Abstract
IMPORTANCE
Sleep disturbances are most prevalent among older adults and often go untreated. Treatment options for sleep disturbances remain limited, and there is a need for community-accessible programs that can improve sleep.
OBJECTIVE
To determine the efficacy of a mind-body medicine intervention, called mindfulness meditation, to promote sleep quality in older adults with moderate sleep disturbances.
DESIGN, SETTING, AND PARTICIPANTS
Randomized clinical trial with 2 parallel groups conducted from January 1 to December 31, 2012, at a medical research center among an older adult sample (mean [SD] age, 66.3 [7.4] years) with moderate sleep disturbances (Pittsburgh Sleep Quality Index [PSQI] >5).
INTERVENTIONS
A standardized mindful awareness practices (MAPs) intervention (n = 24) or a sleep hygiene education (SHE) intervention (n = 25) was randomized to participants, who received a 6-week intervention (2 hours per week) with assigned homework.
MAIN OUTCOMES AND MEASURES
The study was powered to detect between-group differences in moderate sleep disturbance measured via the PSQI at postintervention. Secondary outcomes pertained to sleep-related daytime impairment and included validated measures of insomnia symptoms, depression, anxiety, stress, and fatigue, as well as inflammatory signaling via nuclear factor (NF)–κB.
RESULTS
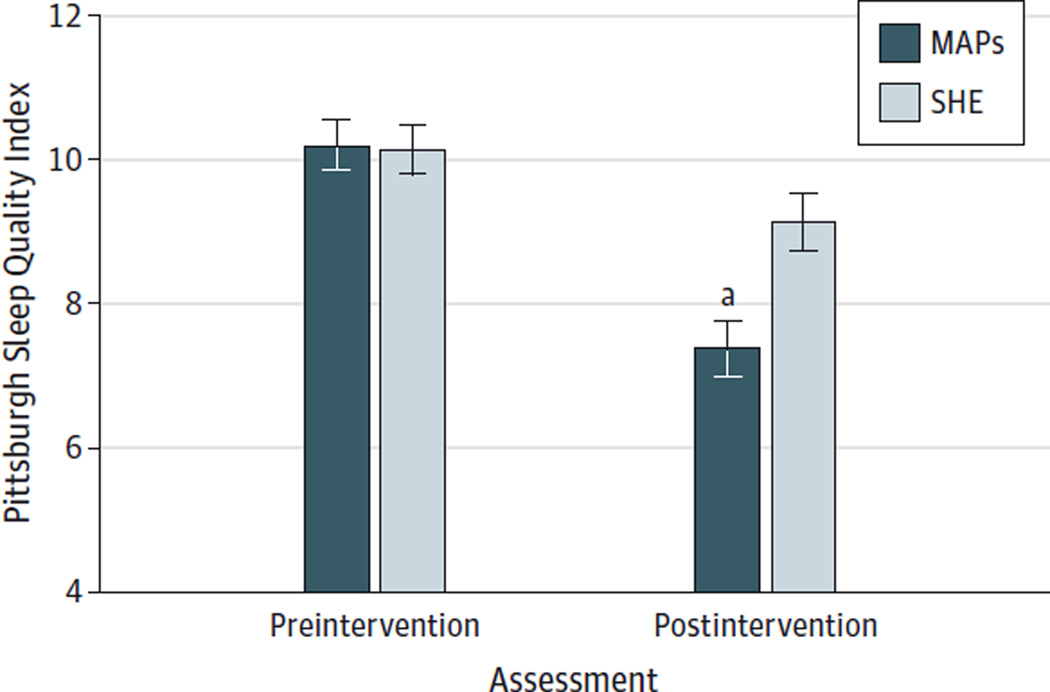
Using an intent-to-treat analysis, participants in the MAPs group showed significant improvement relative to those in the SHE group on the PSQI. With the MAPs intervention, the mean (SD) PSQIs were 10.2 (1.7) at baseline and 7.4 (1.9) at postintervention. With the SHE intervention, the mean (SD) PSQIs were 10.2 (1.8) at baseline and 9.1 (2.0) at postintervention. The between-group mean difference was 1.8 (95%CI, 0.6–2.9), with an effect size of 0.89. The MAPs group showed significant improvement relative to the SHE group on secondary health outcomes of insomnia symptoms, depression symptoms, fatigue interference, and fatigue severity (P < .05 for all). Between-group differences were not observed for anxiety, stress, or NF-κB, although NF-κB concentrations significantly declined over time in both groups (P < .05).
