the myenteric plexus in acardia, ataxia-telangiectasia, cystic fibrosis, extrahepatic biliary atresia, pediatric AIDS and Werdnig-Hoffmann disease. Pediatr Pathol. 1992;12(3):385–395. doi: 10.3109/15513819209023317. [DOI] [PubMed] [Google Scholar]
- 38.Le TT, McGovern VL, Alwine IE, Wang X, Massoni-Laporte A, Rich MM, Burghes AH. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet. 2011;20(18):3578–3591. doi: 10.1093/hmg/ddr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank DU, Carter KL, Thomas KR, Burr RM, Bakker ML, Coetzee WA, Tristani-Firouzi M, Bamshad MJ, Christoffels VM, Moon AM. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc Natl Acad Sci U S A. 2012;109(3):E154–E163. doi: 10.1073/pnas.1115165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88(3):333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neary MT, Mohun TJ, Breckenridge RA. A mouse model to study the link between hypoxia, long QT interval and sudden infant death syndrome. Dis Model Mech. 2013;6(2):503–507. doi: 10.1242/dmm.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, Rosenbaum DS, Van Wagoner DR, Kirsch GE, Wang Q. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res. 2004;61(2):256–267. doi: 10.1016/j.cardiores.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Invest. 2009;119(2):267–277. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinsky L, Digeorge AM. Cleft Palate in the Mouse: A Teratogenic Index of Glucocorticoid Potency. Science. 1965;147(3656):402–403. doi: 10.1126/science.147.3656.402. [DOI] [PubMed] [Google Scholar]
- 45.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranda-Carus ME, Boutjdir M, Tseng CE, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161(11):5886–5892. [PubMed] [Google Scholar]
- 47.Charpentier F, Bourge A, Merot J. Mouse models of SCN5A-related cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98(2–3):230–237. doi: 10.1016/j.pbiomolbio.2008.10.012. [DOI] [PubMed] [Google Scholar]