Abstract
Rationale: Obesity, especially truncal obesity, is a risk factor for asthma incidence, prevalence, and severity. Chitinase 3–like-1 (Chi3l1) is an evolutionarily conserved moiety that plays a critical role in antipathogen and Th2 responses. However, the mechanisms that underlie the association between asthma and obesity and the role(s) of Chi3l1 in fat accumulation have not been defined.
Objectives: To determine whether Chi3l1 is regulated by a high-fat diet (HFD) and simultaneously plays an important role(s) in the pathogenesis of asthma and obesity.
Methods: We evaluated the regulation of Chi3l1 by an HFD and Th2 inflammation. We also used genetically modified mice to define the roles of Chi3l1 in white adipose tissue (WAT) accumulation and Th2 inflammation and blockers of sirtuin 1 (Sirt1) to define its roles in these responses. Finally, the human relevance of these findings was assessed with a case–controlstudy involving obese and lean control subjects and those with asthma.
Measurements and Main Results: These studies demonstrate that an HFD and aeroallergen challenge augment the expression of WAT and pulmonary Chi3l1. Chi3l1 also played a critical role in WAT accumulation and lung Th2 inflammation. In addition, Chi3l1 inhibited Sirt1 expression, and the deficient visceral fat and Th2 responses in Chi3l1 null mice were reversed by Sirt1 inhibition. Finally, serum and sputum Chi3l1 were positively associated with truncal adiposity, and serum Chi3l1 was associated with persistent asthma and low lung function in obese subjects with asthma.
Conclusions: Chi3l1 is induced by an HFD and Th2 inflammation, and simultaneously contributes to the genesis of obesity and asthma.
Keywords: chitinase 3–like-1, adipose tissue, asthma, allergic Th2 inflammation, sirtuin 1
At a Glance Commentary
Scientific Knowledge on the Subject
Epidemiologic studies have highlighted a significant association between obesity and asthma prevalence, incidence, and severity. However, the mechanistic link between these two pandemic diseases is still enigmatic. Chitinase 3–like-1 (Chi3l1)/YKL-40 is an evolutionarily conserved member of the 18 glycosyl hydrolase family that plays a major role in antipathogen responses, inflammation, and remodeling. However, the mechanistic connections between Chi3l1, asthma, and obesity have not been defined.
What This Study Adds to the Field
These are the first studies to demonstrate that Chi3l1 plays a critical role in both visceral fat accumulation and adaptive Th2 inflammation, and highlight the roles of sirtuin 1 in these responses and the correlations between circulating and sputum Chi3l1 and measures of human obesity.
The world is currently experiencing contemporaneous epidemics of obesity and asthma. In the United States, the prevalence of obesity (defined as a body mass index [BMI] ≥ 30 kg·m−2) increased from approximately 15% in 1975 to 35% in 2010 (1). Epidemiologic studies have consistently demonstrated that obesity is a risk factor for asthma incidence (2, 3) and prevalence (4), as well as increased asthma severity and poor disease control (1, 5, 6). In many of these studies, the most impressive associations were between asthma and measures of abdominal or visceral obesity (1, 5, 6). Overall, the association between obesity and asthma is so striking that obese subjects with asthma are now considered to represent a “unique” asthma phenotype that does not respond well to inhaled corticosteroids (4, 7, 8). The mechanistic basis for the obesity–asthma association, however, has not been adequately defined (3, 9–11). Specifically, although past studies have hypothesized that the mechanical and adipokine-related effects of visceral adiposity cause or worsen asthma (4, 12), the alternative possibility, that asthma and obesity share a common pathogenetic mechanism(s) that is activated in these patients, has not been addressed.
Chitinase 3–like-1 (Chi3l1; a chitinase-like protein, also called YKL-40 in humans and breast regression protein-39 in rodents) is a member of the 18 glycosyl hydrolase gene family, which binds to but does not degrade chitin (13). The retention over species and evolutionary time has led to the belief that some of these moieties play essential roles in biology (14, 15). In support of this speculation, recent studies from our laboratory and others have demonstrated that Chi3l1 plays a major role in antipathogen, antigen-induced, and oxidant-induced inflammatory, repair, and remodeling responses by regulating a variety of essential biologic processes, including oxidant injury, apoptosis, pyroptosis, inflammasome activation, Th1/Th2 inflammatory balance, M2 macrophage differentiation, transforming growth factor-β1 elaboration, dendritic cell accumulation, and the activation of mitogen-activated protein kinase, Akt, and Wnt/β-catenin signaling (4, 16–21). Studies from our laboratory and others have also identified significant correlations between dysregulated Chi3l1 and the development, severity, and/or progression of a number of diseases, including asthma and obesity (as reviewed in Refs. 13, 22). These studies strongly suggest that Chi3l1 plays an important role(s) in the biology that underlies asthma, obesity, and other disorders. However, the mechanistic contributions of Chi3l1 in obesity and asthma have not been adequately defined.
Sirtuin 1 (Sirt1) is a histone deacetylase that regulates a number of transcription factors that are essential for metabolism, endocrine signaling, and inflammation, including peroxisome proliferator–activated receptor-γ, forkhead transcription factor, p53, and nuclear factor (NF)-κB (23, 24). Sirt1 is believed to be an important regulator of the energy metabolism associated with obesity and allergen-induced airway inflammation and airway hyperresponsiveness (25–27). However, its roles in these disorders have not been adequately defined, and its relationship to Chi3l1 has not been addressed.
We hypothesized that Chi3l1 simultaneously plays a causal role in asthma-like Th2 inflammation and visceral fat accumulation. To test this hypothesis, we characterized the expression and roles of Chi3l1 in animal models of asthma and obesity using wild-type (WT) and Chi3l1 null mutant mice. These studies demonstrate that Chi3l1 is readily detected and significantly induced in the lung and visceral adipose tissue after allergen-challenge and/or or a high-fat diet (HFD), respectively. They also demonstrate that, in the absence of Chi3l1, allergen-induced Th2 inflammation and visceral fat accumulation are significantly reduced compared with WT controls and that these effects are mediated by Sirt1. Finally, we confirmed our murine findings in humans where serum and sputum Chi3l1/YKL-40 correlated with parameters of truncal obesity and serum Chi3l1 was associated with persistent asthma and low lung function in obese subjects with asthma.
Methods
A detailed description of the methods is provided in the online supplement.
Mice Used for the Experiments
Chi3l1/breast regression protein-39 null mutant (Chi3l1−/−) mice and transgenic mice in which Chi3l1/YKL-40 was targeted to the lung using the CC10 promoter (YKL-40 transgenic mice [Tg]) were generated and characterized in our laboratory, as previously described (19). All murine procedures were approved by the Institutional Animal Care and Use Committee at Yale University (New Haven, CT).
Obesity Studies
Age- and sex-matched Chi3l1−/− male mice and WT controls were fed regular chow (RC) or an HFD (TD88137; Harlan Inc. South Easton, MA) ad libitum for 12–24 weeks.