CONCLUSIONS AND RELEVANCE
The use of a community-accessible MAPs intervention resulted in improvements in sleep quality at immediate postintervention, which was superior to a highly structured SHE intervention. Formalized mindfulness-based interventions have clinical importance by possibly serving to remediate sleep problems among older adults in the short term, and this effect appears to carry over into reducing sleep-related daytime impairment that has implications for quality of life.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01534338
Sleep disturbances pose a significant medical and public health concern for our nation’s aging population. An estimated 50% of persons 55 years and older have some form of sleep problem, including initiating and maintaining sleep.1–5 Older adults report the highest prevalence of sleep problems compared with younger age groups when quantified by self-report and by biological assessment.1,6,7 Moderate sleep disturbances in older adults are often associated with deficits in daytime functioning including elevated levels of fatigue; disturbed mood, such as depressive symptoms; and reduced quality of life and lead to the onset of clinical insomnia.1,4,8 Addressing moderate sleep disturbances and sleep-related daytime dysfunction using community-accessible programs is a promising public health approach.9
Despite the medical consequences of sleep problems, they often go untreated in older adults, especially among those with moderate sleep disturbances.10 However, when clinical insomnia is present, several treatment options are available. In-somniais often treated with pharmacotherapy.11 Although such treatments provide only temporary remediation of sleep disturbance for some persons, the benefits often diminish after drug discontinuation, and there is risk for residual daytime effects and dependency syndrome.12–14 Psychobehavioral therapies are also recognized nonpharmacological treatments for insomnia.15 One universal behavioral program is sleep hygiene education (SHE), which targets the modification of day-to-day behavioral and environmental factors that contribute to poor sleep.16 Of the standard clinical treatments, cognitive behavior therapy focuses on modulating sleep needs and correcting expectations, attitudes, and beliefs about sleep.14 These nonpharmacological treatments have advantages over pharmacotherapy in that they are somewhat effective at improving sleep for both short-term and long-term periods and have no serious contraindications.12 However, clinical interventions, such as cognitive behavior therapy, are intensive, require administration by highly trained therapists, and are intended for patients with insomnia.17 Little is known about the efficacy of alternative behavioral treatments that might target moderate sleep disturbances in older adults, which have the potential to prevent insomnia and related consequences.
Limitations of current treatments for sleep problems highlight the need for scalable community-accessible treatments that can improve sleep in older adults with moderate sleep disturbances. Mindfulness-based interventions (MBIs) hold the potential to possibly meet these needs. The MBIs consist of evidence-based programs for stress-related ailments18 that train one in the systematic practice of attending to moment-by-moment experiences, thoughts, and emotions from a nonjudgmental perspective.19 Evidence from previous studies20,21 provides preliminary yet mixed support for the use of MBIs for sleep disturbances in adults, and a review of research on MBIs for sleep problems highlighted a spectrum of research gaps.22 Mainly, sleep has been tested as a secondary outcome to a primary disease state that can affect sleep,23,24 so the findings are confounded by changes in the primary ailment. Other MBI experimental studies20,25 have focused on mixed-age populations diagnosed as having insomnia.
Previous research has shown that a movement-based meditation, called tai chi, can improve sleep quality in older adults.26,27 However, to our knowledge, there are no reports of the use of a nonmovement form of meditation for sleep problems in older adults to examine the effects of mental practice alone on improvements in sleep quality. Therefore, we conducted a randomized clinical trial (RCT) to examine the effect of a low-cost community-accessible MBI known as mindful awareness practices (MAPs) for daily living compared with a structured SHE program on moderate sleep disturbances in older adults. We hypothesized that MAPs relative to SHE would confer superior improvements in sleep quality. Sleep-related daytime impairment and the peripheral blood inflammatory transcription factor nuclear factor (NF)–κB concentration were secondary outcomes. The NF-κB concentration was assessed given that sleep disturbance can induce an inflammatory response, which has implications for chronic disease risk in older adults.28–32 It has previously been shown that mind-body interventions in older adults can reduce or attenuate activation of NF-κB,33,34 but the association of these changes with improvements in sleep is unknown.33
Methods
Trial Design and Randomization
This was a single-site, parallel-group RCT. The trial protocol appears in the Supplement. The randomization sequence was obtained via computerized random number generation before the start of the trial in blocks of 2 conditions (1:1 MAPs to SHE). The statistician (R.O.) who directed the randomization was provided with random identification numbers to assign to a condition, and he never interacted with participants before or after assignment to a condition. The project manager informed participants of start dates. Blocks of 12 participants stratified by age, sex, and sleep quality (Pittsburgh Sleep Quality Index [PSQI]) were used to ensure that randomized treatment groups were filled within 1½ months of screening. Individuals were the unit of randomization. The University of California, LosAngeles institutional review board approved all procedures. Participants provided written informed consent.
Setting and Participants
The study was conducted in LosAngeles from January 1 toDecember31, 2012. Older adults (≥55years)were recruited through an advertisement in the local newspaper and flyers posted at the UCLA medical research center, as well as at community centers located in LosAngeles. Inclusion criteria were age of at least 55 years, agreement to the randomization, and active sleep disturbance as indicated by a PSQI exceeding 5 at screening.35 Exclusion criteria were current smoking, substance dependence, inability to speak English, depression (Patient Health Questionnaire 9 score >14),36 cognitive impairment (Mini-Mental State Examination score <23),37 significant current practice of any form of meditation (>15minutes per day), and obesity (body mass index [calculated as weight in kilograms divided by height in meters squared] >34.9). Also excluded were those who reported a current inflammatory disorder, sleep apnea, restless legs syndrome, illness, or infection (eg, autoimmune disease, type 1 diabetes mellitus, hepatitis C, cancer, or acute infection in the past 2 weeks).
