Abstract
The effect of nitrogen and sulfur nutrition on sulfate permease and O-acetylserine sulfhydrylase was studied in tobacco cells.
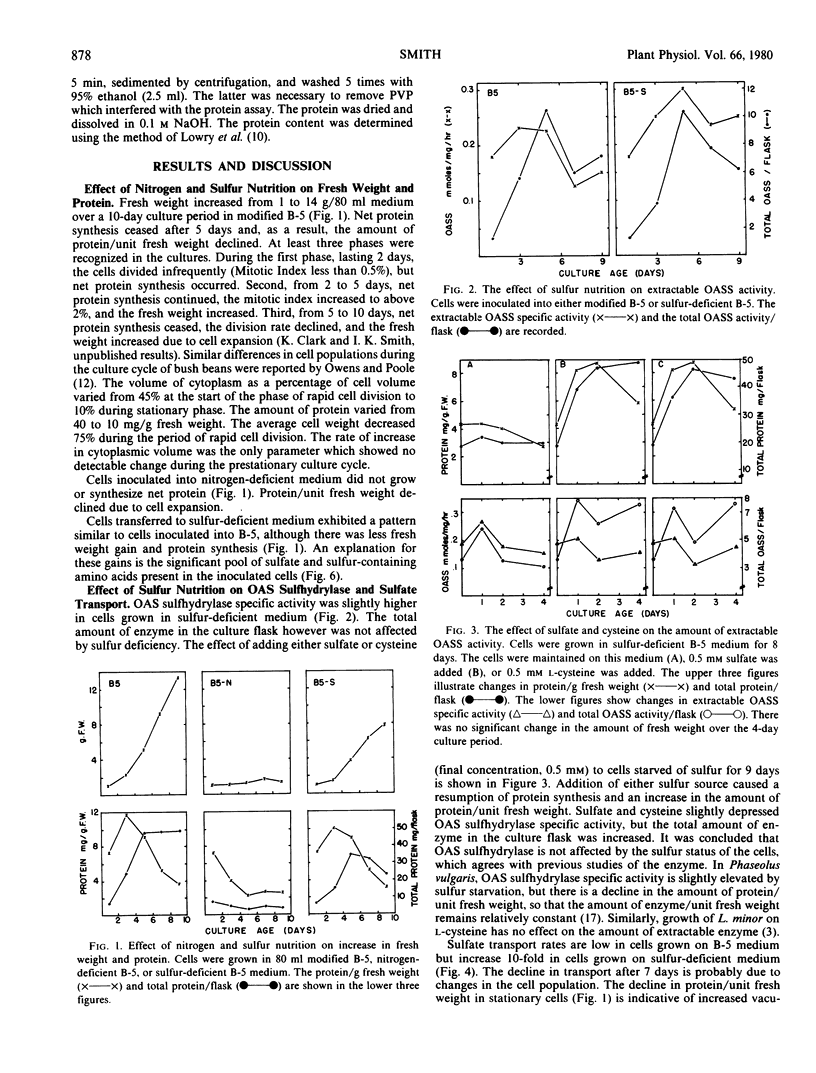
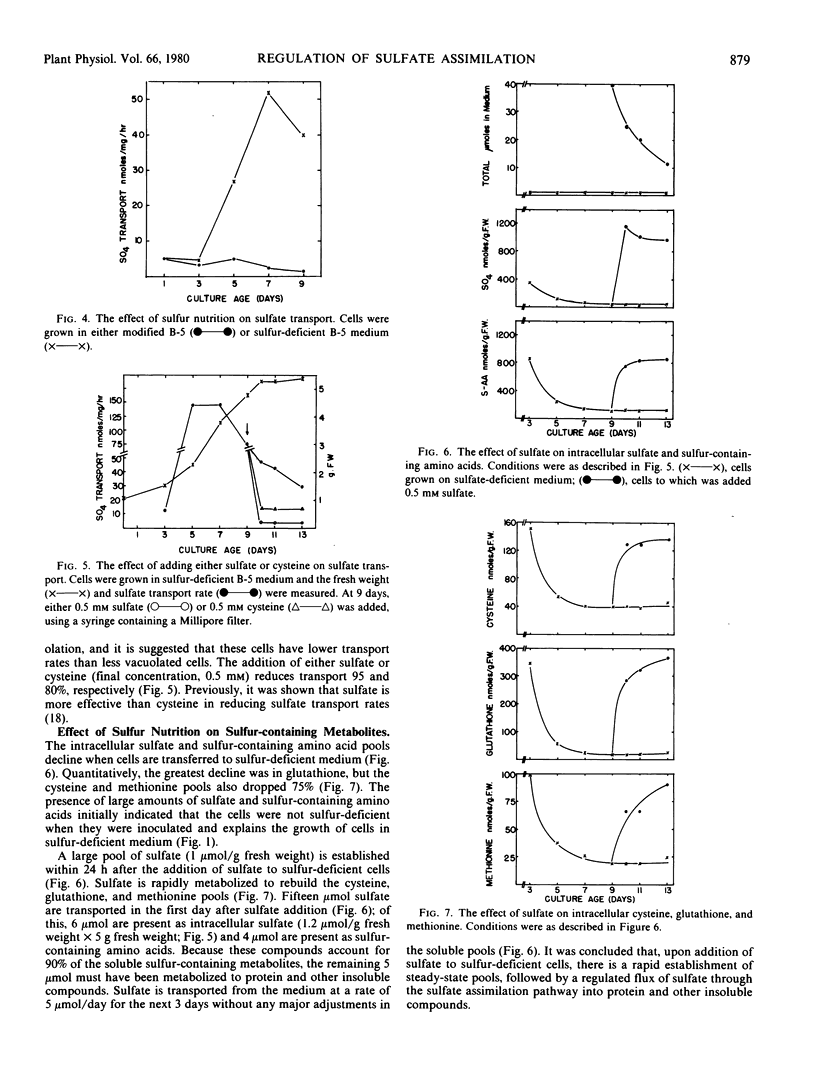
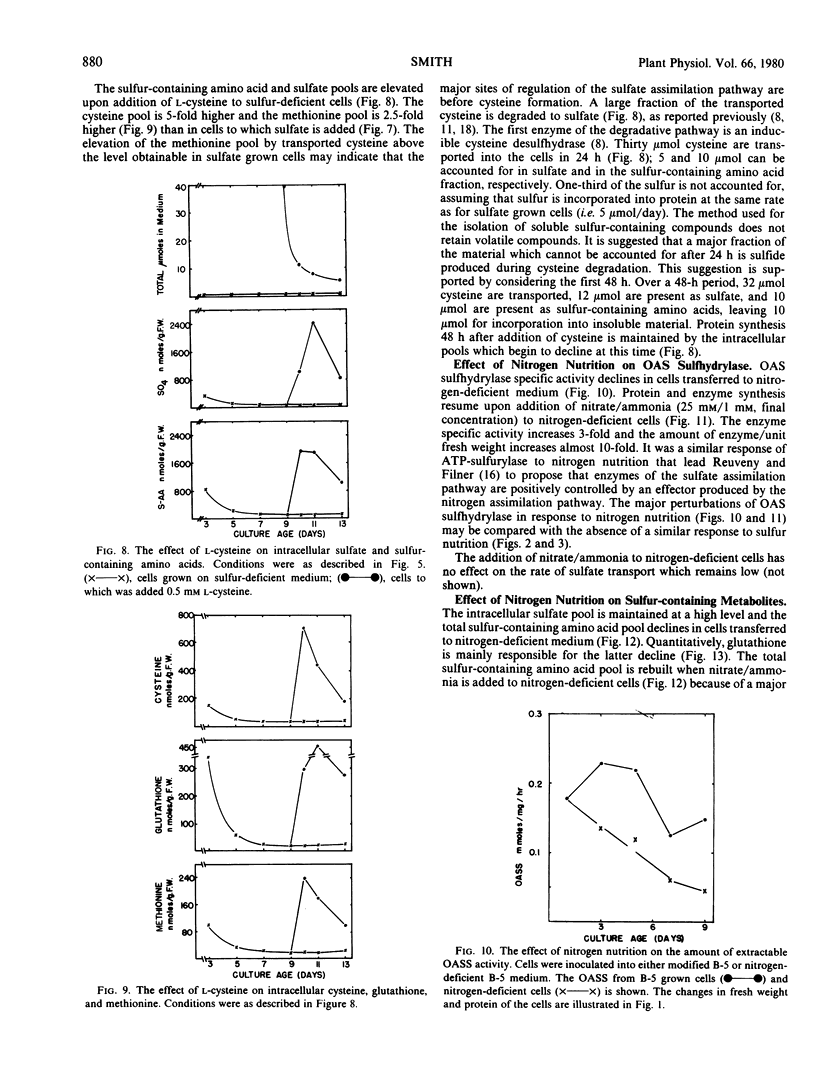
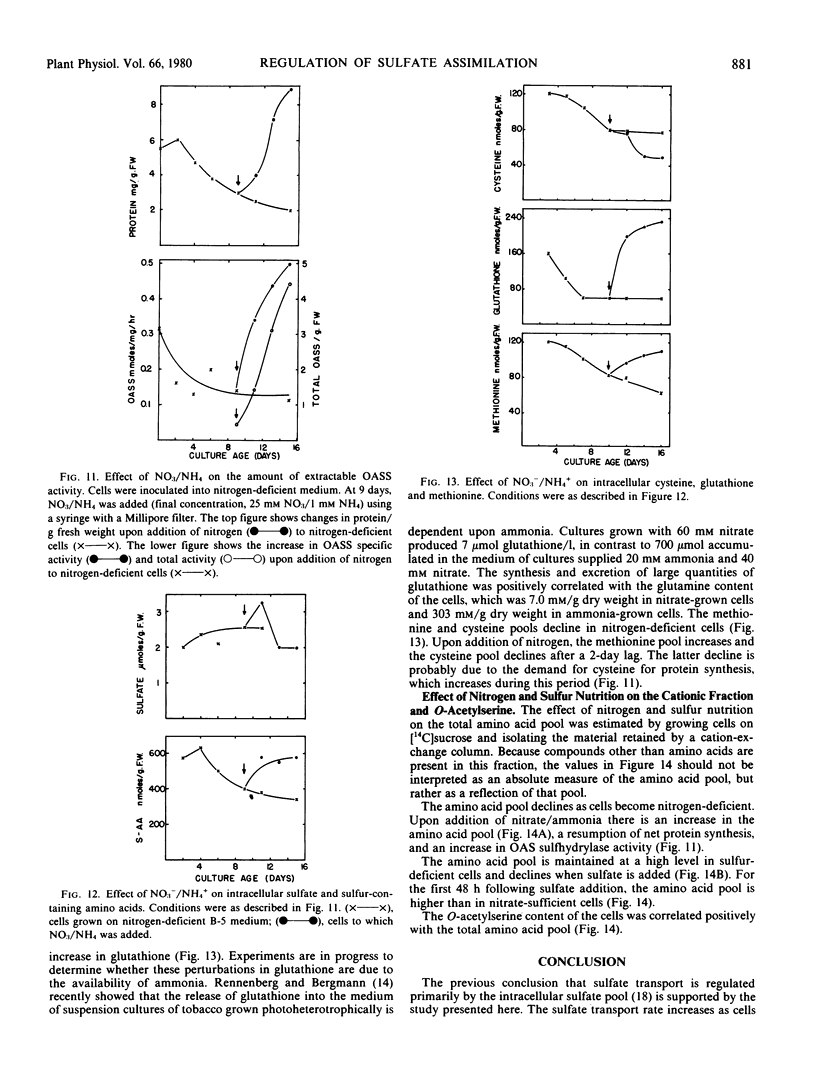
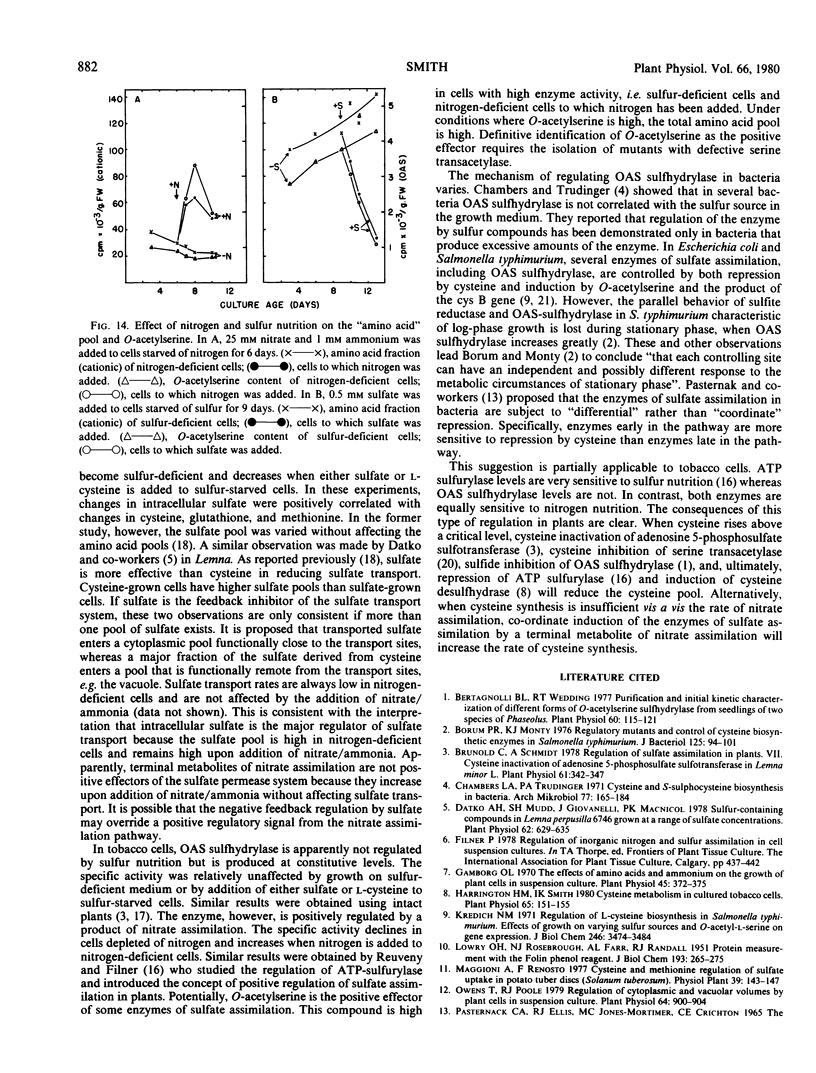
Sulfate transport rates increased 10-fold in cells transferred to sulfur-deficient B-5 medium. The addition of either sulfate or l-cysteine reduced transport 95 and 80%, respectively. The pools of sulfate, cysteine, glutathione, and methionine declined in sulfur-starved cells. The addition of either sulfate or l-cysteine increased the pools of sulfur-containing compounds, but major quantitative differences were measured. Nitrogen-starved cells had low transport rates which were not increased by addition of nitrate/ammonia. The pools of sulfate, cysteine, and