Abstract
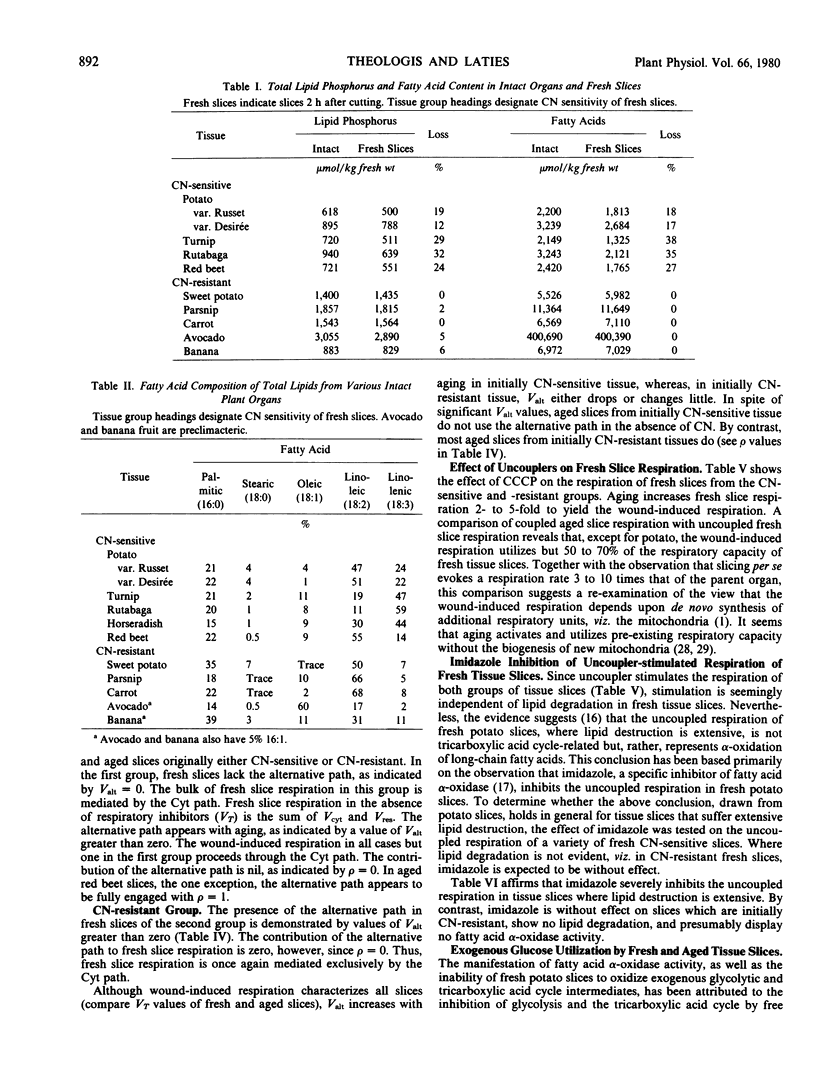
A study of a variety of bulky storage organs and fruits reveals that fresh slices fall into two categories with respect to their sensitivity to CN. Fresh slices in the first class are CN-sensitive, whereas slices of the second class are resistant to, and often stimulated by, CN. In tissue slices which are initially CN-sensitive, cutting initiates a burst of lipolytic activity. In CN-resistant fresh slices, there is no measurable lipid breakdown.