Procedures
Participants who responded to the advertisement underwent 2 assessment phases before enrollment. The first phase was a 15-minute telephone interview by a trained data collector who evaluated eligibility. The second phase included an in-person visit. Trained data collectors then administered the Mini-Mental State Examination to rule out cognitive impairment. Participants were blinded to the randomization until the start of the intervention. Data collectors were unaware of group assignment at baseline assessment and were instructed to treat all participants in the same manner. Participants completed self-report questionnaires by themselves in a private room to negate assessor effects on outcomes. Eight visits to the study site were requested, including 1pretreatment assessment visit, 6 intervention sessions, and 1 posttreatment assessment visit. Pretreatment and posttreatment visits included height and weight assessment, administration of questionnaires, and blood draw. Assessments were completed at the UCLA medical research center within 10 days before and after the intervention. Participants were compensated up to $50 (allocated as gift cards) and received parking vouchers for each visit.
Interventions
MAPs for Daily Living
The MAPs for daily living is a weekly 2-hour, 6-session group-based course in mindfulness meditation that is available for residents to take in person within the Los Angeles area or to anyone online (http://marc.ucla.edu).Acertified teacher with more than 20 years of mindfulness practice delivered the formalized program curriculum to participants. Mindfulness exercises included mindful sitting meditation, mindful eating, appreciation meditation, friendly or loving-kindness meditation, mindful walking, and mindful movement. Participants engage in a mean of 10 to 30 minutes of mindful experiential practice in each class in addition to the teacher-delivered didactic material and group discussion. Participants are also provided with a book on mindfulness accompanied by a guided meditation compact disc.38 Mindfulness practice homework began with 5minutes daily and then progressively advanced to 20 minutes daily by session 6.
SHE Program
The SHE program is a weekly 2-hour, 6-session group-based course in sleep hygiene and education developed by the lead author (D.S.B.) that matched the MAPs condition for time, attention, group interaction, and expectancy of benefit effects. The SHE program was selected as a highly active control group to account for nonspecific effects (eg, time, attention, teacher, and group support) and participant or staff expectancy of a benefit on sleep. A trained health educator who held an MPH degree in health education delivered the program curriculum to participants. Active components included knowledge of sleep biology, characteristics of healthy and unhealthy sleep, sleep problems, stress biology and stress reduction, self-monitoring of sleep behavior, relaxation methods for improving sleep, and weekly behavioral sleep hygiene strategies. The SHE educational and behavioral content is based on National Institutes of Health39 and National Sleep Foundation40 tips for better sleep (eg, changing poor sleep habits and establishing a bedtime routine). Homework included practicing sleep hygiene and weekly reading, with in class group discussion to match the homework assigned in the MAPs group.
Outcome and Assessments
Self-assessments were administered before and after the intervention, which comprised a 10-week interval. The primary outcome measure was the PSQI,35 a widely used and validated 19-item self-report questionnaire of sleep disturbances experienced over the past month. Cronbach α = .83 for the PSQI,35 and test-retest reliability41 is 0.87, with high sensitivity (99%) and specificity (84%)to identify sleep disturbances.42 Planned a priori, secondary outcomes were daytime impairment commonly related to sleep disruption and included the validated Athens Insomnia Scale,43 Beck Depression Inventory II,44 Beck Anxiety Inventory,45 Perceived Stress Scale,46 and Fatigue Symptom Inventory.47 A summed score of 9 items for the Fatigue Symptom Inventory–Interference (ie, the degree to which fatigue interfered with daily life in the past week, such as normal work activity and ability to concentrate) and 4 items for the Fatigue Symptom Inventory–Severity (ie, the severity of fatigue in the past week) was calculated, with lower scores indicating less fatigue. The Five Facet Mindfulness Questionnaire short form48 is a validated 24-item self-report of the tendency to be mindful in daily life, with a reference to the past 2 weeks. A mean score is calculated, with lower scores indicating less mindfulness. In the MAPs group, participants recorded time spent meditating in a daily log. Logs captured the total number of practice minutes and the number of unique meditation sessions during the intervention period.
Inflammatory Signaling
Inflammatory signaling via activated NF-κBin peripheral blood mononuclear cells was quantified after venipuncture between 8:30 and 11:30 AM.34 The mean NF-κB concentrations from triplicate determinations were expressed as nanograms of p65 per microgram of total protein using recombinant p65 (ActiveMotif) as the reference standard. The detectable range of the assay was 0.08 to 5.00 ng of NF-κB p65 per microgram of total protein. The NF-κB activation in peripheral blood mononuclear cells is a precursor to increased concentrations of inflammatory markers in plasma, and sleep disruption increases NF-κB activation.49,50
Calculation
An a priori power analysis was conducted in G*Power (http://www.gpower.hhu.de/en.html). An estimated final total sample size of 42 was needed to detect a PSQI between-group effect (f = 0.2, or of medium size) of difference across time based on previous MBI trials at postintervention with 80% power,20 two-sided P < .05, two assessment points, and 0.60test-retest reliability for the PSQI.41 A 10% attrition rate was anticipated, making our target enrollment 24 per group.
