Abstract
Streptococcus pneumoniae is the leading cause of community-acquired pneumonia and sepsis, with adult hospitalization linked to approximately 19% incidence of an adverse cardiac event (e.g., heart failure, arrhythmia, infarction). Herein, we review the specific host–pathogen interactions that contribute to cardiac dysfunction during invasive pneumococcal disease: (1) cell wall–mediated inhibition of cardiomyocyte contractility; (2) the new observation that S. pneumoniae is capable of translocation into the myocardium and within the heart, forming discrete, nonpurulent, microscopic lesions that are filled with pneumococci; and (3) the bacterial virulence determinants, pneumolysin and hydrogen peroxide, that are most likely responsible for cardiomyocyte cell death. Pneumococcal invasion of heart tissue is dependent on the bacterial adhesin choline-binding protein A that binds to laminin receptor on vascular endothelial cells and binding of phosphorylcholine residues on pneumococcal cell wall to platelet-activating factor receptor. These are the same interactions responsible for pneumococcal translocation across the blood–brain barrier during the development of meningitis. We discuss these interactions and how their neutralization, either with antibody or therapeutic agents that modulate platelet-activating factor receptor expression, may confer protection against cardiac damage and meningitis. Considerable collagen deposition was observed in hearts of mice that had recovered from invasive pneumococcal disease. We discuss the possibility that cardiac scar formation after severe pneumococcal infection may explain why individuals who are hospitalized for pneumonia are at greater risk for sudden death up to 1 year after infection.
Keywords: Streptococcus pneumoniae, invasive pneumococcal disease, heart failure, invasion, pathogenesis
At a Glance Commentary
Scientific Knowledge on the Subject
Adverse cardiac events are a common occurrence during severe community-acquired pneumonia. These contribute substantially to the associated risk for mortality.
What This Study Adds to the Field
Streptococcus pneumoniae is the leading cause of community-acquired pneumonia. It is now understood that the pneumococcus has direct cardiotoxic effects. The particular molecular mechanisms by which S. pneumoniae leads to heart failure have not been reviewed.
Pneumonia is the most common cause of death in children under 5 years of age (1). In European adults, pneumonia-associated deaths are predicted to peak in 2023, when one-third of the population will be over 65 years of age (2). In the United States, over 5 million individuals develop pneumonia annually (3), with pneumonia currently ranked as the eighth leading cause of death (4). Although hospitalization for and 30-day mortality after pneumonia have declined in the United States during the last decade (5, 6), due in large part to introduction of the 7- and 13-valent conjugate vaccine (5), individuals hospitalized for pneumonia have higher all-cause mortality rates than patients hospitalized for all other reasons (7). This increased likelihood for death is particularly evident in the elderly (>65 yr). Kaplan and colleagues (7) documented that almost one-half of elderly patients admitted for community-acquired pneumonia (CAP) experience mortality during the subsequent year, most deaths occurring after hospital discharge. It is now recognized that adverse cardiac events (i.e., myocardial infarction, arrhythmia, and heart failure) are a major contributing factor to mortality during hospitalization of the elderly for pneumonia and thereafter (8). Cumulative rates of new or worsening heart failure during hospitalization of adults for CAP have been reported to be as high as 33%, arrhythmias at 11%, and acute coronary syndromes at 11% (9). Adverse cardiovascular events occur most frequently in individuals who present with severe disease, usually those who required hospitalization and admittance to an intensive care unit (8).
There are multiple hypotheses as to the underlying mechanism(s) responsible for the increased risk of cardiovascular events seen during pneumonia. These include pre-existing cardiac conditions, raised inflammatory cytokines that destabilize atherosclerotic plaques (10), platelet activation and thrombosis (11), side effects from antimicrobial therapy (12–15), and increased myocardial demand at a time when oxygenation is compromised (16). However, most clinical studies are not pathogen specific, and it is highly likely that the mechanism of cardiovascular involvement differs with differing pathogens, particularly as pneumonia severity can differ substantially between etiological agents (17). Streptococcus pneumoniae (the pneumococcus) is the most frequent cause of CAP, bacteremia, and sepsis (18). Pneumococcal infection is associated with greater disease severity and increased risk of death compared with other causes of pneumonia; in one study, the mortality risk was almost threefold higher (19). Infection with S. pneumoniae has also been directly associated with adverse cardiac events. In a 2007 study by Musher and colleagues (20), 19.4% of 170 admitted adult patients experienced some form of an adverse cardiac event; most importantly, those who experienced an adverse cardiac event during pneumonia were at significantly higher risk for death than those with pneumococcal pneumonia alone. Herein, we review the pathogenic mechanisms by which S. pneumoniae directly inhibits cardiac contractility. In addition, we review the new finding that the pneumococcus is capable of translocation into the heart, and forms discrete, nonpurulent cardiac microlesions that may directly disrupt contractility.
