Abstract
In recent years, there has been significant progress in the clinical development and application of antiangiogenic therapies in renal cell carcinomas, particularly inhibitors of the vascular endothelial growth factor (VEGF) pathway. Despite this progress, no validated methods are currently available for identifying which patients are most likely to respond to treatment or experience toxic effects, selecting the optimal dose, or determining whether the intended molecular target has been effectively inhibited. However, recent studies have suggested that some of the biomarkers currently under investigation in clear cell renal cell carcinoma for VEGF pathway inhibitors are promising. These biomarkers include circulating proangiogenic factors and receptors; markers of hypoxia and endothelial damage; and cellular populations in peripheral blood, such as circulating endothelial cells. Further preclinical and translational validation studies are still needed to determine their practical utility in the clinical setting.
Keywords: blood-based biomarkers, vascular endothelial growth factor, angiogenesis inhibitors, renal cell carcinoma, circulating endothelial cells
During the past decade, antiangiogenic therapy has moved from theory to clinical practice. Bevacizumab, amonoclonal antibody directed against vascular endothelial growth factor (VEGF), has been demonstrated to provide clinical benefit when combined with chemotherapy for colorectal, lung, and breast cancer. Furthermore, bevacizumab and multitargeted inhibitors blocking the VEGF receptor (VEGFR) pathway, such as sunitinib and sorafenib, have demonstrated significant clinical activity in chemotherapy-refractory tumors, such as renal cell carcinoma (RCC).1–3 Despite these advances, the biologic activity of these and newer agents remains difficult to assess. Given the large number of targeted agents now entering clinical testing in RCC and the escalating costs of drug development, it is generally not feasible to perform large randomized trials for drugs without extra evidence of biologic activity early in their development. Consequently, a real risk remains that drugs that could ultimately benefit patients may not be developed and even that many patients might be treated with less effective drug doses or schedules in phase 2 or 3 trials because of a lack of correlates of activity in earlier clinical testing. Therefore, surrogate biomarkers are clearly needed to advance the clinical development of VEGF inhibitors. These markers may serve some, or all, of the following uses: 1) assessment of expected biologic activity; 2) optimization of dosing; 3) identification of patients most likely, or least likely, to benefit from a given treatment; and/or 4) monitoring response to treatment and investigating potential mechanisms of resistance.
SOLUBLE MARKERS IN SERUM AND PLASMA
Circulating Angiogenic Growth Factors, Inhibitors, and Related Vascular Molecules
Most patients with advanced RCC demonstrate clear cell histology, which is typically characterized by von Hippel-Lindau gene (VHL) inactivation. This process leads to an abnormal accumulation of hypoxia-inducible factor (HIF) and a deregulated HIF-1 activity that results in the transcription of >200 hypoxia-inducible genes, including mediators of angiogenesis such as VEGF, platelet- derived growth factor, transforming growth factor–α, erythropoietin, and carbonic anhydrase IX (CAIX).4 Several molecules implicated in angiogenesis can be detected in meaningful amounts in circulation (serum or plasma) and other biologic fluids in patients with RCC and serve as biomarkers for monitoring anti-VEGF therapies.
