Abstract
The activity of a bacterial agglutinin from soybean seed [Glycine max (L.) Merrill cv. Clark] against two bacterial pathogens, Pseudomonas glycinea (causal agent of bacterial blight) and Xanthomonas phaseoli var. sojensis (causal agent of bacterial pustule) was determined. The agglutinin was active against several strains of X. phaseoli var. sojensis grown on nutrient agar, but there was no correlation between pathogenicity and agglutination. Agglutination was affected by the age of the bacterial cells and the growth medium used. None of seven strains of P. glycinea was agglutinated.
Bacterial agglutination was inhibited by both purified lipopolysaccharide and extracellular polysaccharide from five strains of X. phaseoli var. sojensis. The lipopolysaccharides and extracellular polysaccharides from other species of bacteria were ineffective.
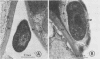
Ultrastructural studies showed that an avirulent strain of X. phaseoli var. sojensis was attached to leaf mesophyll cell walls of the susceptible cultivar Clark by 34 hours after vacuum infiltration. Cells of this avirulent strain were enveloped by fibrillar and granular material at the mesophyll cell wall. In contrast, cells of a virulent strain were not attached or enveloped, and they remained free to multiply in the intercellular spaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal A. K., Shantharam S., Ratnam S. Ultrastructure of Rhizobium japonicum in relation to its attachment to root hairs. J Bacteriol. 1978 Mar;133(3):1393–1400. doi: 10.1128/jb.133.3.1393-1400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDMAN W. F. Comparison of slime from tomato and banana strains of Pseudomonas solanacearum. Nature. 1959 Dec 19;184(Suppl 25):1969–1970. doi: 10.1038/1841969a0. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- De Zoeten G. A., Gaard G. Possibilities for inter- and intracellular translocation of some icosahedral plant viruses. J Cell Biol. 1969 Mar;40(3):814–823. doi: 10.1083/jcb.40.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Sequeira L. A New Bacterial Agglutinin from Soybean: I. ISOLATION, PARTIAL PURIFICATION, AND CHARACTERIZATION. Plant Physiol. 1980 Nov;66(5):847–852. doi: 10.1104/pp.66.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. L., Sequeira L., Huang T. S. Bacterial lipopolysaccharides as inducers of disease resistance in tobacco. Appl Environ Microbiol. 1977 Oct;34(4):424–432. doi: 10.1128/aem.34.4.424-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lippincott B. B., Whatley M. H., Lippincott J. A. Tumor induction by agrobacterium involves attachment of the bacterium to a site on the host plant cell wall. Plant Physiol. 1977 Mar;59(3):388–390. doi: 10.1104/pp.59.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N., Katchalski E. Soybean hemagglutinin, a plant glycoprotein. I. Isolation of a glycopeptide. J Biol Chem. 1966 Feb 10;241(3):684–689. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: II. Distribution of Soybean Lectin in Tissues of Glycine max (L.) Merr. Plant Physiol. 1978 May;61(5):779–784. doi: 10.1104/pp.61.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]