Adaptive Th2 Inflammation
Age- and sex-matched mice were sensitized and challenged with ovalbumin (OVA) as previously described (19). At 1 day after the last challenge, the mice were killed, bronchoalveolar lavage was undertaken, and tissue responses were evaluated.
Administration of Sirtinol
Sirtinol, a Sirt1 inhibitor (Enzo Life Sciences, Farmingdale, NY) or vehicle control (0.05% dimethyl sulofoxide) were diluted with phosphate-buffered saline and administered via an intraperitoneal route.
Histologic Evaluation, Messenger RNA, and Western Analysis
The reagents and assessment procedures are described in the online supplement.
Adipocyte Isolation
Epididymal fat pads from Chi3l1−/− and WT controls were removed and adipocytes were isolated as previously described (28–30).
Human Studies
A case–control study design was used, in which 180 subjects were included: 93 control subjects and 87 subjects with asthma. Asthma was defined by a “provider diagnosis” and a “positive” (provocative concentration of methacholine causing a 20% drop in FEV1 of ≤16 mg/m) methacholine challenge test. The methacholine challenge test was performed as per the American Thoracic Society guidelines (31, 32). Additional details on the subjects are described in the online supplement.
Statistical Analysis
Spearman correlations and regression analyses were mainly used in human studies. Mouse data are expressed as means (±SEM). A P value of 0.05 or less was considered to be significant. Statistical methods are described in the online supplement.
Results
Regulation of Murine Adipose Tissue and Pulmonary Chi3l1 by an HFD
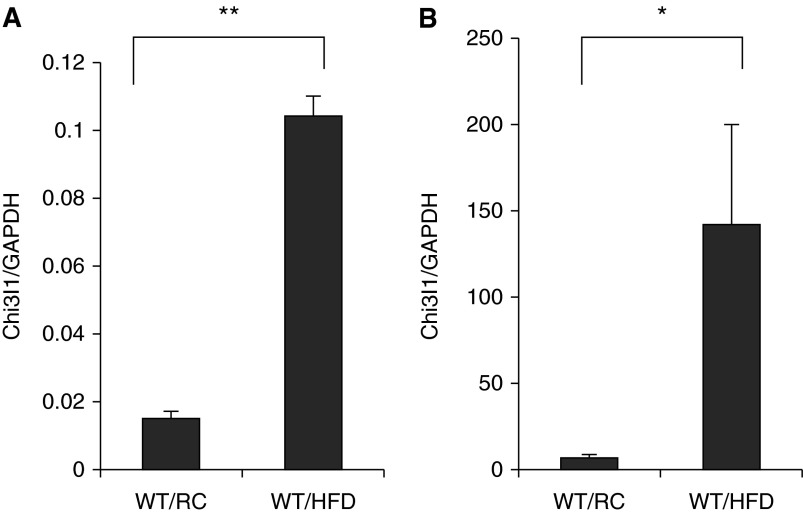
In these experiments, we compared the expression of Chi3l1 in white adipose tissue (WAT) and pulmonary tissues in mice on RC or an HFD. In mice on RC, Chi3l1 gene expression was readily appreciated in WAT and lung tissues (Figures 1A and 1B). After 12–24 weeks on an HFD, a significant increase in the expression of Chi3l1 was seen in WAT and pulmonary tissues (Figures 1A and 1B). These studies demonstrate that Chi3l1 is expressed by WAT and lung tissues, and that this expression is significantly enhanced by an HFD in both tissue compartments.
Figure 1.
High-fat diet (HFD) regulation of chitinase 3–like-1 (Chi3l1) gene expression. Wild-type (WT) mice received HFD or regular chow (RC). (A) The levels of messenger RNA encoding Chi3l1 in epididymal fat pad. (B) The levels of mRNA encoding Chi3l1 in total lung tissue. The noted values represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05, **P ≤ 0.01. GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Chi3l1 Plays a Critical Role in Murine Visceral Fat Accumulation
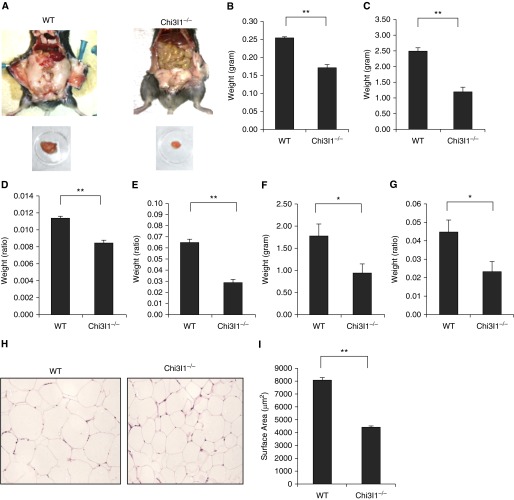
To define the role(s) of Chi3l1 in these tissues, we first compared the levels of visceral fat in Chi3l1 null mice and WT controls. As can be seen in Figure 2, abdominal visceral fat accumulation was diminished in Chi3l1 null mice compared with WT controls (Figure 2A). In accord with these findings, the epididymal fat pads from Chi3l1 null mice were also smaller than those from controls (Figure 2A). In contrast, the transgenic overexpression of Chi3l1/YKL-40 augmented visceral fat accumulation (see Figure E1 in the online supplement). Because reduced visceral fat mass was seen in Chi3l1 null mice, both on RC and on HFD (Figures 2B and 2C, respectively), these alterations were not the result of diet. These alterations were also not related to differences in the size of the animals, because the differences remained significant when total body weight was accounted for (Figures 2D and 2E). The alterations were also not limited to epididymal fat pads, because perirenal fat mass size (Figures 2F and 2G) was similarly altered. Interestingly, the reduction in visceral fat mass in Chi3l1 null mice was due, at least in part, to significantly smaller adipocyte size in Chi3l1 null mice versus controls (Figures 2H and 2I), even when body weight was accounted for (Figures E2A and E2B). These studies demonstrate that Chi3l1 plays a significant role in the accumulation of visceral fat in mice.
Figure 2.
Chitinase 3–like-1 (Chi3l1) regulation of visceral fat accumulation in mice. (A) A representative photograph showing abdominal fat and epididymal fat pads from wild-type (WT) and Chi3l1−/− mice after 12 weeks on a regular diet (regular chow [RC]). (B and C) The weight of epididymal fat pads in WT and Chi3l1−/− mice on RC or a high-fat diet (HFD), respectively. (D and E) The ratio of epididymal fat pad to the total body weight of mice on RC or an HFD, respectively. (F) The weight of perirenal fat pads from WT and Chi3l1−/− mice on an HFD. (G) The ratio of perirenal fat to the total body weight of mice on an HFD. (H) Hematoxylin and eosin photomicrograph of epididymal fat pads from WT and Chi3l1−/− mice. (I) Morphometric evaluation of adipocyte cell size after 12 weeks on an HFD. The noted values in B, C, D–G, and I represent the mean ± SEM of a minimum of five animals (*P ≤ 0.05, **P ≤ 0.01). (A and H) Representative of a minimum of three similar evaluations.