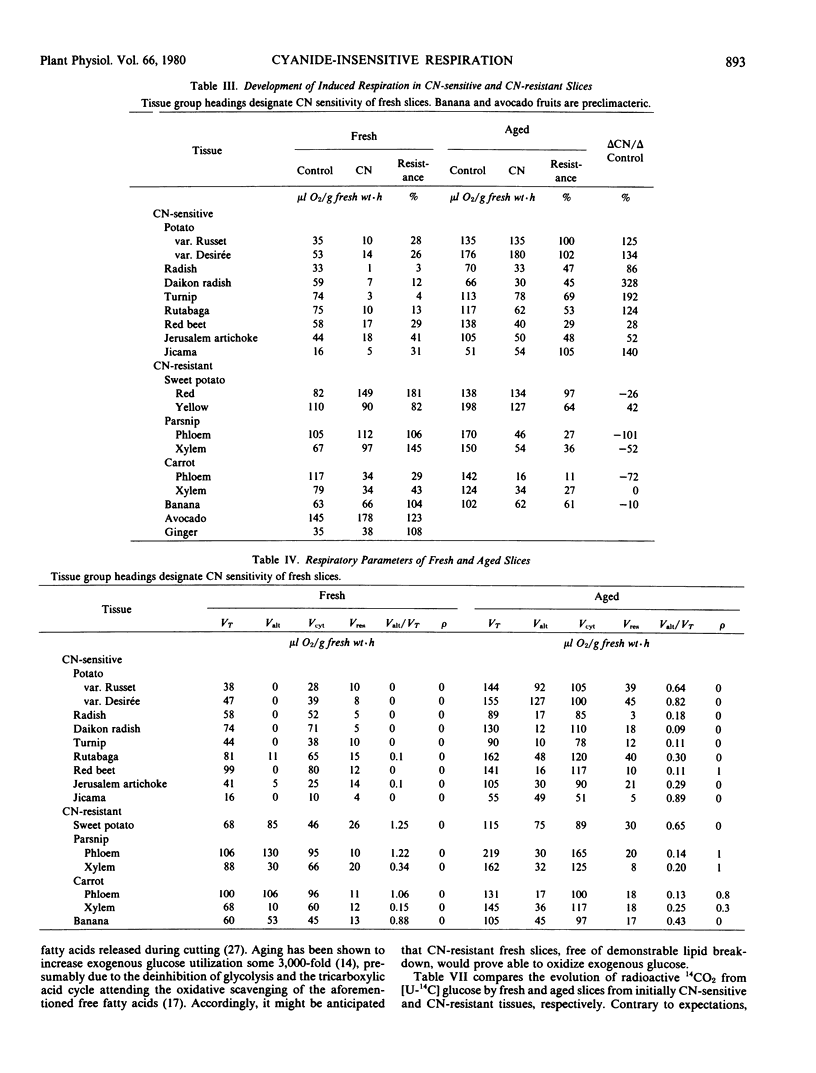
Slicing evokes the wound-respiration which is 5- to 10-fold that of the parent organ. Slice aging, in turn, evokes a further 2- to 3-fold respiratory increase, the wound-induced respiration, whether fresh slice respiration is CN-sensitive or -resistant. Estimation of the contribution by the cytochrome and alternative paths shows that the wound respiration in both groups is mediated by the cytochrome path. On the other hand, the wound-induced respiration in the first class is cytochrome path mediated, whereas, in some members of the second group, both pathways are utilized. Uncouplers of oxidative phosphorylation elicit a CN-sensitive increment in fresh slices as great or greater than the wound-induced respiration. Accordingly, de novo synthesis of mitochondria is ruled out as an explanation of the latter.
The integrity of endomembranes, perhaps including mitochondrial membranes, is seemingly a prerequisite for the operation of the alternative path, that is, alternative path activity is lost concomitantly with membrane lipid breakdown. The development of the wound-induced respiration is not co-extensive with the development of the CN-resistant path in all tissue slices. The fundamental process of aging appears to involve activation of pre-existing respiratory capacity.