The majority of antiangiogenic drugs used in clinical practice or currently under development in RCC target the VEGF signaling pathway directly or indirectly. Patients with RCC demonstrate increased VEGF levels compared with healthy controls.5 High serum VEGF levels have been associated with tumor stage, tumor grade, disease progression, and poor prognosis.5–7 The Groupe Français d’Immunotherapie recently presented data indicating an independent correlation between VEGF and event-free and overall survival in metastatic RCC patients with good and intermediate prognostic characteristics.8 Similar results were found in patients treated with placebo or bevacizumab in combination with interferon-α in the phase 3 TARGET (sorafenib) and AVOREN clinical trials, respectively. Above–median VEGF concentrations were found to be correlated with significantly shorter progression-free survival (PFS),7,9 supporting the notion of VEGF being a prognostic biomarker in clear cell RCC. Interestingly, patients with high and low pretreatment VEGF levels benefited equally from sorafenib (5.5 months in terms of PFS) in the TARGET trial.7
Plasma and serum VEGF levels are also currently being actively investigated as biomarkers of activity of VEGF inhibitors. In preclinical models, blood plasma levels of VEGF are rapidly and significantly increased by VEGFR-2 blockade in a dose-dependent manner,10 with maximum values peaking when doses previously determined to be optimal for therapy were used. The mechanisms responsible for these fluctuations in serum or plasma VEGF are not known. Likewise, VEGF levels increase during VEGF-targeted therapy in RCC patients,7,11,12 but the extent of VEGF modulation varies widely in different individuals and depends on the specific drug, its potency, and its mechanism of action.13
VEGF-A binds to 2 receptor tyrosine kinases: VEGFR-1 (Flt-1) and VEGFR-2 (KDR, flk-1). Naturally occurring, soluble forms of VEGFR-1 (sVEGFR-1), VEGFR-2, and VEGFR-3 (which is involved in lymphangiogenesis) have been described previously.13–15 Soluble VEGFR-1 has been studied as a surrogate marker for inhibition of angiogenesis in RCC.16 The functional significance of sVEGFR-2 and sVEGFR-3 remains unclear, but decreases in plasma sVEGFR-2 appear to be related to VEGF-induced down-regulation from the cell surface.17 VEGF and sVEGFR-2 change in a reciprocal manner during the treatment of patients with RCC and other cancers with tyrosine kinase inhibitors.7,13,18–20 Initial experiences in assessing changes in serum or plasma levels of these factors as a surrogate for exposure to anti-VEGF agents and even patient benefit suggest a clear potential value. Most of the available results in RCC come from sunitinib-treated patients, but similar data have been shown for sorafenib and pazopanib (Table 1). Overall, larger changes in VEGF, sVEGFR-2, and sVEGFR-3 levels have been observed in patients achieving treatment response, suggesting their potential value as biomarkers of pharmacologic and clinical activity.13,21
Table 1.
Reported Effects of Tyrosine Kinase Inhibitors on VEGF and sVEGFR-2 in Renal Cell Carcinoma Patients
| Drug | Target | VEGF | sVEGFR-2 | Study |
|---|---|---|---|---|
| Sorafenib | VEGF-1, VEGF-2, VEGF-3, PDGFR, RET, Raf | ↑ | ↓ | Bukowski 20077 |
| Sunitinib | VEGF-1, VEGF-2, PDGFR, KIT, RET | ↑ | ↓ | Motzer 200612 Deprimo 200713 George 200721 |
| Axitinib | VEGF-1, VEGF-2, VEGF-3, PDGFR, KIT | ↑ | ↓ | Rixe 200519 |
| Pazopanib | VEGF-1, VEGF-2, VEGF-3, PDGFR | ↑ | ↓ | Hutson 200818 |
| Cediranib | VEGF-1, VEGF-2, VEGF-3, PDGFR, KIT | NA | ↓ | van Herpen 200720 |
VEGF indicates vascular endothelial growth factor; sVEGFR, soluble vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; NA, not applicable.
Other Molecules Regulatory of Angiogenesis
Circulating serum and/or plasma levels of fibroblast growth factor–2, placental growth factor, hepatocyte growth factor, thrombospondin-1, soluble Tie-2, and interleukin–8 have been explored for their potential as surrogates of angiogenesis and to monitor response to VEGF-targeted therapies. In RCC, elevations in plasma levels of placental growth factor have been reported after therapy with sunitinib.12,13,21
Markers of Endothelial Cell Damage and Hemostasis
VEGF inhibition markedly increases the thromboembolic risk in cancer patients.22–25 Markers of endothelial cell function or damage include von Willebrand factor, soluble thrombomodulin, soluble tissue factor, and soluble E-selectin. To our knowledge, few data are available regarding modulation of these factors by antiangiogenic therapy in RCC.