Statistical Analysis
Between-group change in the mean PSQI at postintervention was the primary outcome in the intent-to-treat population. Analyses were performed in SPSS, version 21 (IBM Corporation). Between-group contrasts in outcomes across the intervention period were tested using generalized linear mixed modeling (MIXED command with full information maximum likelihood estimation to allow for analysis of participants with missing data) with pairwise comparisons, adjusted for preintervention levels of the outcome. Estimated mean differences and effect sizes (Cohen d with Hedges bias correction for small sample size)with their 95%CIs are provided. We assessed whether data provided evidence of a benefit of MAPs vs SHE on the PSQI and secondary daytime impairment and NF-κB signaling outcomes. Baseline PSQIs between those with vs without missing data at postintervention were compared using t tests.
Results
Participant Flow and Characteristics
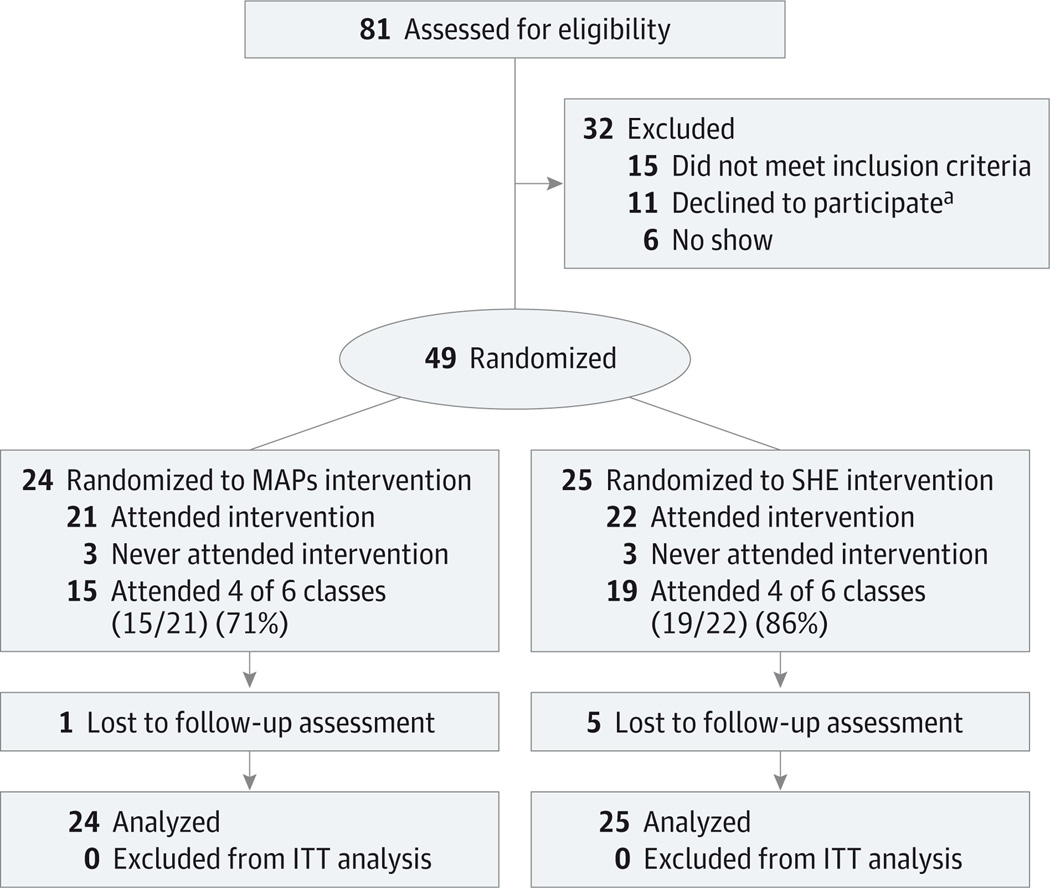
Participant flow through enrollment, randomization, follow-up, and analysis phases of the trial is shown in Figure 1. After being screened for eligibility, 49 older adults were randomized to treatment (a 6-week intervention [2 hours per week]), and 43 of them attended treatment visits in the interval between March and December 2012. The mean (SD)session attendance was similar across the MAPs(4.2 [1.9]) and SHE(4.7 [1.5]) groups(t = 0.79, P = .31). In the MAPs group, the median meditation practice time across the intervention period was 17.2 hours (range, 3.7–65.3 hours),and the median number of unique practice sessions was 111 (range, 37–179).No adverse events related to participation in the study were reported.
Figure 1. Consolidated Standards of Reporting Trials Flow Diagram of the Single-Site, Parallel-Group Randomized Clinical Trial of MAPs Compared With SHE for Sleep Problems in Older Adults.