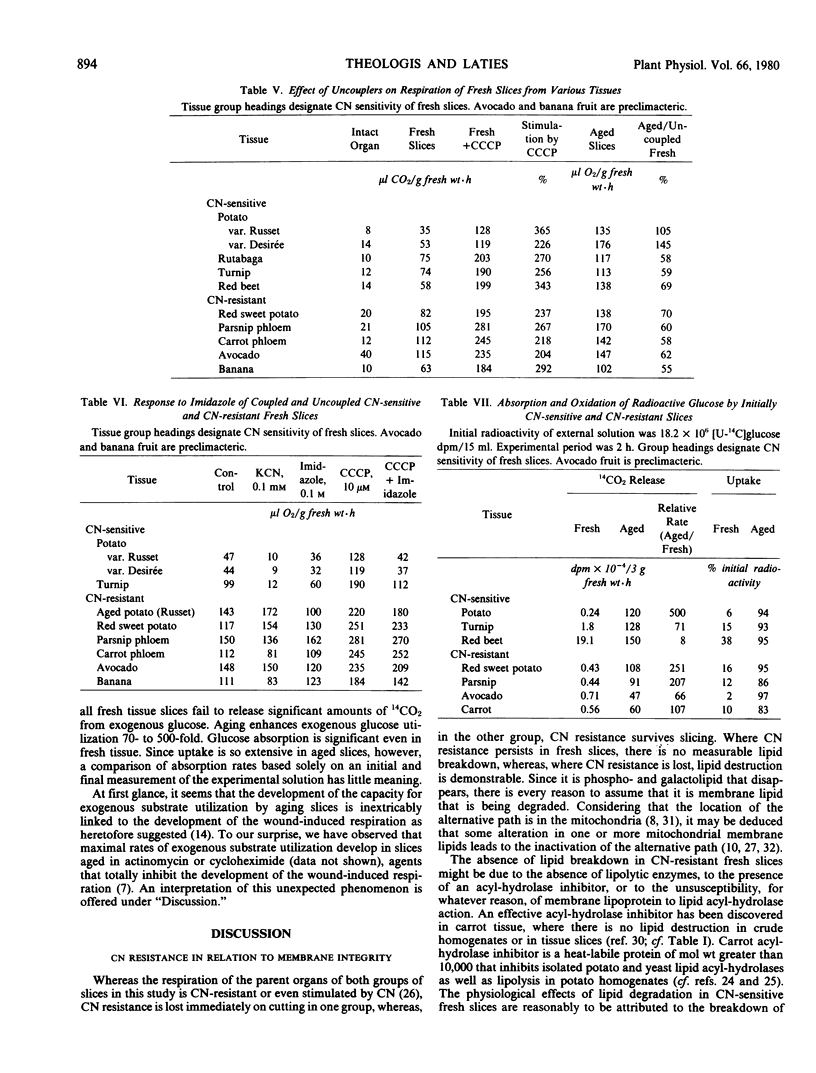
Fresh slices from whatever source fail to utilize exogenous 14C-labeled glucose, whereas aged slices do so readily. A transport lesion is indicated, the healing of which does not depend on the development of the wound-induced respiration but does depend on fatty acid, and presumably membrane lipid, biosynthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizengremel P., Lance C. Control of Changes in Mitochondrial Activities during Aging of Potato Slices. Plant Physiol. 1976 Aug;58(2):147–151. doi: 10.1104/pp.58.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Smith B. N., Epstein S., Laties G. G. The prevalence of carbon-13 in respiratory carbon dioxide as an indicator of the types of endogenous substrate. The change from lipid to carbohydrate during the respiratory rise in potato slices. J Gen Physiol. 1970 Jan;55(1):1–17. doi: 10.1085/jgp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E., Reed D. J. Metabolism of Red Beet Slices II. Effects of Aging. Plant Physiol. 1966 Apr;41(4):661–669. doi: 10.1104/pp.41.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G., Hoelle C. Malonate and Cyanide Insensitivity in Relation to Respiratory Compensation in Potato Slices. Plant Physiol. 1965 Jul;40(4):757–764. doi: 10.1104/pp.40.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G. The Onset of Tricarboxylic Acid Cycle Activity with Aging in Potato Slices. Plant Physiol. 1964 Jul;39(4):654–663. doi: 10.1104/pp.39.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAPSON L. W., BURTON W. G. The terminal oxidases of the potato tuber. Biochem J. 1962 Jan;82:19–25. doi: 10.1042/bj0820019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Induction of ethylene of cyanide-resistant respiration. Biochem Biophys Res Commun. 1976 May 17;70(2):663–671. doi: 10.1016/0006-291x(76)91098-6. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Cyanide-resistant Respiration in Fresh and Aged Sweet Potato Slices. Plant Physiol. 1978 Aug;62(2):243–248. doi: 10.1104/pp.62.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson P. F., Moreland D. E. Cyanide-resistant Respiration of Sweet Potato Mitochondria. Plant Physiol. 1975 Feb;55(2):365–369. doi: 10.1104/pp.55.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Dependence of Wound-induced Respiration in Potato Slices on the Time-restricted Actinomycin-sensitive Biosynthesis of Phospholipid. Plant Physiol. 1977 Jul;60(1):5–10. doi: 10.1104/pp.60.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Laties G. G. Inhibition of the Development of Induced Respiration and Cyanide-insensitive Respiration in Potato Tuber Slices by Cerulenin and Dimethylaminoethanol. Plant Physiol. 1977 Jul;60(1):11–16. doi: 10.1104/pp.60.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]