Markers of Hypoxia
Hypoxia promotes the production of proangiogenic factors, many of which are regulated by the HIF-1α pathway.4 High levels of HIF-1α have been shown in RCC.26 Because VEGF inhibitors may induce tumor hypoxia,27 the role of the HIF-1α pathway in the response to antiangiogenics is an area of active investigation. Data preliminarily presented at the 2008 annual meeting of the American Society of Clinical Oncology suggest that high tumor levels of HIF-2a as assessed by Western blot analysis are predictive of sunitinib response in patients with metastatic RCC.28 Levels of the circulating hypoxia-regulated proteins osteopontin and soluble CAIX have been shown to have prognostic value in RCC29 and hold promise for monitoring the effects of VEGF-targeted therapies. High levels of CAIX expression in tissue (along with GLUT-1, another HIF-regulated marker) have been preliminarily linked to response to sorafenib and improved PFS in RCC patients.30
In summary, several circulating angiogenic growth factors and related vascular molecules appear to have a role as pharmacodynamic biomarkers of exposure to and effect of anti-VEGF therapies. More studies, however, are needed to evaluate their role in response prediction and to validate these initial findings.
CIRCULATING ENDOTHELIAL CELLS AND OTHER CELLULAR MARKERS IN PERIPHERAL BLOOD
Rationale
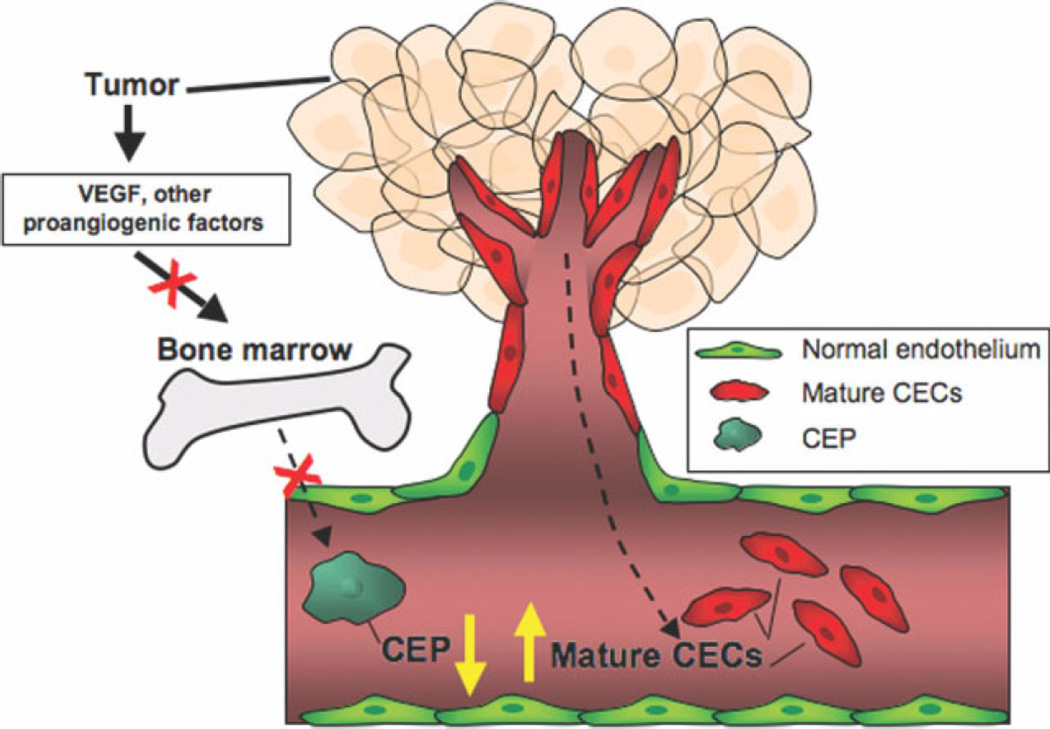
It has been recognized for more than 30 years that cells bearing characteristics of endothelial cells can be detected in peripheral blood. The majority of these cells, termed circulating endothelial cells (CECs), appear to arise from blood vessel walls and are increased after vascular damage.31–33 However, in recent years it has been demonstrated that a subset of CECs derived from bone marrow, the so-called circulating endothelial precursors (CEPs), can differentiate into mature endothelial cells and contribute to neovascularization in both murine models and humans.34 The contribution of CEPs to the tumor vasculature appears to be highly variable and depends on both host and tumor contexts.34–37 In humans, mature CECs and CEPs are distinguished from other peripheral blood mononuclear cells based on surface markers such as VEGFR-2 (KDR) and CD133.38 The rationale for investigating these cells as a potential biomarker is as follows. First, CECs are known to be increased in cancer patients and are known to be associated with disease progression.39,40 Second, these cells are known to be mobilized in response to VEGF in both murine models35,41 and humans42,43 and to express molecular targets for many of the angiogenesis inhibitors currently in development, including VEGFR-1 and VEGFR-2. Finally, CECs may serve as a marker of damage to tumor vasculature and, therefore, reflect activity and potential benefit. Therefore, distinguishing between CEPs and mature CECs is important because one would expect VEGFR inhibitors to decrease the number of CEPs by inhibiting their mobilization but increase the number of sloughed vessel wall derived CECs (Fig. 1).