Chi3l1 Plays a Critical Role in Murine Visceral Fat Cytokine Elaboration
To determine if Chi3l1 regulates visceral fat cytokine expression and elaboration, we compared the cytokine production by visceral WAT from Chi3l1 null mice and WT controls. When compared with equal amounts of WAT from WT mice, the levels of messenger RNA encoding tumor necrosis factor-α, IL-10, and IL-6 were significantly lower in WAT from Chi3l1 null mice (Figures 3A–3C). In accord with these findings with whole adipose tissue, adipocytes isolated from Chi3l1 null mice also produced significantly less IL-1β compared with cells from controls (Figures 3D and 3E). These studies demonstrate that Chi3l1 production is associated with enhanced fat accumulation and adipocyte cytokine production.
Figure 3.
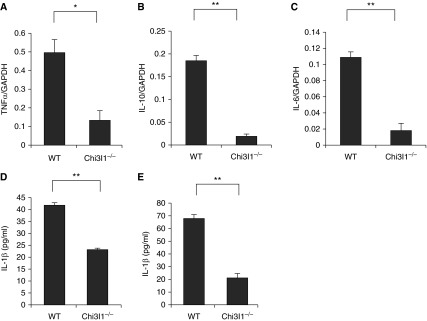
Chitinase 3–like-1 (Chi3l1) regulation of murine epididymal fat pad cytokines. (A–C) The levels of messenger RNA encoding the noted cytokines in fat pads from wild-type (WT) and Chi3l1−/− mice after 12 weeks on a high-fat diet: (A) tumor necrosis factor (TNF)-α; (B) IL-10; and (C) IL-6. (D and E) We assessed the levels of supernatant IL-1β released by adipocytes and epididymal fat pads (100 mg), respectively, from WT and Chi3l1−/− mice after 24 hours in culture. The noted values represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05, **P ≤ 0.01. GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Chi3l1 Plays an Essential Role in Murine Pulmonary Th2 Inflammation
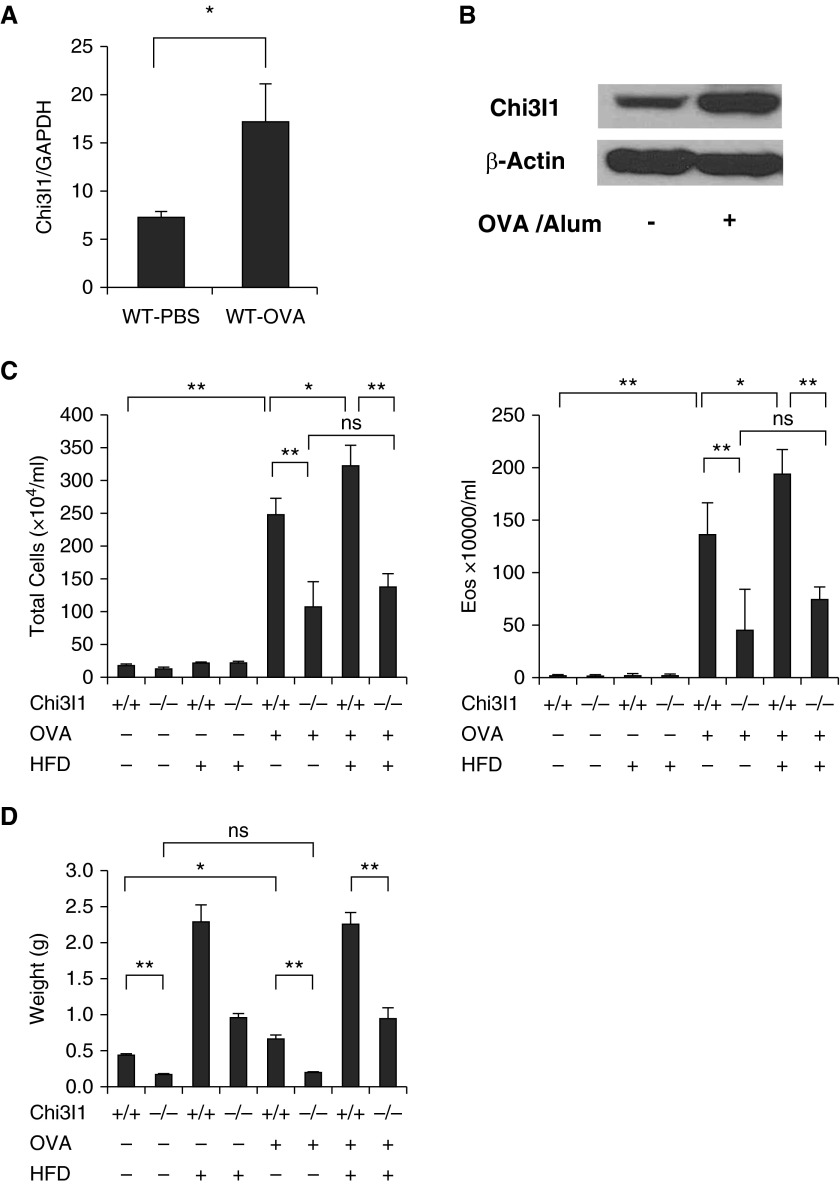
Because visceral fat mass correlates with asthma severity (33), studies were undertaken to define the role of Chi3l1 in the regulation of adaptive Th2 inflammation in the lung. Aeroallergen sensitization and challenge stimulated the expression and production of Chi3l1 (Figures 4A and 4B). In keeping with prior observations (19) with OVA and house dust mite antigens, pulmonary aeroallergen-induced Th2 inflammation was significantly decreased in Chi3l1 null mice versus WT controls (Figure 4C). When viewed in combination, these studies demonstrate that Chi3l1 plays a critical role in adaptive Th2 pulmonary inflammation.
Figure 4.
Chitinase 3–like-1 (Chi3l1) in murine allergen (ovalbumin [OVA])– and high-fat diet (HFD)–stimulated pulmonary inflammation visceral fat accumulation. Wild-type (WT) mice were treated with vehicle (phosphate-buffered saline [PBS]) or sensitized and challenged with aeroallergen (OVA). (A) The levels of Chi3l1 messenger RNA in total lung tissue. (B) Chi3l1 protein detected by Western blot from total lung tissues. (C) Total cell and eosinophil recovery in bronchoalveolar lavage fluids from WT and Chi3l1−/− mice that had been sensitized and challenged with OVA (OVA+) or vehicle control (OVA−). Comparisons are made of mice on an HFD (HFD+) with those on regular chow (RC; HFD−). (D) The weight of epididymal fat pads from the mice that had been sensitized and challenged with OVA (OVA+) or vehicle control (OVA−). Comparisons are made of mice on an HFD (HFD+) with those on RC (HFD−). The values in A, C, and D represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05, **P ≤ 0.01. Eos = eosinophils; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; ns = not significant.