ITT indicates intent-to-treat; MAPs, mindful awareness practices; and SHE, sleep hygiene education.
aReasons included time limitations (n = 10) and upcoming surgery (n = 1), reportedly unrelated to sleep.
Table 1 lists summary descriptive statistics for the study groups at baseline. None of the baseline variables differed across treatment groups by chance (P < .05 for all). At baseline, the mean(SD) age of participants was 66.3 (7.4 years), and 67% (33 of 49) were female. The mean (SD) PSQI of 10.2 (3.2) indicated a sample with moderate or greater sleep disturbances. The mean (SD) scores of 11.0 (9.1) on the BeckDepression Inventory II and 13.7 (9.3) on the Beck Anxiety Inventory were consistent with a nonclinical sample based on mental health status.
Table 1.
Baseline Demographic and Clinical Characteristics by Intervention Group and Total Sample
| Variable | Total Sample (N = 49) |
MAPs (n = 24) |
SHE (n = 25) |
|---|---|---|---|
| Age, mean (SD), y | 66.3 (7.4) | 66.5 (6.3) | 66.1 (8.5) |
| Education level, mean (SD), y | 16.6 (3.0) | 16.1 (2.6) | 15.6 (6.1) |
| Not employed, No. (%) | 34 (69) | 14 (58) | 20 (80) |
| Married, No. (%) | 25 (51) | 11 (46) | 14 (56) |
| Female sex, No. (%) | 33 (67) | 16 (67) | 17 (68) |
| Hispanic, No. (%) | 5 (10) | 3 (13) | 2 (8) |
| Race/ethnicity, No. (%) | |||
| Asian | 1 (2) | 0 | 1 (4) |
| Black | 2 (4) | 1 (4) | 1 (4) |
| White | 41 (84) | 21 (88) | 20 (80) |
| Pacific Islander | 1 (2) | 0 | 1 (4) |
| Other | 4 (8) | 2 (8) | 2 (8) |
| BMI, mean (SD) | 24.8 (3.9) | 25.0 (4.2) | 24.7 (3.8) |
| Waist to hip ratio, mean (SD) | 0.90 (0.11) | 0.92 (0.13) | 0.88 (0.09) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MAPs, mindful awareness practices; SHE, sleep hygiene education.
As assessed with the Five Facet Mindfulness Questionnaire, the mean (SD) level of mindfulness significantly increased in the MAPs group (3.4 [0.2] at baseline and 3.6 [0.2] at postintervention) but did not change in the SHE group (3.3 [0.3] at baseline and 3.4 [0.3] at postintervention) across the intervention period. The between-group mean difference was 0.2 (95%CI, 0.1–0.3), with an effect size of 0.76 (P = .008), indicating that mindfulness levels changed only in the MAPs group, as intended. Forty-three participants (88%) completed the postintervention assessment. Those lost to follow- up showed PSQI baseline scores statistically equivalent to those of the retained sample, indicating that the level of sleep disruption was independent of dropout.
Primary Outcome
Primary and secondary outcome intent-to-treat analyses included all participants (N = 49) according to original group assignment regardless of program attendance or missing data. The PSQI of the MAPs group improved by a mean of 2.8 and of the SHE group by a mean of 1.1, indicating greater improvement in the MAPs group (between-group mean difference, 1.8; 95%CI, 0.6–2.9) (Table 2 and Figure 2). The PSQI changes were correlated with Five Facet Mindfulness Questionnaire nonreactivity change scores in the MAPs group (r = −0.46, P = .04) but not in the SHE group (r = −0.03, P = .91). The same correlation pattern was replicated for Five Facet Mindfulness Questionnaire nonreactivity and the secondary sleep measure (Athens Insomnia Scale) (r = −0.53, P = .02 for the MAPs group and r = −0.23, P = .35 for the SHE group).
Table 2.
Intent-to-Treat Model Estimates for Primary and Secondary Outcome Measuresa
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| MAPs (n = 24) |
SHE (n = 25) |
Value (95% CI) | ||||
| Outcome, Scale Range |
Baseline | Postintervention | Baseline | Postintervention | Difference in Change Values |
Effect Sizeb |
| Primary Outcome | ||||||
| Pittsburgh Sleep Quality Index, 0–21 | 10.2 (1.7) | 7.4 (1.9)c | 10.2 (1.8) | 9.1 (2.0)d | 1.8 (0.6 to 2.9) | 0.89 (0.28 to 1.50) |
| Secondary Outcomes | ||||||
| Athens Insomnia Scale, 0–24 | 11.6 (2.2) | 9.1 (2.4)c | 11.7 (2.2) | 10.7 (2.5) | 1.6 (0.2 to 3.0) | 0.65 (0.07 to 1.22) |
| Beck Depression Inventory II, 0–63 | 10.9 (4.9) | 7.0 (5.4)c | 11.0 (4.9) | 10.9 (5.6) | 3.8 (0.7 to 6.9) | 0.68 (0.11 to 1.26) |
| Beck Anxiety Inventory, 0–63 | 13.5 (4.5) | 10.6 (4.9)d | 13.4 (4.5) | 10.8 (5.2)d | 0.3 (−2.6 to 3.1) | 0.05 (−0.61 to 0.51) |
| Perceived Stress Scale, 0–40 | 22.0 (3.7) | 16.9 (4.1) | 22.1 (3.7) | 18.3 (4.3) | 1.4 (−1.0 to 3.8) | 0.33 (−0.89 to 0.23) |
| Fatigue Symptom Inventory– Interference, 0–90 | 33.2 (9.0) | 22.6 (9.9)c | 33.1 (9.0) | 33.1 (10.4) | 10.5 (4.7 to 16.3) | 1.02 (0.43 to 1.62) |
| Fatigue Symptom Inventory–Severity, 0–40 | 17.3 (3.5) | 13.6 (3.8)c | 17.1 (3.5) | 19.5 (4.0)c | 6.0 (3.7 to 8.2) | 1.50 (0.86 to 2.13) |
| NF-κB p65 ng/µg of total protein | 0.48 (0.10) | 0.36 (0.10)c | 0.48 (0.05) | 0.38 (0.10)c | 0.03 (−0.07 to 0.02) | 0.20 (−0.76 to 0.36) |
Abbreviations: MAPs, mindful awareness practices; NF, nuclear factor; SHE, sleep hygiene education.