The Cardiodepressant Effect of Pneumococcal Cell Wall Is Platelet-Activating Factor Receptor Dependent
During pneumococcal infection, bacterial cell wall is routinely shed and binds to the surface of epithelial cells in the lungs and endothelial cells in the vasculature. Pneumococcal cell wall has also been detected within the hearts of experimentally infected mice (21). S. pneumoniae is recognized by heterodimers of Toll-like receptor (TLR) 1/2, which recognize diacylated lipoproteins found in its cell wall, TLR4 that recognizes the toxin pneumolysin, and TLR9 that recognizes bacterial DNA (22–24). Nonetheless, and in contrast to numerous studies that show a central role for TLRs in cardiac dysfunction during infection (25–28), our experimental results instead suggest that the dominant negative effects of S. pneumoniae and its cell wall on heart function are TLR independent. Although inhibition of cardiomyocyte contractility in vitro and intact heart contractility ex vivo occurred after exposure to purified S. pneumoniae cell wall, this could be protected against by treatment with an antagonist for platelet-activating factor (PAF) receptor (PAFR) (29). Pneumococcal cell wall contains phosphorylcholine (ChoP) residues that mirror the structural aspects of phospholipid activator, PAF. ChoP, thereby, functions as a PAF mimetic and a bacterial adhesin to PAFR (21).
PAF is an inflammatory phospholipid produced by macrophages, neutrophils, platelets, endothelial cells, and cardiomyocytes in response to injury, and contributes to the development of inflammatory reactions (30–33). PAF induces cellular activation by binding to PAFR, which is a ubiquitous G protein–coupled receptor. PAFR interaction with PAF on different cell types has varied results that include activation of the proinflammatory transcription factor, nuclear factor (NF)-κB (34, 35), vasodilation (36), vasoconstriction (37), superoxide production (38), increased permeability (38, 39), augmentation of arachidonic acid metabolism, and the release of proinflammatory factors and cytokines (40, 41), all of which may directly or indirectly affect cardiac function (26, 27, 42, 43). In cardiomyocytes, direct treatment with PAF has been shown to have at first a positive, but then long-lasting negative and arrhythmogenic, effect on contractility (44, 45). This is most likely due in part to its endogenous activation of NF-κB, which has been shown to be inhibitory of cardiac contractility (25–28), as well as PAF-induced endogenous mediators.
Of note, exposure of cardiomyocytes to S. pneumoniae cell wall does not result in their death (46). What is more, cardiac depression as a result of NF-κB activation seems to be reversible, with flagellin-induced TLR5-mediated increases in end-systolic and end-diastolic left ventricular volumes, along with a diminished ejection fraction being transient (47). Thus, the negative effects of pneumococcal cell wall on cardiac function are most likely restricted to the acute and early convalescent phase of the infection (i.e., during hospitalization). Importantly, and as inferred previously here, TLRs play a central role in cardiac dysfunction during other types of infection and cardiac injury. In addition to detecting conserved structural motifs found in micro-organisms (i.e., pathogen-associated molecular patterns), TLRs also detect endogenous molecules released from necrotic or dying cells (i.e., danger-associated molecular patterns) and activate the host response (48). Engagement of a TLR by its ligand leads to the transduction of a proinflammatory signal through either the adaptor molecules, myeloid differentiation marker-88 or Toll/IL-1R domain–containing adaptor inducing IFN, resulting in activation of NF-κB or IFN regulatory factor-3/-7, respectively. TLRs that signal through myeloid differentiation marker-88, along with cytokines that activate NF-κB, have been linked to cardiac depression during acute infection, and it is therefore likely that these pathways work alongside cell wall–mediated PAFR activation during S. pneumoniae invasive disease to suppress cardiac function.