Chi3l1 Plays a Critical Role in Allergic Inflammation and Epididymal Fat Accumulation in Mice on an HFD
To further define the role(s) of Chi3l1 as a molecule that links obesity and asthma, we evaluated the aeroallergen-induced airway inflammatory responses and visceral fat accumulation in WT and Chi3l1−/− mice on a regular diet or an HFD. These studies demonstrated that HFD-fed mice manifest a significant increase in OVA-induced bronchoalveolar lavage total cell and eosinophil recovery compared with regular diet–fed mice (Figure 4C). Importantly, the OVA-induced responses in mice on a regular diet or an HFD were both significantly ameliorated in Chi3l1 null mice (Figure 4C). An HFD and, to a lesser degree, OVA sensitization and challenge both also significantly enhanced epididymal fat accumulation (Figure 4D). Importantly, these augmented fat responses were also significantly decreased in mice that lacked Chi3l1 (Figure 4D). These studies demonstrate that Chi3l1 plays a critical role in the augmented adaptive Th2 inflammation and the fat accumulation in mice on RC or an HFD.
Chi3l1 Inhibits Sirt1 in Murine Lung and Adipose Tissue
Studies using WT and genetically modified mice revealed an interesting relationship between Chi3l1 and Sirt1. Specifically, messenger RNA encoding Sirt1 was readily appreciated in lungs from WT mice (Figures 5A). In contrast, enhanced levels of pulmonary Sirt1 gene expression were seen in lung tissues from Chi3l1 null mice (Figure 5A). This difference was also augmented after aeroallergen-induced sensitization and challenge (Figure 5B). Similarly, Sirt1 was readily appreciated in visceral WAT, and the levels of expression were increased in in the absence of Chi3l1 in tissues from mice on either RC or an HFD (Figures 5C and 5D). These studies demonstrate that Chi3l1 inhibits Sirt1 in both lung tissues and visceral fat.
Figure 5.
Chitinase 3–like-1 (Chi3l1) regulation of sirtuin 1 (Sirt1) gene expression. (A) Sirt1 messenger RNA (mRNA) levels in lungs from wild-type (WT) and Chi3l1−/− mice. (B) Sirt1 mRNA levels in the lungs from WT and Chi3l1−/− mice after ovalbumin (OVA) sensitization and challenge. (C) Sirt1 mRNA levels in epididymal fat pads from mice on regular chow (RC). (D) Sirt1 mRNA levels in epididymal fat pads from mice on a high-fat diet (HFD). The noted values represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05. GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Sirt1 Interacts with Chi3l1 to Mediate Proinflammatory and Antiinflammatory Effects on Murine Adaptive Th2 Inflammation
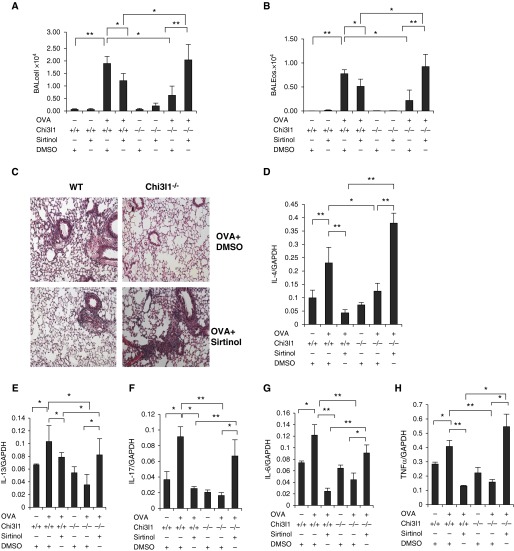
Studies were next undertaken to determine if Sirt1 played important roles in the Th2 and related responses in lungs from WT and Chi3l1 null mice. As noted above, Sirt1 gene expression was readily appreciated in lungs from WT mice and augmented in the setting of Th2 inflammation. Interestingly, systemic Sirt1 blockade in WT mice diminished aeroallergen-induced Th2 inflammation and Th2, IL-17 and IL-6 cytokine production and mucus production in the lung (Figures 6A–6H and Figure E3). As also noted previously here, when compared with WT controls, the levels of Th2 inflammation were markedly decreased, whereas Sirt1 expression was enhanced, in Chi3l1 null mice. In these mice, systemic Sirt1 blockade restored aeroallergen-induced Th2 inflammation and Th2, IL-17, and IL-6 cytokine expression, and airway mucus to levels that approached those in WT mice (Figures 6A–6H and Figure E3). In keeping with reports in the literature, these studies demonstrate that Sirt1 has both proinflammatory and antiinflammatory effects on pulmonary Th2 inflammation, and that the effect that is seen is regulated by Chi3l1. Specifically, they demonstrate that Sirt1 augments Th2 inflammation in the presence of Chi3l1 and inhibits Th2 inflammation in the absence of Chi3l1.
Figure 6.
The effects of the sirtuin 1 inhibitor (sirtinol) on lung Th2 inflammation. Wild-type (WT) and chitinase 3–like-1 (Chi3l1)−/− mice were sensitized and challenged with ovalbumin (OVA+) or phosphate-buffered saline (OVA−) and were treated with sirtinol (sirtinol+) or its vehicle (dimethyl sulofoxide [DMSO]; sirtinol−). Each animal received drug (0.4 mg/kg body weight/d) or vehicle 1 hour before every antigen challenge. (A and B) Bronchoalveolar lavage (BAL) total cell and eosinophil recovery. (C) Hematoxylin and eosin photomicrographs of lungs from WT and Chi3l1−/− mice treated with sirtinol or vehicle after OVA challenge. (D–H) The levels of messenger RNA encoding IL-4, IL-13, IL-17, IL-6, and tumor necrosis factor (TNF)-α in the lungs of WT and Chi3l1−/− mice treated with sirtinol or DMSO. The values in A, B, and D–H represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05, **P ≤ 0.01. Eos = eosinophils; GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Sirt1 Regulation of Murine Visceral Adipose Tissue
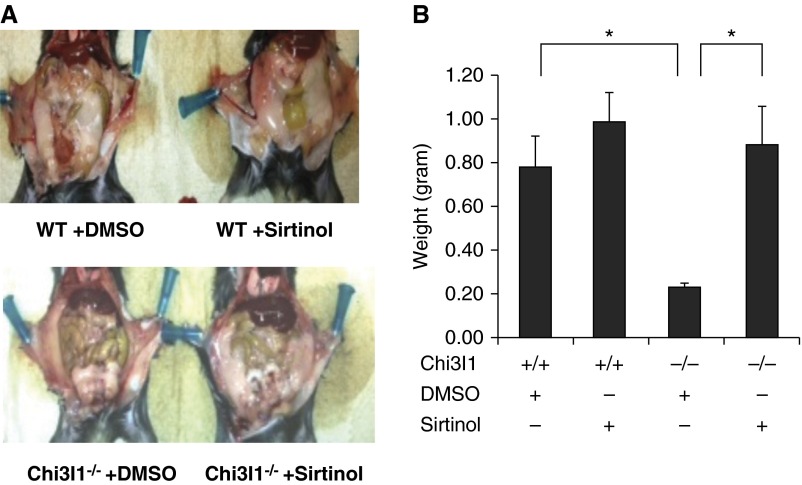
The studies noted previously demonstrate that visceral fat accumulation is markedly diminished, whereas Sirt1 expression is increased, in mice that lack Chi3l1. To define the role(s) of Sirt1 in these phenotypes systemic Sirt1 blockade was employed. As can be seen in Figure 7, Sirt1 blockade did not significantly alter the levels of visceral fat in WT mice. In contrast, Sirt1 blockade restored the levels of visceral fat in Chi3l1 null mice to levels that approached those in WT animals (Figures 7A and 7B). These studies demonstrate that Sirt1 inhibits visceral fat accumulation in mice that lack Chi3l1.