methionine were high in nitrogen-starved cells and remained high upon addition of a nitrogen source. The results show that sulfate transport is regulated by the intracellular sulfate pool.
O-Acetylserine sulfhydrylase was not affected by sulfur nutrition. The extractable activity was high in B-5-grown cells, sulfur-deficient cells, and cells to which either sulfate or l-cysteine had been added. In contrast, the enzyme declined in cells transferred to nitrogen-deficient medium and the amount of enzyme/g fresh weight increased 10-fold when nitrate/ammonia was added. The addition of nitrate/ammonia had no effect on the cysteine or methionine pools but increased the total amino acid pool. The amount of O-acetylserine was positively correlated with extractable enzyme activity. This enzyme is positively regulated by an effector (possibly O-acetylserine) which is high under conditions of net nitrate assimilation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertagnolli B. L., Wedding R. T. Purification and initial kinetic characterization of different forms of o-acetylserine sulfhydrylase from seedlings of two species of phaseolus. Plant Physiol. 1977 Jul;60(1):115–121. doi: 10.1104/pp.60.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borum P. R., Monty K. J. Regulatory mutants and control of cysteine biosynthetic enzymes in Salmonella typhimurium. J Bacteriol. 1976 Jan;125(1):94–101. doi: 10.1128/jb.125.1.94-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunold C. Regulation of Sulfate Assimilation in Plants: 7. Cysteine Inactivation of Adenosine 5'-Phosphosulfate Sulfotransferase in Lemna minor L. Plant Physiol. 1978 Mar;61(3):342–347. doi: 10.1104/pp.61.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers L. A., Trudinger P. A. Cysteine and S-sulphocysteine biosynthesis in bacteria. Arch Mikrobiol. 1971;77(2):165–184. doi: 10.1007/BF00408609. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970 Apr;45(4):372–375. doi: 10.1104/pp.45.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980 Jan;65(1):151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Owens T., Poole R. J. Regulation of cytoplasmic and vacuolar volumes by plant cells in suspension culture. Plant Physiol. 1979 Nov;64(5):900–904. doi: 10.1104/pp.64.5.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z. Derepression of ATP sulfurylase by the sulfate analogs molybdate and selenate in cultured tobacco cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):619–622. doi: 10.1073/pnas.74.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Sulfate transport in cultured tobacco cells. Plant Physiol. 1975 Feb;55(2):303–307. doi: 10.1104/pp.55.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]