Figure 1.
Potential changes in circulating endothelial progenitors (CEPs) and mature circulating endothelial cells (CECs) in response to treatment with antiangiogenic agents. Angiogenesis inhibitors may decrease the mobilization of bone marrow-derived CEPs induced by vascular endothelial growth factor (VEGF) and other proangiogenic cytokines but at least transiently increase the shedding of mature endothelial cells by preferentially targeting fragile tumor endothelium (2-compartment model).66
A variety of different methods have been used to detect CECs. Most recent studies applied within the clinical setting have used multicolor flow cytometry using a panel of antibodies to endothelial, hematopoietic, and progenitor cells.44,45 Flow cytometry-based methods have significant advantages in that they permit multiparametric analyses and high-speed measurement. There are, however, significant methodologic issues related to differences in the antibodies and markers used (cell preparation; use of nucleated, activated, replicating, or viable cell staining; absolute or relative counts; and gating strategies) that make it difficult to compare results among different investigators and studies. Furthermore, to the best of our knowledge, no single antigen identified to date is entirely specific to endothelium. A variety of definitions of these different populations have been proposed based on different markers. Most commonly, CD133 has been used as a marker of CEPs; with CD31, CD146, VEGFR-2, and VE-cadherin being common endothelial markers and CD45 being used to identify hematopoietic cells. Clearly, ongoing efforts standardizing these different methods will help compare results from different investigators and from different studies.47
Clinical Results: Changes in CECs in Response to Treatment
CECs (including both mature CECs and CEPs) have been shown to be elevated in RCC patients compared with healthy controls. Ebbinghaus et al investigated blood samples from 77 RCC patients and 19 healthy subjects and found significantly higher CEC and CEP counts in cancer patients.48 More recently, Bhatt et al49 analyzed blood samples from patients with von Hippel-Lindau (VHL) syndrome and RCC and found an increased ratio of CEPs and CECs only in RCC patients with and without VHL syndrome compared with those with VHL syndrome with no RCC and healthy controls.
