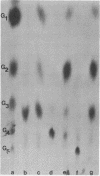
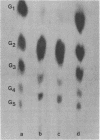
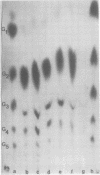
Abstract
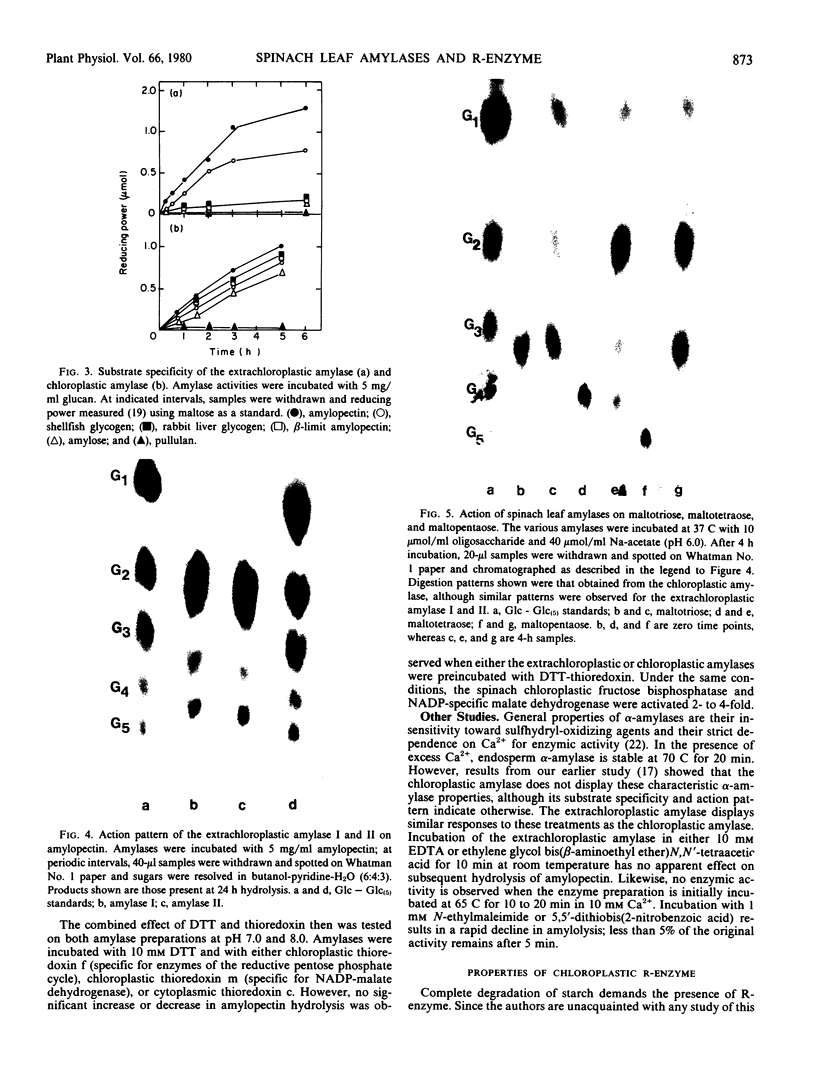
The properties of two amylase activities which differ in their substrate specificity and subcellular location as well as a chloroplast-associated R-enzyme (debranching activity) are reported. An extrachloroplastic amylase is resolved by gel filtration chromatography into two activities of 80,000 and 40,000 daltons. Both extrachloroplastic activities hydrolyze amylopectin and shellfish glycogen and only slowly hydrolyze rabbit liver glycogen, β-limit amylopectin, and amylose. In contrast, the major chloroplastic amylase attacks all of these glucans at comparable rates. Glucan hydrolysis by both the extrachloroplastic and chloroplastic amylase generates not only maltose but appreciable amounts of other oligosaccharides, whereas maltotetraose hydrolysis produces glucose, maltose, and maltotriose. The action patterns displayed by the amylase activities indicate that both are endoamylases, although they lack the typical Ca2+ requirement or heat stability of seed endosperm α-amylases. Dithiothreitol, glutathione (oxidized or reduced), ascorbate, dehydroascorbate, and dithiothreitol plus thioredoxin have no effect on either the chloroplastic or extrachloroplastic amylase activities.