Figure 7.
The effect of sirtuin 1 inhibition on fat accumulation. Wild-type (WT) and chitinase 3–like-1 (Chi3l1)−/− mice were treated with sirtinol or its vehicle (dimethyl sulofoxide [DMSO]), and fat accumulation was assessed. Each animal received drug (0.4 mg/kg body weight/d) or vehicle twice per week for 5 weeks. (A) A representative photograph illustrating abdominal fat accumulation. (B) The weight of epididymal fat pads from WT and Chi3l1−/− mice. (A) Representative of at least three similar evaluations. The values in B represent the mean ± SEM of a minimum of five animals. *P ≤ 0.05.
Serum and Sputum Chi3l1/YKL-40 Are Positively and Independently Associated with Truncal Adiposity in Humans
Studies were next undertaken to define the human relevance of our murine findings. This was first undertaken by characterizing the levels of serum Chi3l1/YKL-40 and anthropometric and dual-energy X-ray absorptiometry (DEXA) parameters in patients who were lean or obese with or without asthma (as detailed in Table E1). These studies demonstrate that serum levels of Chi3l1/YKL-40 were positively associated with generally all mass measures, both overall (Table 1) as well as within the four individual groups (asthma or controls; lean or obese; Figure E4). Among all anthropometric measures, waist circumference was most strongly positively associated with serum Chi3l1/YKL-40 in the overall population, by a stepwise regression analysis (Table 1). After adjustment for standard covariates and asthma status, waist circumference was still significantly associated with serum Chi3l1/YKL-40 (Table 1). When all DEXA mass measures and BMI were instead included in a stepwise regression, the resulting statistical model included truncal lean mass, peripheral lean mass, and arm fat mass indices as the significant predictors (P ≤ 0.006; Table 2). After adjustment for standard covariates, asthma status, and the other two DEXA mass indices, truncal lean mass was still positively associated with serum Chi3l1/YKL-40 in the overall population (P < 0.001; Table 2). These studies highlight associations between Chi3l1/YKL-40 and truncal obesity in subjects with and those without asthma. They are in accord with a prior study that showed that truncal lean mass (possibly measuring intravisceral fat) is the strongest obesity measure predicting asthma in women (34).
Table 1.
Association between Serum Chi3l1/YKL-40 and Anthropometric Measures in Humans
| BMI/Skinfold Thickness Measure Variable | Univariate Analysis
(n = 180) |
Multivariable Analysis
(n = 180)* |
||
|---|---|---|---|---|
| Standardized β | P Value | Standardized β | P Value | |
| Waist circumference†‡ | 0.37 | <0.001 | 0.37 | <0.001 |
| Hip circumference | 0.29 | <0.001 | ||
| Waist to hip ratio | 0.31 | <0.001 | ||
| Subscapular skinfold thickness | 0.22 | 0.003 | ||
| Triceps skinfold thickness | 0.20 | 0.006 | ||
| Chest skinfold thickness | 0.30 | <0.001 | ||
| Midaxillary skinfold thickness | 0.32 | <0.001 | ||
| Abdominal skinfold thickness | 0.27 | <0.001 | ||
| Suprailiac skinfold thickness | 0.23 | 0.002 | ||
| Thigh skinfold thickness | 0.18 | 0.02 | ||
| BMI | 0.33 | <0.001 | ||
Definition of abbreviations: BMI = body mass index; Chi3l1 = chitinase 3–like-1.
In the fully adjusted multivariable model, asthma status and standard covariates were included.
Waist circumference was the strongest predictor in a stepwise regression, and therefore the multivariable analysis was performed only for this measure. Additional adjustment for sputum Chi3l1/YKL-40 concentrations in the multivariable analysis did not change the results (standardized β = 0.46, P < 0.001).
A graphic illustration of the linear positive relationship between (logarithmically transformed) serum YKL-40 concentrations and waist circumference is presented in Figure E4. This finding was verified by using a quadratic fit model as well.
Table 2.
Association between Serum Chi3l1/YKL-40 and Dual-Energy X-Ray Absorptiometry Fat and Lean Mass Measures in Humans
| BMI/DEXA Mass Measure Variable | Univariate Analysis
(n = 174) |
Multivariable Analysis
(n = 174)* |
||
|---|---|---|---|---|
| Standardized β | P Value | Standardized β | P Value | |
| Total fat mass index | 0.27 | <0.001 | ||
| Truncal fat mass index | 0.31 | <0.001 | ||
| Peripheral fat mass index | 0.21 | 0.004 | ||
| Arm fat mass index† | 0.29 | <0.001 | 0.27 | 0.006 |
| Leg fat mass index | 0.18 | 0.02 | ||
| Total lean mass index | 0.26 | <0.001 | ||
| Truncal lean mass index† | 0.34 | <0.001 | 0.51 | <0.001 |
| Peripheral lean mass index† | 0.13 | 0.09 | −0.41 | 0.001 |
| Arm lean mass index | 0.08 | 0.27 | ||
| Leg lean mass index | 0.13 | 0.08 | ||
| BMI | 0.33 | <0.001 | ||
Definition of abbreviations: BMI = body mass index; Chi3l1 = chitinase 3–like-1; DEXA = dual-energy X-ray absorptiometry.
All indices were obtained by dividing the weight of that measure by the square of height (kg/m2). Six of 180 eligible individuals did not have DEXA evaluations.
In the fully adjusted multivariable model, asthma status, standard covariates, and the three DEXA mass indices were included.
Truncal lean mass, peripheral lean mass, and arm fat mass indices were the strongest predictors in a stepwise regression, and therefore the multivariable analysis was performed only for these measures. The separate relationships between truncal lean mass, peripheral lean mass, and arm fat mass indices with serum YKL-40 were generally unchanged after adding sputum Chi3l1/YKL-40 in the multivariable model (standardized β = 0.57, −0.29, and 0.22, respectively with P < 0.001, 0.04, and 0.053, respectively).