CECs have been evaluated after treatment with angiogenesis inhibitors in several clinical studies in RCC. It is not yet possible to draw definitive conclusions from those studies to date because they have been of modest size and used different detection methods and phenotypic markers. Nevertheless, they suggest that CECs may be a useful marker that merits further investigation in larger trials (Table 2). In 1 case, baseline levels of CECs were shown to stratify patients with advanced RCC who were receiving treatment with the thrombospondin-1 mimetic peptide ABT-510 with regard to time to disease progression.48 Patients with higher baseline CEC levels had a significantly shorter time to disease progression compared with those with low and stable CECs counts. However, in a smaller study of 21 RCC patients receiving treatment with sunitinib or sorafenib, elevated baseline levels of CECs and low CEPs were found to be correlated with improved treatment response, whereas high CEC counts by Day 14 of treatment predicted poor response.50 A sunitinib- induced increase in CEPs has also been shown to be predictive of treatment benefit.51
Table 2.
Studies Describing Detection of CECs and CEPs by Flow Cytometry in Metastatic Renal Cell Carcinoma Patients
| Study | Cell Phenotype | Drug | No. | Association |
|---|---|---|---|---|
| Ebbinghaus 200548 | CD45−CD31+CD146+LDS751+ | ABT-510 | 77 | CEC <15/µL–longer TTP |
| Escudier 200850 | CECs: | Sunitinib | 21 | High BL CEC–response |
| CD45−CD31+CD146+7AAD− | Sorafenib | High BL CEP–poor prognosis | ||
| CEPs: | High C1 on D 14 CEC–poor response | |||
| CD45dimCD34+VEGFR2+7AAD− | ||||
| Vroling 200851 | CEPs: | Sunitinib | 23 | Increase in CEP C1–response |
| CD45−CD133−CD34bright |
CECs indicates circulating endothelial cells; CEPs, circulating endothelial progenitors; −, negative; +, positive; TTP, time to disease progression; BL, baseline; C1, cycle 1; D 14, Day 14.
These and additional studies in other cancers illustrate that CECs change in a dynamic manner after treatment with an angiogenesis inhibitor and that the kinetics of change, as well as the specific population of cells that are changing, are critical parameters to assess. The development and standardization of robust, reproducible, and multiparametric analytical methods are clearly an important unmet need in the field.
Other Cellular Populations in Peripheral Blood as Potential Biomarkers
Although it was initially assumed that the contribution of bone marrow-derived cells to angiogenesis was through CEPs, it recently has been appreciated that circulating cell populations with a clear hematopoietic origin may contribute as well. Such cells have in common their commitment to the myeloid lineage in a more or less advanced stage of differentiation.
The majority of available data are related to myelomonocytic cells that home to areas of vascular injury and contribute to angiogenesis and metastasis by secreting angiogenic growth factors, facilitating extracellular matrix remodeling, promoting tumor cell motility, and providing structural support to the adjacent endothelial cells by establishing themselves as mural cells or pericyte precursors.52–54 These results suggest that distinct populations of hematopoietic cells may functionally contribute to angiogenesis and metastasis. Also relevant are recent data implicating CD11b+ Gr-1+ cells, a phenotype that corresponds to murine immunosuppressive myeloid cell populations,55 in therapeutic resistance to VEGF pathway inhibition in animal models of cancer.56
However, it is unknown in humans whether, after treatment with antiangiogenic agents, these cell populations may serve as a target or as a biomarker or both. Circulating myeloid-derived cells with immunosuppressive activity, defined as arginase-producing or displaying a CD15+ CD14− CD33+ HLA-DR− phenotype, have been found to be significantly increased in the peripheral blood of patients with metastatic RCC compared with healthy controls.57–59 As a step toward identifying new potential cellular biomarkers for the VEGF pathway, we screened peripheral blood for cellular populations binding VEGF and the expression of VEGFRs. We found that most VEGF-binding cells in peripheral blood were VEGFR-1+ cells coexpressing the monocyte marker CD14 (CECs also expressed VEGFR-1 and VEGFR-2, but were present at lower levels).60 Next, we monitored changes in CEC and monocyte numbers during treatment with sunitinib in patients with metastatic gastrointestinal stromal tumors and correlated these findings with clinical benefit. Mean monocyte numbers decreased by 54% from baseline to Day 14 of treatment, and there was a greater decrease noted in the progressive disease group compared with the clinical benefit group (58% vs 48%). Monocyte levels rebounded toward baseline during a rest period and then repeated the pattern when sunitinib therapy was restarted. In patients with metastatic RCC, initial experience suggests that treatment with sunitinib reverses the accumulation of myeloid-derived suppressor cells.58,59 Therefore, accumulating data indicate that myelomonocytic cells in peripheral blood hold potential as a pharmacodynamic marker for sunitinib activity and clinical benefit in cancer patients, including those with RCC. Because such cells express VEGFR-1 and other potential targets for angiogenesis inhibitors such as platelet-derived growth factor receptor-β, Kit, and Tie-2,60–65 we are currently assessing distinct subpopulations to determine whether that may serve as a more specific biomarker of activity.