Lower scores for all scales indicate improvement.
Bias-corrected Cohen d (0.2 is small, 0.5 is medium, and 0.8 is large).
Significant change from baseline to postintervention at P < .05.
Significant change from baseline to postintervention at P < .09.
Figure 2. Estimated Pittsburgh Sleep Quality Index at Preintervention and Postintervention.
Data are given as means (SEs). MAPs indicates mindful awareness practices; SHE, sleep hygiene education.
aP = .002 for difference between groups, covarying for preintervention Pittsburgh Sleep Quality Index.
Secondary Outcomes
Daytime Impairment
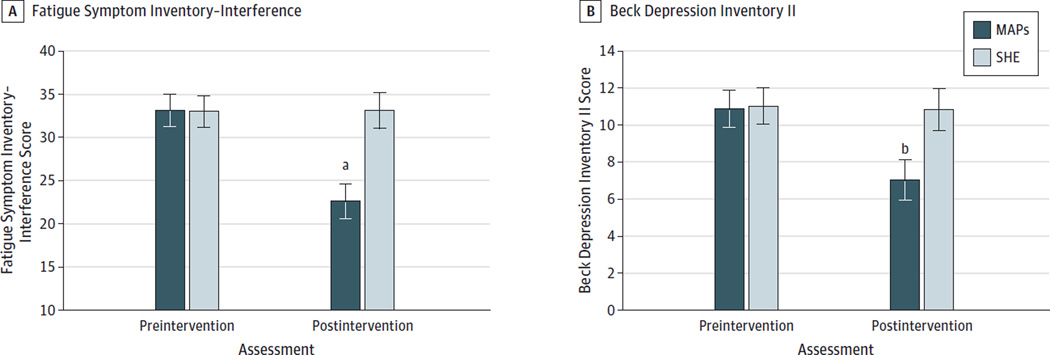
Table 2 lists the summary results for the sleep-related daytime impairment outcomes. The Athens Insomnia Scale (P = .03), Fatigue Symptom Inventory–Interference (P = .001 in Figure 3A), Fatigue Symptom Inventory–Severity (P < .001), and Beck Depression Inventory II (P = .02 in Figure 3B) scores in the MAPs group showed improvement relative to the SHE group at immediate posttreatment. Reductions in Beck Anxiety Inventory scores over time were significant (P < .05), but changes were equivalent between groups (P = .85).
Figure 3. Estimated Fatigue Symptom Inventory–Interference and Beck Depression Inventory II Scores at Preintervention and Postintervention.
Data are given as means (SEs). MAPs indicates mindful awareness practices; SHE, sleep hygiene education.
aP = .001 for difference between groups, covarying for pretreatment Fatigue Symptom Inventory–Interference score.
b P = .02 for difference between groups, covarying for pretreatment Beck Depression Inventory II score.
Inflammatory Signaling
Significant mean reductions over time were statistically equivalent across treatment groups for NF-κB (P = .26). Although the reduction was slightly greater in the MAPs group, this difference was not significant (Table 2).