To further understand the relationships between Chi3l1/YKL-40 and obesity, we also measured the levels of sputum Chi3l1/YKL-40 and characterized its relationship to anthropometric and DEXA parameters in our cohort. These studies demonstrated that the levels of sputum Chi3l1/YKL-40 were positively associated with generally all mass measures, both overall (Tables E2 and E3) as well as within the four populations. Among all anthropometric measures, waist circumference was most strongly positively associated with sputum Chi3l1/YKL-40 in the overall population by a stepwise regression analysis. Importantly, serum and sputum levels of Chi3l1/YKL-40 were separately correlated with parameters of truncal obesity, and these associations were independent of each another. Specifically, when the sputum levels were accounted for, serum levels of Chi3l1/YKL-40 were still correlated with parameters of obesity and vice versa (Table 1 and 2 footnotes). Thus, in keeping with our murine studies, the present human study demonstrates that the levels of serum and sputum Chi3l1/YKL-40 correlate with truncal adiposity.
Serum Chi3l1/YKL-40 Is Positively Associated with Persistent Asthma in Humans
Studies were next undertaken to define the relationship between serum Chi3l1/YKL-40 and asthma. This was done by stratifying the subjects with asthma into patients with intermittent and persistent asthma and comparing to control subjects. This study revealed that serum Chi3l1/YKL-40 concentrations were positively associated with persistent asthma (Table E4), with P ≤ 0.04 without and with adjustment for standard covariates. Interestingly, this association was not explained by sputum Chi3l1/YKL-40, as inclusion of this variable in the statistical model did not change the results.
Serum Chi3l1/YKL-40 Is Inversely Associated with FEV1, Only in Obese Subjects with Asthma
We next evaluated the correlation in humans between serum Chi3l1/YKL-40 and prebronchodilator FEV1, a measure of asthma control and severity. When analyzed as four groups (obese asthma, normal-weight asthma, obese control subjects, normal-weight control subjects; Table 3), we found negative correlations between serum Chi3l1/YKL-40 concentrations and FEV1 % predicted values in the obese asthma group only (ρ = −0.34; P = 0.01; Table 3). Thus, serum Chi3l1/YKL-40 concentrations were inversely associated with FEV1 only when obesity and asthma were both present, but not in obesity without asthma or in asthma without obesity (P value for three-way multiplicative interaction between asthma, obesity, and serum Chi3l1/YKL-40 on FEV1 is 0.03 after adjustment for standard covariates). Finally, the association between FEV1 and serum Chi3l1/YKL-40 in obese subjects with asthma was not explained away by sputum Chi3l1/YKL-40 (Table 3 footnote).
Table 3.
Spearman Correlations between Serum Chi3l1/YKL-40 Concentrations with Prebronchodilator FEV1 % Predicted Values
| Obese Subjects with Asthma (n = 51) | Normal-Weight Subjects with Asthma (n = 34) | Obese Control Subjects (n = 44) | Normal-Weight Control Subjects (n = 48) | |
|---|---|---|---|---|
| FEV1 % predicted, unadjusted | −0.34 (P = 0.01) | 0.01 (P = 0.97) | 0.09 (P = 0.56) | 0.03 (P = 0.86) |
| FEV1 % predicted, adjusted for standard covariates | −0.40 (P = 0.005) | −0.19 (P = 32) | 0.26 (P = 0.12) | 0.003 (P = 0.99) |
Definition of abbreviation: Chi3l1 = chitinase 3–like-1.
Subjects with asthma included those with intermittent disease as well as persistent disease. The above correlations appeared stronger in obese subjects with persistent asthma than obese subjects with intermittent asthma (ρ = −0.35, adjusted P = 0.049 versus ρ = −0.03, adjusted P = 0.93), although the interaction was not significant (P = 0.50). The correlation between serum Chi3l1/YKL-40 concentrations with FEV1 % predicted values for obese subjects with asthma remained unchanged in separate analyses for standard covariates plus body mass index (BMI; ρ = −0.35; P = 0.02; n = 51) and for standard covariates plus waist circumference (ρ = −0.31; P = 0.04; n = 51) when compared to either the unadjusted value or the value after adjustment for standard covariates only. Correlations in the other three groups remained nonsignificant. Standard covariates included sex, race, ethnicity, and atopic status. There was a three-way multiplicative interaction between asthma status, obesity status, and serum YKL-40 concentrations on baseline FEV1 % predicted (P = 0.03) after adjustment for standard covariates. When the three-way interaction analysis was additionally adjusted for BMI or waist circumference (in separate analyses), the P value remained significant. When adjusted for sputum Chi3l1/YKL-40, the correlation between serum Chi3l1/YKL-40 and FEV1 in obese asthmatic group was unchanged from the unadjusted value (ρ = −0.39; P = 0.009; n = 44). Correlations in the other three groups remained nonsignificant. However, an independent association between sputum Chi3l1/YKL-40 and FEV1 % predicted was not seen in unadjusted or adjusted analyses (as detailed in Table E6).
Associations of Sputum Chi3l1/YKL-40 with Asthma Outcomes Do Not Mirror Those of Serum Chi3l1/YKL-40
Unlike serum Chi3l1/YKL-40, sputum Chi3l1/YKL-40 is neither associated with asthma status (Table E5) nor with FEV1 % predicted (Table E6). Furthermore, unlike serum Chi3l1/YKL-40, sputum Chi3l1/YKL-40 is associated with greater systemic lipid oxidant stress (measured by urinary 8-isoprostanes) in obese subjects with asthma, but not in normal-weight subjects with asthma (interaction between obese status and sputum Chi3l1/YKL-40 on urinary 8-isoprostanes among subjects with asthma, P = 0.04). These results are provided in Table E7, and their potential clinical relevance is discussed in the online supplement. Overall, these studies demonstrate that, although sputum Chi3l1/YKL-40 is not associated with asthma or pulmonary function, it is associated with systemic oxidant stress in the obese asthma subgroup.
Discussion
Obesity has reached epidemic proportions in the United States, and obesity-related illnesses have become a leading preventable cause of morbidity and even mortality (35). Asthma is one of the most prominent contributors to this morbidity and mortality, because obesity augments asthma prevalence and severity and decreases responsiveness to therapy. We hypothesized that the asthma–obesity association is due, in part, to shared regulation by Chi3l1/YKL-40. To address this hypothesis, we used murine modeling systems and a human case–control study to characterize the regulation and roles of Chi3l1 in the generation and maintenance of visceral WAT and pulmonary Th2 inflammation in mice and humans. The murine studies demonstrated that Chi3l1 is induced in visceral WAT and pulmonary tissues by an HFD and Th2 inflammation, respectively. Importantly, they also demonstrated that Chi3l1 plays a critical role in the pathogenesis of WAT accumulation and Th2 inflammation, and that the blunted Th2 response and decrease in visceral fat accumulation that are seen in the absence of Chi3l1 are mediated, at least in part, by Sirt1. The human studies confirmed the relevance of the murine studies to humans, because serum and sputum Chi3l1/YKL-40 both correlated with truncal obesity, serum Chi3l1/YKL-40 was associated with persistent asthma, and serum Chi3l1/YKL-40 correlated with lower FEV1 or worse impairment in subjects who had both obesity and asthma. When viewed in combination, these findings allow for the exciting hypothesis that a high-fat Western diet simultaneously augments visceral adiposity and asthma by stimulating the Chi3l1 pathway.