FUTURE DIRECTIONS
In part due to recent randomized phase 3 trials in RCC demonstrating that angiogenesis inhibitors can benefit cancer patients, there has been an explosion in the number and variety of these agents currently under development and clinical testing. These advances, although welcomed, present a new set of challenges and obstacles for the field. First, there is a major yet unmet need for standardized, validated, noninvasive markers for evaluating the activity of these agents and for assessing the degree of target inhibition caused by treatment. This is needed for both selecting drugs to advance in clinical development and choosing the optimal dose for a given patient. Second, it will be necessary to identify the mechanisms by which tumors become resistant to these agents and to develop markers to monitor for this resistance. Biomarkers will play a critical role in the rational design of regimens, including combinations of different angiogenesis inhibitors. Third, it will be critical to develop robust predictive markers to guide the selection of the most appropriate agent or combination for the individual patient before initiating therapy. For all these reasons, biomarkers are likely to play an increasingly important role in the clinical development of VEGF inhibitors and other antiangiogenic agents in RCC and the cancer field in general.
Acknowledgments
Conflict of Interest Disclosures
The program was made possible by educational grants provided by Genentech; Novartis Pharmaceuticals, Pfizer, Inc., and Wyeth Pharmaceuticals. Program management and CME sponsorship were provided by InforMEDical Communications, Inc., Carlisle, Mass.
OPEN DISCUSSION
The questions and discussion below follow from the oral presentation given at the Third Cambridge Conference on Innovations and Challenges in Renal Cancer and do not correspond directly to the written article, which is a more general review.
Dr. Bernard Escudier: How can we standardize biomarker thresholds?
Dr. Amado J. Zurita: Only those markers that showed statistically significant interaction with the treatment arm were chosen.
Dr. Gary Hudes: Don’t you need to look at each biomarker separately and determine whether it is a mean value or whether there is a relationship between a certain cutoff value and whatever your endpoint is ?
Dr. Zurita: We did that for the univariate analysis.
Dr. Jeffrey Sosman: Have you looked further at what changes occur with therapy ?
Dr. Zurita: Yes. We accessed changes in biomarker concentrations from baseline to 4 weeks and 8 weeks on treatment.
Dr. Michael Atkins: This multiplex bead profiling technology can be applied to a lot of different diseases. The ideal place to look at this is with antiangiogenic and targeted agents in kidney cancer, where these treatments work. You can gain a sense of what the treatments are doing, you can look at the various pathways and how they change, and you can determine whether they might predict for a particular therapy. We have an advantage in kidney cancer relative to other diseases and an opportunity to test this technology.