Discussion
This RCT examined the effect of a structured mindfulness meditation program on moderate sleep disturbances in older adults. Our main findings indicate that the program, which is available to the general community, resulted in improvement in sleep quality at postintervention relative to a highly active and standardized SHE program. The effect size of 0.89 for improvement in sleep quality was large and of clinical relevance considering that effect sizes obtained from all types of behavioral interventions on self-reported sleep quality outcomes averaged 0.76 among older adults.51 Meta-analyses13,52 comparing treatment modalities indicate that the mean effect sizes for self-reported sleep improvements resulting from pharmacotherapy (effect size, 0.87) (ie, benzodiazepines and benzodiazepine receptor agonists) and cognitive behavior therapy (effect size, 0.96) are of medium to large magnitude in mixed-age adult samples with primary insomnia. Therefore, our observed changes are consistent with previous findings and are at the level of a minimally important difference for insomnia severity.53 The MAPs for daily living also yielded relative improvements in sleep-related daytime impairment of depression and fatigue that were of medium to large effect size. According to our findings, mindfulness meditation appears to have a role in addressing the prevalent burden of sleep problems among older adults1–5 by remediating their moderate sleep disturbances and deficits in daytime functioning, with short-term effect sizes commensurate with the status quo of clinical treatment approaches for sleep problems.13,52
Mindfulness meditation is believed to function on arousal and neurocognitive processes that mediate the relationship between perception of stimuli and appraisal. Structural and functional brain changes support the role of mindfulness meditation in modulating these two processes.54,55 Froma cognitive behavior perspective, sleep problems stem from automatic arousal, dysfunctional cognitions, and consequential distress.56 Previous studies indicate that mindfulness meditation can attenuate such automatic responses and increase the relaxation response through its function of increasing attentional factors that impart control over the autonomic nervous system,57 reduce worry and rumination,58,59 and alleviate mood disturbances.18,60 The potential mechanisms of our MBI based mindfulness meditation include cognitive behavior and intrapsychic processes that influence arousal and reactivity in a manner that improves sleep, daytime functioning, or perceptions of sleep and wakefulness.
A previous RCT20 showed that a MBI outperformed a Food and Drug Administration–approved sleep aid on the PSQI in persons with primary insomnia at postintervention (mean difference, 1.69).We extend this work by using a larger RCT with a nonpharmacological comparison condition adequately controlled for time, attention, social support, and other nonspecific effects. In our older adult sample with moderate sleep disturbances, we observed a similar effect size on PSQIs at postintervention in a population with subsyndromal insomnia. Our study also differed because we found relative improvements in depression score with the Beck Depression Inventory II in older adults, while previous work found no changes in depression score.20 We further extend the literature by showing relative improvements in daytime fatigue.
Strengths of our study include that it is the first RCT to date to examine the effect of an MBI on sleep disturbances and solely among older adults. Enrollment of older adults has a large potential health impact because this population reports among the highest prevalence of sleep problems. The validated measures used are reproducible in future work and are correlated with sleep diary reports, insomnia diagnosis, and actigraphy readings.61,62 MAPs is a fully developed community-offered program, and our SHE program can be viewed as a highly active psychoeducational program. Evaluation of NF-κB concentration was a novel method to explore an immune signal that is responsive to sleep disruption. Reductions in NF-κB concentrations after meditation corroborate the results from previous studies with older adults.33,34 The SHE program also reduced NF-κB concentrations over time, suggesting that this approach to addressing moderate sleep disturbances might also confer some anti-inflammatory benefit, but future trials are needed to elucidate this potential.
Our study has some potential limitations. Despite rigorous efforts to obtain data from all enrollees, 12% (6 of 49) did not complete the postintervention assessment. An intent-to-treat approach was used to obtain pragmatic estimates for the groups as originally randomized to conditions. The preponderance of female participants (67%[33 of 49]) and those with a higher educational level might constrain the external validity of our findings; however, the prevalence of sleep disturbances is highest among women,63 and the findings herein need to be replicated for racial/ethnic and socioeconomic diversity. The results are limited to older adults with normal cognitive abilities. Our focus on adults with moderate sleep disturbances does not lend itself to primary and tertiary levels of care. Studies need to examine whether MBIs are effective for preventing future sleep problems in older adult healthy sleepers and for treating older adults diagnosed as having insomnia. Objective measures (eg, actigraphy) were omitted because of funding constraints, and future studies should add objective protocols.
Conclusions
The findings from our study suggest that mindfulness meditation may be introduced to older adults as a short-term solution to remediate their moderate sleep disturbances, although research is needed to determine possible longer-term effects on sleep. Given that standardized mindfulness programs are readily delivered in many communities, dissemination efforts do not serve as a barrier in this instance. Therefore, older adults often have immediate access to these programs, which are offered at low cost. Pending future replication of these findings, structured mindfulness meditation training appears to have at least some clinical usefulness to remediate moderate sleep problems and sleep-related daytime impairment in older adults
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant P30-AG028748 from the University of California, Los Angeles Older Americans Independence Center; grants UL1TR000124 and 1UL1RR033176 from the University of California, Los Angeles Clinical and Translational Science Institute; grant 5T32-MH019925 from the National Institute of Mental Health (Dr Black); grants R01-AG034588, R01-AG026364, R01-CA160245, R01-CA160245- 01, R01-CA119159, R01-HL095799, R01-DA032922, R01-DA032922-01, and P30-AG028748 from the National Institutes of Health (Dr Irwin); the Cousins Center for Psychoneuroimmunology; the Pettit Family Foundation; and the Furlotti Family Foundation.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank our research assistants Samira Sayari, MPH (Cedars-Sinai Medical Center), and Deena Margolin, BS (University of Southern California), the experienced mindfulness teachers Marvin Belzer, PhD, and Diana Winston, BS (both at University of California, Los Angeles), Christian Perez, BS (University of California, Los Angeles), for the performance of NF-κB assays, and the study participants for their time.