Pneumococcal Invasion into the Heart
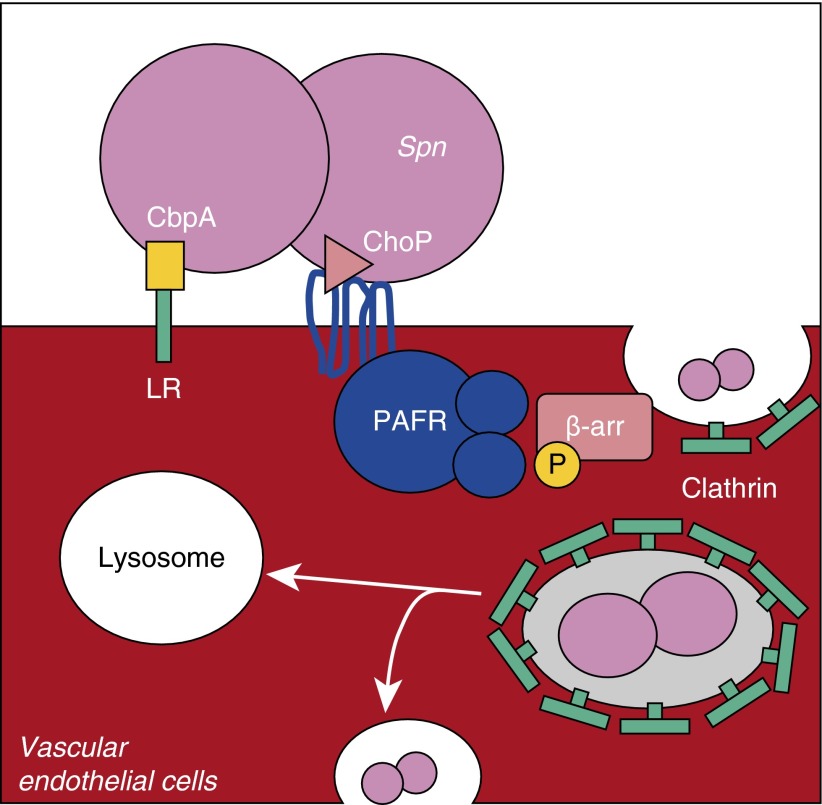
S. pneumoniae is the leading cause of bacterial meningitis, and considerable research has been directed toward determining the molecular mechanisms by which this pathogen gains access to the central nervous system (CNS). It is now recognized that two key interactions are required for translocation across the blood–brain barrier. First, bacterial adhesion to vascular endothelial cells is mediated by the bacterial adhesion, choline-binding protein A (CbpA), which binds to laminin receptor (LR) (49). Second, ChoP-mediated interactions with PAFR lead to bacterial uptake and translocation across these cells (21). In a TLR2-independent manner, PAFR ligation by pneumococcal cell wall induces the activation of the scaffold protein, β-arrestin-1, in turn causing endocytosis and uptake of bacteria or cell wall into a clathrin-coated vesicle (50). Contents of this vesicle are either destroyed after fusion with a lysosome or receptor recycling takes place, at which point the bacteria has an opportunity to exit the cell to the basolateral surface, and thus escape from the bloodstream into the CNS (Figure 1) (51).
Figure 1.
Translocation across vascular endothelial cells requires platelet-activating factor receptor (PAFR)/lipoteichoic acid–bound phosphorylcholine (ChoP) and choline-binding protein A (CbpA)/laminin receptor (LR) interactions. To translocate across the vascular endothelium, Streptococcus pneumoniae (Spn) first binds LR with its adhesin CbpA. This facilitates the interaction of bacterial cell wall ChoP residues on teichoic and lipoteichoic acid with PAFR on endothelial cells. Activation of this G protein–coupled receptor results in the phosphorylation (P) and activation of β-arrestin. The activation of β-arrestin (β-arr), a scaffold protein, leads to clathrin recruitment and the uptake of the pneumococcus or cell wall fragment in a clathrin-coated vesicle. This vesicle either fuses with lysosomes, killing internalized bacteria, or is recycled to the surface, allowing, in some instances, release of live pneumococci to the basolateral side.
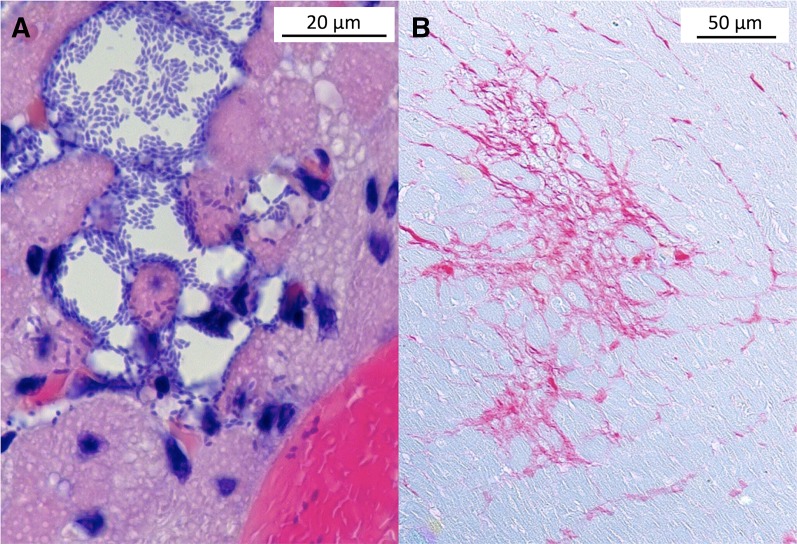
Our laboratory has recently made the observation that S. pneumoniae in the bloodstream is also capable of translocation into the myocardium. Within the ventricles, S. pneumoniae forms discrete bacteria-filled lesions approximately 10–100 μm in diameter (i.e., microlesions). Microlesions are distinct from Staphylococcus aureus cardiac abscesses (52) in that there is a stark absence of infiltrating immune cells (Figure 2A). In experimentally infected mice, pneumococcal microlesion formation occurred both in the left and right ventricles, was often found adjacent to blood vessels, and was concomitant with development of abnormal electrophysiology, as determined by ECG analysis. Importantly, in antibiotic-rescued animals, microlesion formation resulted in substantial cardiac scarring thereafter, as evidenced by the deposition of collagen at former lesions sites (Figure 2B). Evidence for microlesion formation was also observed in experimentally infected nonhuman primates, and in two of nine human autopsy cardiac samples from individuals who succumbed to invasive pneumococcal disease (IPD) (46). Thus, cardiac microlesion formation is a potential additional explanation for why adverse cardiac events occur during severe infections, and the scarring that occurs thereafter may explain the elevated incidence of sudden death in convalescent individuals.