Footnotes
Innovations and Challenges in Renal Cancer: Proceedings of the Third Cambridge Conference, Supplement to Cancer
References
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen J, Rasmuson T, Grankvist K, Ljungberg B. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol. 2000;163:343–347. [PubMed] [Google Scholar]
- 6.Negrier S, Perol D, Menetrier-Caux C, et al. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6-from the Groupe Francais d’Immunotherapie. J Clin Oncol. 2004;22:2371–2378. doi: 10.1200/JCO.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski RM, Eisen T, Szczylik C, et al. Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: survival and biomarker analysis. J Clin Oncol. 2007;25(18 suppl):5023. [Google Scholar]
- 8.Negrier S, Chabaud S, Escudier B, et al. Serum level of vascular endothelial growth factor (VEGF) as an independent prognostic factor in metastatic renal cell carcinoma (MRCC) J Clin Oncol. 2007;25(18 suppl):5044. [Google Scholar]
- 9.Escudier BJ, Ravaud A, Negrier S, et al. Update on AVOREN trial in metastatic renal cell carcinoma (mRCC): efficacy and safety in subgroups of patients (pts) and pharmacokinetic (PK) analysis. J Clin Oncol. 2008;26:5025. [Google Scholar]
- 10.Bocci G, Man S, Green SK, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 13.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebos JM, Bocci G, Man S, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor-2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315–326. [PubMed] [Google Scholar]
- 16.Harris AL, Reusch P, Barleon B, Hang C, Dobbs N, Marme D. Soluble Tie2 and Flt1 extracellular domains in serum of patients with renal cancer and response to antiangiogenic therapy. Clin Cancer Res. 2001;7:1992–1997. [PubMed] [Google Scholar]
- 17.Ebos JM, Lee CR, Bogdanovic E, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 18.Hutson TE, Davis ID, Machiels JH, et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multikinase angiogenesis inhibitor. J Clin Oncol. 2008;26:5046. [Google Scholar]
- 19.Rixe O, Meric J, Bloch J, et al. Surrogate markers of activity of AG-013736, a multi-target tyrosine kinase receptor inhibitor, in metastatic renal cell cancer (RCC) J Clin Oncol. 2005;23(16 suppl):3003. [Google Scholar]
- 20.van Herpen C, Drevs J, van Cruijsen H, et al. Evaluation of AZD2171, an oral, highly potent and selective VEGFR signaling inhibitor, in renal cell carcinoma (RCC): combined results from 2 phase I studies. J Clin Oncol. 2007;25(18 suppl):3560. [Google Scholar]
- 21.George DJ, Michaelson MD, Rosenberg JE, et al. Phase II trial of sunitinib in bevacizumab-refractory metastatic renal cell carcinoma (mRCC): updated results and analysis of circulating biomarkers. J Clin Oncol. 2007;25(18 suppl):5035. [Google Scholar]
- 22.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Francia G, Viloria-Petit A, et al. In vitro procoagulant activity induced in endothelial cells by chemotherapy and antiangiogenic drug combinations: modulation by lower-dose chemotherapy. Cancer Res. 2005;65:5365–5373. doi: 10.1158/0008-5472.CAN-04-3156. [DOI] [PubMed] [Google Scholar]
- 24.Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–844. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 26.Wiesener MS, Munchenhagen PM, Berger I, et al. Constitutive activation of hypoxia-inducible genes related to over-expression of hypoxia-inducible factor-1alpha in clear cell renal carcinomas. Cancer Res. 2001;61:5215–5222. [PubMed] [Google Scholar]
- 27.Franco M, Man S, Chen L, et al. Targeted anti-vascular endothelial growth factor receptor-2 therapy leads to shortterm and long-term impairment of vascular function and increase in tumor hypoxia. Cancer Res. 2006;66:3639–3648. doi: 10.1158/0008-5472.CAN-05-3295. [DOI] [PubMed] [Google Scholar]
- 28.Patel PH, Chadalavada RS, Ishill NM, et al. Hypoxiainducible factor (HIF) 1α and 2α levels in cell lines and human tumor predicts response to sunitinib in renal cell carcinoma (RCC) J Clin Oncol. 2008;26:5008. [Google Scholar]
- 29.Ramankulov A, Lein M, Kristiansen G, Meyer HA, Loening SA, Jung K. Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J Cancer Res Clin Oncol. 2007;133:643–652. doi: 10.1007/s00432-007-0215-z. [DOI] [PubMed] [Google Scholar]