Obesity has recently been identified as a major risk factor in the development of asthma, and asthma in obese individuals tends to be more severe and refractory to treatment. However, the mechanism(s) linking obesity and asthma has not been clearly defined (2). The relationship between asthma and obesity has been assessed in a number of human studies. In many cases, this association was most striking when indices of truncal obesity were employed (1, 5, 6). In accord with these observations, our murine studies demonstrated that Chi3l1 plays a critical role in asthma and obesity, and our human studies revealed impressive correlations between Chi3l1/YKL-40 and truncal versus other obesity parameters. These findings can be accounted for via a number of mechanisms. First, Chi3l1 could play critical, but independent, roles in the pathogenesis of asthma and obesity. Alternatively, it is possible that the effects are not independent of one another, with obesity being a critical intermediary of Chi3l1 in obesity-associated asthma.
Abdominal obesity is known to be a strong predictor of low lung function (36). However, our studies demonstrated that the levels of circulating Chi3l1 correlate with abnormal lung function in obese subjects with asthma, but not obese control subjects without asthma. This suggests that these findings are not simply due to the mechanical effects of abdominal fat, and that there is, instead, a significant three-way interaction between obesity status, asthma status, and serum YKL-40 on FEV1. It is tempting to speculate that this is due, at least in part, to the inflammatory, remodeling, and oxidant injury–regulating effects of Chi3l1.
It should be noted that there are still controversies in regard to the roles of eosinophils in the pathogenesis of asthma and obesity. In asthma, eosinophils are presumed to be detrimental, and therapies are aimed at reducing their numbers or state of activation. In contrast, recent studies suggest that adipose tissue eosinophils play a physiologic role, where they control and improve metabolic homeostasis (37). Interestingly, in subjects with asthma, only the number of airway tissue eosinophils, but not the number of circulating, sputum, or adipose tissue eosinophils, directly correlate with BMI (37). In addition, the number of adipose tissue eosinophils is also decreased in obese versus lean mice (37, 38). When viewed in combination, these studies suggest that eosinophils have different roles in different tissue compartments (38). They also raise the possibility that eosinophil redistribution plays an important role in obesity-associated asthma (38). The present studies provide insights into the mechanisms that may underlie these responses. By demonstrating that aeroallergen-induced eosinophilic inflammation and WAT accumulation in mice on an HFD are abrogated in the absence of Chi3l1, they raise the interesting possibility that Chi3l1 is an intermediary molecule that links pulmonary Th2 eosinophilic inflammation and visceral fat accumulation. It is tempting to suggest that the ability of Chi3l1 to regulate eosinophil cell death (19) may be important, and that Chi3l1 may have similar effects on adipocytes. Additional investigation, however, will be required to address this possibility and other ways that Chi3l1 might contribute to eosinophil redistribution and adipogenesis.
Dysregulated Chi3l1 is well documented in a variety of inflammatory and remodeling disorders (13, 22). In the majority of these studies, only serum Chi3l1 was evaluated, and the source(s) of circulating Chi3l1 was not assessed. Our studies evaluated the relationships between serum and sputum Chi3l1 and obesity. Interestingly, although both correlated with truncal obesity, these associations were independent of one another. In contrast, only serum Chi3l1/YKL-40 concentrations inversely associated with FEV1, and only sputum Chi3l1 correlated with systemic lipid peroxidation in obese subjects with asthma. This is the first demonstration of the compartmentalization of Chi3l1 effects in humans. It raises the possibility that Chi3l1 is regulated differently, and may have different effector responses in different bodily compartments. Additional experimentation is needed to fully understand the contributions of Chi3l1 in the lung versus the serum.
Sirt1 is a nicotinamide adenine dinucleotide+-dependent histone deacetylase that influences a diverse assortment of cellular processes through interactions with targets, such as NF-κB, p53, Forkhead transcription factors, and histones, as well as epigenetic programming (39). It is a nutrient-sensing protein that induces hepatic gluconeogenesis, fatty acid oxidation, and adiponectin production, while repressing lipogenesis and inflammation (39). However, the contribution of Sirt1 in lung inflammation is still ambiguous. Some studies suggest that it has an antiinflammatory role by inhibiting proinflammatory transcription factors, such as NF-κB (23). Other studies show the opposite, with pharmacological inhibition of Sirt1 dampening lung Th2 inflammation by repressing peroxisome proliferator–activated receptor-γ (25) or modulating vascular endothelial growth factor expression (26). Our murine studies highlight novel relationships between Chi3l1 and Sirt1 and, in so doing, provide at least a partial explanation for the paradox in the literature. Specifically, they demonstrate that the effect of Sirt1 blockade that is seen depends on the levels of Chi3l1, with Sirt1 exerting proinflammatory effects in the presence of Chi3l1 and antiinflammatory effects in the absence of Chi3l1. They also demonstrate that, in the absence of Chi3l1, Sirt1 inhibits WAT accumulation. One can readily see how interventions that alter Chi3l1, its receptor(s), and or Sirt1 can be useful in efforts to control asthma, obesity, and, specifically, obesity-associated asthma.
Because Chi3l1 has been retained over species and evolutionary time, it is assumed to play important roles in biology. To survive over evolutionary time, early cave dwellers needed to be able to mount effective antipathogen responses. In addition, regardless of the time since their last successful hunting foray, they also needed to be able to store enough fuel to be able to initiate “energetically expensive acts,” such as immune activation (39). In accord with these concepts, studies from our laboratory have demonstrated that Chi3l1 plays a critical role in antipathogen responses, where it augments pathogen clearance and heightens disease tolerance (4, 21). Our present studies add to this evolutionary conceptualization of Chi3l1 by demonstrating that, in accord with these evolutionary principles, Chi3l1 also augments fat accumulation. However, unlike the early hunter-gatherers who lived in a world where food was scarce, modern Western humans live in a world of fat-rich food abundance. Thus, one can see how the ability of Chi3l1 to augment fat/energy storage helped early humans, and how it can contribute to the generation of diseases like obesity-associated asthma in a modern Western society.
Many of the large epidemiologic studies examining the association of obesity with asthma have noted that the effect sizes for obesity are larger in women than in men (40). One explanation may be that, despite its lower amount, the visceral fat in women is more metabolically active than fat from men, resulting in disproportionate inflammatory effects on the lungs of women. It is also possible that female reproductive hormones regulate the Chi3l1/Sirt1 pathways. Previous studies from our laboratory have demonstrated that many of the effects of Chi3l1 are mediated by a ligand–receptor interaction with IL-13 receptor α 2 (IL-13Rα2) (41). Interestingly, IL-13Rα2 is encoded by a gene on chromosome X. Although our small human study was not powered to detect sex interactions, it is possible that Chi3l1 contributes to the sex differences in the obesity–asthma association by interacting with IL-13Rα2.