Footnotes
Author Contributions: Drs Black and Olmstead had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Black, Breen, Irwin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Black, O’Reilly, Breen, Irwin.
Critical revision of the manuscript for important intellectual content: Black, Olmstead, Breen, Irwin.
Statistical analysis: Black, O’Reilly, Olmstead, Irwin.
Obtained funding: Black, Irwin.
Administrative, technical, or material support: Black, Breen, Irwin.
Study supervision: Black, Breen, Irwin.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14(2):95–103. doi: 10.1097/01.JGP.0000196627.12010.d1. [DOI] [PubMed] [Google Scholar]
- 2.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Prinz PN. Sleep and sleep disorders in older adults. J Clin Neurophysiol. 1995;12(2):139–146. doi: 10.1097/00004691-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Gramling SE. Sleep patterns and aging: comparison of older adults with and without insomnia complaints. Psychol Aging. 1989;4(3):290–294. doi: 10.1037//0882-7974.4.3.290. [DOI] [PubMed] [Google Scholar]
- 7.Benca RM. Sleep in psychiatric disorders. Neurol Clin. 1996;14(4):739–764. doi: 10.1016/s0733-8619(05)70283-8. [DOI] [PubMed] [Google Scholar]
- 8.McCrae CS, Rowe MA, Tierney CG, Dautovich ND, Definis AL, McNamara JP. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60(4):182–189. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- 9.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280. [PubMed] [Google Scholar]
- 10.National Sleep Foundation. Aging and sleep. [Accessed February 4, 2014]; http://www.sleepfoundation.org/article/sleep-topics/aging-and-sleep. [Google Scholar]
- 11.Vitiello MV. Sleep disorders and aging. Curr Opin Psychiatry. 1996;9(4):284–289. [Google Scholar]
- 12.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 13.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, III, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278(24):2170–2177. [PubMed] [Google Scholar]
- 14.Silber MH. Clinical practice: chronic insomnia. N Engl J Med. 2005;353(8):803–810. doi: 10.1056/NEJMcp043762. [DOI] [PubMed] [Google Scholar]
- 15.Morgenthaler T, Kramer M, Alessi C, et al. American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update: an American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 16.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2002;(2):CD003161. doi: 10.1002/14651858.CD003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 20.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore (NY) 2011;7(2):76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbling A, Reilly-Spong M, Kreitzer MJ, Gross CR. How mindfulness changed my sleep: focus groups with chronic insomnia patients. BMC Complement Altern Med. 2014;14(1):50. doi: 10.1186/1472-6882-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore (NY) 2007;3(6):585–591. doi: 10.1016/j.explore.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Andersen SR, Wurtzen H, Steding-Jessen M, et al. Effect of mindfulness-based stress reduction on sleep quality: results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52(2):336–344. doi: 10.3109/0284186X.2012.745948. [DOI] [PubMed] [Google Scholar]
- 24.Britton WB, Haynes PL, Fridel KW, Bootzin RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72(6):539–548. doi: 10.1097/PSY.0b013e3181dc1bad. [DOI] [PubMed] [Google Scholar]
- 25.Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore (NY) 2009;5(1):30–36. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of tai chi chih. Sleep. 2008;31(7):1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J AmGeriatr Soc. 2004;52(6):892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 28.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16(5):503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 29.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 30.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65(12, pt 2):S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84(8):2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 33.Black DS, Cole SW, Irwin MR, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38(3):348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black DS, Irwin MR, Olmstead R, Ji E, Crabb Breen E, Motivala SJ. Tai chi meditation effects on nuclear factor-κB signaling in lonely older adults: a randomized controlled trial. Psychother Psychosom. 2014;83(5):315–317. doi: 10.1159/000359956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Smalley SL, Winston D. Fully Present: The Science, Art, Practice of Mindfulness. Boston, MA: Da Capo Press; 2010. [Google Scholar]
- 39.Patlak M. Your Guide to Healthy Sleep. Washington, DC: US Dept of Health and Human Services; 2005. [Google Scholar]
- 40.National Sleep Foundation. Sleep tools & tips. [Accessed December 26, 2014]; http://sleepfoundation.org/sleep-tools-tips. [Google Scholar]
- 41.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 42.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 43.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 45.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 46.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 47.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 48.Bohlmeijer E, ten Klooster PM, Fledderus M, Veehof M, Baer R. Psychometric properties of the Five Facet Mindfulness Questionnaire in depressed adults and development of a short form. Assessment. 2011;18(3):308–320. doi: 10.1177/1073191111408231. [DOI] [PubMed] [Google Scholar]
- 49.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 50.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 52.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 53.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 54.Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? a systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 56.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 57.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–1585. [PubMed] [Google Scholar]
- 58.Black DS, Milam J, Sussman S. Sitting-meditation interventions among youth: a review of treatment efficacy. Pediatrics. 2009;124(3):e532–e541. doi: 10.1542/peds.2008-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 60.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- 63.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.