- 30.Signoretti S, Regan M, Atkins M. Carbonic anhydrase IX as a predictive biomarker of response to kidney cancer therapy. BJU Int. 2008;101(suppl 4):31–35. doi: 10.1111/j.1464-410X.2008.07646.x. [DOI] [PubMed] [Google Scholar]
- 31.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George F, Brouqui P, Boffa MC, et al. Demonstration of Rickettsia conorii-induced endothelial injury in vivo by measuring circulating endothelial cells, thrombomodulin, and von Willebrand factor in patients with Mediterranean spotted fever. Blood. 1993;82:2109–2116. [PubMed] [Google Scholar]
- 34.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 37.Peters BA, Diaz LA, Polyak K, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 38.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 39.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 40.Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 42.Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 43.Hattori K, Dias S, Heissig B, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry. 2005;64:1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 45.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 46.Duda DG, Cohen KS, di Tomaso E, et al. Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol. 2006;24:1449–1453. doi: 10.1200/JCO.2005.04.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancuso P, Antoniotti P, Quarna J, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 48.Ebbinghaus SW, Hussain M, Tannir NM, et al. A randomized phase 2 study of the thrombospondin-mimetic peptide ABT-510 in patients with previously untreated advanced renal cell carcinoma. J Clin Oncol. 2005;23(16 suppl):4607. [Google Scholar]
- 49.Bhatt RS, Norden-Zfoni A, O’Neill A, et al. Circulating endothelial cells are a potential biomarker for patients with renal cell carcinoma (RCC) with and without von Hippel Lindau (VHL) syndrome. J Clin Oncol. 2008;26:5098. [Google Scholar]
- 50.Escudier BJ, Taylor M, Koscielny S, et al. Circulating endothelial cells and progenitor cells in metastatic renal cell carcinoma: predictive value during antiangiogenic therapy [abstract]. Proceedings of the 2008 Genitourinary Cancers Symposium; February 14–16, 2008; San Francisco, Ca. Abstract 390. [Google Scholar]
- 51.Vroling L, Van der Veldt AAM, De Haas RR, et al. CD34bright/CD133neg candidate circulating endothelial progenitor cells (ccEPCs) are a potential biomarker during treatment with sunitinib or bevacizumab [abstract]. Proceedings of the 2008 Genitourinary Cancers Symposium; February 14–16, 2008; San Francisco, Ca. Abstract 4956. [Google Scholar]
- 52.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 56.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 57.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 58.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid derived suppressor cell accumulation in metastatic renal cell carcinoma patients [abstract]. Proceedings of the 2008 Genitourinary Cancers Symposium; February 14–16, 2008; San Francisco, Ca. Abstract 125. [Google Scholar]
- 59.Salas RN, Ireland JL, Ko JS, et al. Immune cell changes in the peripheral blood of metastatic renal cell carcinoma (mRCC) patients (pts) treated with sunitinib or temsirolimus. J Clin Oncol. 2008;26:5099. [Google Scholar]
- 60.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 61.George S, Rayman PA, Biswas S, et al. Expression of FLT3 and VEGFR1 on myeloid derived suppressor cells (MDSC) in renal cell carcinoma (RCC) patients (Pts) J Clin Oncol. 2008;26:5108. [Google Scholar]
- 62.Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 63.Dewar AL, Domaschenz RM, Doherty KV, Hughes TP, Lyons AB. Imatinib inhibits the in vitro development of the monocyte/macrophage lineage from normal human bone marrow progenitors. Leukemia. 2003;17:1713–1721. doi: 10.1038/sj.leu.2403071. [DOI] [PubMed] [Google Scholar]
- 64.Inaba T, Shimano H, Gotoda T, et al. Expression of platelet- derived growth factor beta receptor on human monocyte- derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. J Biol Chem. 1993;268:24353–24360. [PubMed] [Google Scholar]
- 65.Venneri MA, De Palma M, Ponzoni M, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 66.Beaudry P, Force J, Naumov GN, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11:3514–3522. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]