In summary, our studies demonstrate that visceral adiposity and asthma share important Chi3l1-dependent pathways. Our findings allow for the exciting hypothesis that a high-fat Western diet simultaneously augments visceral adiposity and asthma, at least in part, via Chi3l1 stimulation. Based on our data, we can also hypothesize that novel preventive and/or therapeutic strategies for obesity-related asthma may include avoidance of fatty foods, inhibitors of Chi3l1/YKL-40 and or its receptor(s), and/or regulators of Sirt1. Additional investigation of the biology of Chi3l1 and other 18 glycosyl hydrolase family members in inflammation and metabolism, and the roles of antagonists and agonists of these pathways in the treatment of asthma, obesity, asthma in obese individuals, and related disorders, is warranted.
Footnotes
Supported by National Institutes of Health grants U01-HL-108638 and R01 HL 093017 (J.A.E.), UH2HL123876 (G.L.C. and J.A.E.), R01 HL115813 (C.G.L.), and K23 HL094531 (A.S.).
Author Contributions: Conception and design—F.A., A.S., S.T., C.G.L., and J.A.E.; data collection—F.A., A.S., B.M., M.S., and C.Q.; analysis and interpretation—F.A., A.S., S.T., M.S., C.Q., C.S.D.C., G.L.C., C.G.L., and J.A.E.; drafting the manuscript for important intellectual content—F.A., A.S., C.G.L., and J.A.E.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201405-0796OC on January 25, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shore SA. Obesity and asthma: location, location, location. Eur Respir J. 2013;41:253–254. doi: 10.1183/09031936.00128812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedón JC, Shore SA American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 3.Farah CS, Salome CM. Asthma and obesity: a known association but unknown mechanism. Respirology. 2012;17:412–421. doi: 10.1111/j.1440-1843.2011.02080.x. [DOI] [PubMed] [Google Scholar]
- 4.Gruchała-Niedoszytko M, Małgorzewicz S, Niedoszytko M, Gnacińska M, Jassem E. The influence of obesity on inflammation and clinical symptoms in asthma. Adv Med Sci. 2013;58:15–21. doi: 10.2478/v10039-012-0082-y. [DOI] [PubMed] [Google Scholar]
- 5.Brumpton B, Langhammer A, Romundstad P, Chen Y, Mai XM. General and abdominal obesity and incident asthma in adults: the HUNT study. Eur Respir J. 2013;41:323–329. doi: 10.1183/09031936.00012112. [DOI] [PubMed] [Google Scholar]
- 6.Collins LC, Hoberty PD, Walker JF, Fletcher EC, Peiris AN. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107:1298–1302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 7.Baruwa P, Sarmah KR. Obesity and asthma. Lung India. 2013;30:38–46. doi: 10.4103/0970-2113.106132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 9.Grotta MB, Squebola-Cola DM, Toro AA, Ribeiro MA, Mazon SB, Ribeiro JD, Antunes E. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013;13:39. doi: 10.1186/1471-2466-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydin M, Koca C, Ozol D, Uysal S, Yildirim Z, Kavakli HS, Yigitoglu MR. Interaction of metabolic syndrome with asthma in postmenopausal women: role of adipokines. Inflammation. 2013;36:1232–1238. doi: 10.1007/s10753-013-9660-9. [DOI] [PubMed] [Google Scholar]
- 11.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107:1287–1300. doi: 10.1016/j.rmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Dietze J, Böcking C, Heverhagen JT, Voelker MN, Renz H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy. 2012;67:1519–1529. doi: 10.1111/all.12031. [DOI] [PubMed] [Google Scholar]
- 13.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aerts JM, van Breemen MJ, Bussink AP, Ghauharali K, Sprenger R, Boot RG, Groener JE, Hollak CE, Maas M, Smit S, et al. Biomarkers for lysosomal storage disorders: identification and application as exemplified by chitotriosidase in Gaucher disease. Acta Paediatr Suppl. 2008;97:7–14. doi: 10.1111/j.1651-2227.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 15.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Areshkov PO, Avdieiev SS, Balynska OV, Leroith D, Kavsan VM. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int J Biol Sci. 2012;8:39–48. doi: 10.7150/ijbs.8.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C-C, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140:268–275. doi: 10.1016/j.clim.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MN, Lee KE, Hong JY, Heo WI, Kim KW, Kim KE, Sohn MH. Involvement of the MAPK and PI3K pathways in chitinase 3-like 1-regulated hyperoxia-induced airway epithelial cell death. Biochem Biophys Res Commun. 2012;421:790–796. doi: 10.1016/j.bbrc.2012.04.085. [DOI] [PubMed] [Google Scholar]
- 19.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2010;182:918–928. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, Ma B, Flavell R, Lee CG, Elias JA. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci. 2008;45:531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 23.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 24.Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab. 2006;17:186–191. doi: 10.1016/j.tem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Legutko A, Marichal T, Fiévez L, Bedoret D, Mayer A, de Vries H, Klotz L, Drion PV, Heirman C, Cataldo D, et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. J Immunol. 2011;187:4517–4529. doi: 10.4049/jimmunol.1101493. [DOI] [PubMed] [Google Scholar]
- 26.Kim SR, Lee KS, Park SJ, Min KH, Choe YH, Moon H, Yoo WH, Chae HJ, Han MK, Lee YC. Involvement of sirtuin 1 in airway inflammation and hyperresponsiveness of allergic airway disease. J Allergy Clin Immunol. 2010;125:449–460.e414. doi: 10.1016/j.jaci.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Zillikens MC, van Meurs JB, Rivadeneira F, Amin N, Hofman A, Oostra BA, Sijbrands EJ, Witteman JC, Pols HA, van Duijn CM, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009;58:2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet—induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–146. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 33.Von Behren J, Lipsett M, Horn-Ross PL, Delfino RJ, Gilliland F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, waist size and prevalence of current asthma in the California Teachers Study cohort. Thorax. 2009;64:889–893. doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sood A, Qualls C, Li R, Schuyler M, Beckett WS, Smith LJ, Thyagarajan B, Lewis CE, Jacobs DR CARDIA Investigators. Lean mass predicts asthma better than fat mass among females. Eur Respir J. 2011;37:65–71. doi: 10.1183/09031936.00193709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Elmquist JK. Tipping the scales early: probing the long-term effects of obesity. J Clin Invest. 2012;122:3840–3842. doi: 10.1172/JCI66409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 37.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, Bafadhel M, Singapuri A, Siddiqui S, Woods J, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188:657–663. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd CM, Saglani S. Eosinophils in the spotlight: Finding the link between obesity and asthma. Nat Med. 2013;19:976–977. doi: 10.1038/nm.3296. [DOI] [PubMed] [Google Scholar]
- 39.Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, et al. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169:963–968. doi: 10.1164/rccm.200303-403WS. [DOI] [PubMed] [Google Scholar]
- 41.He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Reports. 2013;4:830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]