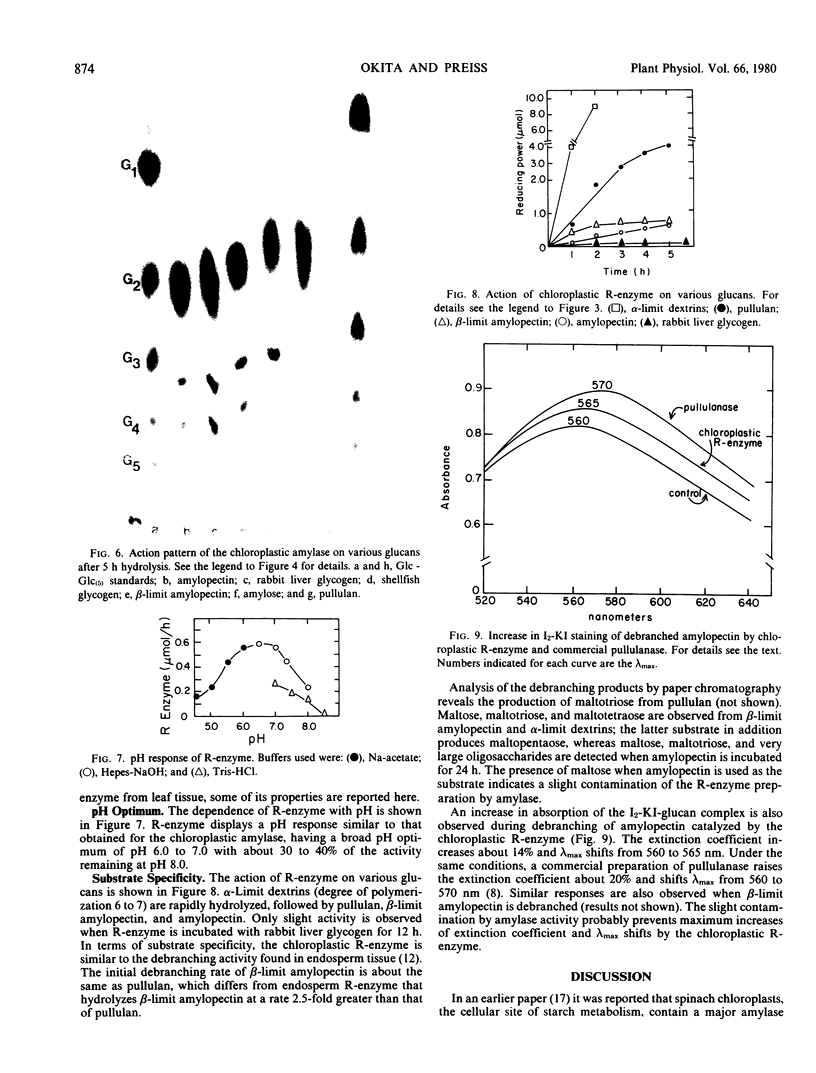
The chloroplastic R-enzyme debranches amylopectin, β-limit amylopectin, pullulan, and α-limit dextrins, but not rabbit liver glycogen. An increase in extinction coefficient and λmax is detected when the debranched amylopectin and β-limit amylopectin form a complex with I2-KI. Based on these properties, the chloroplastic R-enzyme is similar in enzymic activity to the R-enzyme observed in endosperm tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Chapman G. W., Jr, Pallas J. E., Jr, Mendicino J. The hydrolysis of maltodextrins by a -amylase isolated from leaves of Vicia faba. Biochim Biophys Acta. 1972 Aug 28;276(2):491–507. doi: 10.1016/0005-2744(72)91010-8. [DOI] [PubMed] [Google Scholar]
- Cohen P., Picton C., Klee C. B. Activation of phosphorylase kinase from rabbit skeletal muscle by calmodulin and troponin. FEBS Lett. 1979 Aug 1;104(1):25–30. doi: 10.1016/0014-5793(79)81078-9. [DOI] [PubMed] [Google Scholar]
- Evans R. B., Manners D. J. Studies on carbohydrate-metabolising enzymes. XXVII. A comparison of the action of yeast isoamylase and bacterial pullulanase on amylopectin. Carbohydr Res. 1971 Dec;20(2):339–349. doi: 10.1016/s0008-6215(00)81388-9. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISMAN C. R. A method for the colorimetric estimation of glycogen with iodine. Anal Biochem. 1962 Jul;4:17–23. doi: 10.1016/0003-2697(62)90014-3. [DOI] [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- Levi C., Gibbs M. Starch degradation in isolated spinach chloroplasts. Plant Physiol. 1976 Jun;57(6):933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey D. G., Steup M., Gibbs M. Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol. 1977 Aug;60(2):305–308. doi: 10.1104/pp.60.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz P., Beck E. Diurnal oscillation of amylolytic activity in spinach chloroplasts. Plant Physiol. 1978 Nov;62(5):687–689. doi: 10.1104/pp.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. K., Waisman D. M., Brostrom C. O., Soderling T. R. Stimulation of glycogen synthase phosphorylation by calcium-dependent regulator protein. J Biol Chem. 1979 Feb 10;254(3):583–586. [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Wolosiuk R. A., Crawford N. A., Yee B. C., Buchanan B. B. Isolation of three thioredoxins from spinach leaves. J Biol Chem. 1979 Mar 10;254(5):1627–1632. [PubMed] [Google Scholar]