Figure 2.
Streptococcus pneumoniae cardiac microlesion formation and subsequent cardiac scarring. (A) Hematoxylin and eosin–stained mouse cardiac section showing an S. pneumoniae cardiac microlesion adjacent to a blood vessel. Individual diplococci can be seen within the lesion and in adjacent cardiomyocytes. Scale bar = 20 μm. (B) Picro Sirius Red–stained mouse cardiac tissue section after antibiotic treatment highlights collagen deposition (red) and is indicative of scar formation. Scale bar = 50 μm.
Of note, wild-type mice pretreated with neutralizing antibodies against LR and PAFR-deficient mice challenged with S. pneumoniae both failed to develop cardiac microlesions. Likewise, an S. pneumoniae mutant lacking CbpA was attenuated in its ability to form cardiac microlesions (46). Thus, CbpA-mediated adhesion to LR on vascular endothelial cells in the heart and PAFR ligation by cell wall ChoP can be considered a shared pathogenic step with the development of meningitis. Of note, Neisseria meningitidis and Haemophilus influenza, which also express ChoP on their surface and bind PAFR, also bind to LR through meningococcal outer-membrane secretin PilQ and outer membrane protein PorA, and outer membrane protein (OMP) P2 of H. influenzae (49). As such, it is possible that these respiratory pathogens also directly invade the heart.
Why pneumococci within cardiac microlesions do not elicit an immune response is unclear. This stands in stark contrast to the vigorous, even damaging innate immune response that occurs in the lungs, middle ear, and CNS during infection. Interestingly, in hearts with confirmed microlesions, immune cell recruitment could be observed at the pericardium. Likewise, a nonpurulent microlesion was also observed within the gastrocnemius muscle of an infected mouse, suggesting that the absence of an inflammatory response may be due to a specific pneumococcal interaction with striated muscle cells (46). The absence of an immune response is obviously permissive for bacterial replication and, during the course of disease, lesions were observed to increase in size. In addition to directly damaging the heart and causing the release of danger-associated molecular patterns that would lead to TLR activation and cardiosuppressive NF-κB activation, these microlesions presumably directly disrupt electrophysiology, and would serve as a vehicle for direct delivery of pneumococcal cell wall to adjacent, but still viable, cardiomyocytes, in turn affecting the overall capacity of affected hearts to contract.
S. pneumoniae Damages and Kills Cardiomyocytes
Pneumolysin is a cholesterol-dependent cytolysin produced by S. pneumoniae (28). Pneumolysin binds to the cell membrane of eukaryotic cells, oligomerizes, and forms lytic pores in association with lipid rafts (46). At high concentrations, pneumolysin is capable of inducing lysis of the target cell, whereas, at lower concentrations, it can have a potent disruptive effect on cell signaling by causing unregulated ion entry (e.g., Ca2+) and small molecule (e.g., ATP) loss (51). Excitation–contraction coupling in cardiac muscle is dependent on calcium-induced calcium release. Briefly, the calcium influx from the depolarized cardiomyocyte membrane triggers the release of intracellular stores of calcium. This free calcium in the cytosol binds to troponin C by the actin filaments, thereby allowing for cross-bridge cycling by the myosin head and, subsequently, ATP-driven contraction. Thus, unregulated calcium entry through pneumolysin-formed pores would presumably be directly disruptive of contractility. Importantly, pneumolysin has been shown to efficiently kill the HL-1 cardiomyocyte cell line in vitro, and an S. pneumoniae mutant deficient in pneumolysin production was observed to form significantly smaller and fewer cardiac microlesions in mice. Using immunofluorescent microscopy, pneumolysin production by wild-type S. pneumoniae within microlesions has been confirmed to occur (46). Yet, to date, no study has directly examined the effect of pneumolysin on cardiac contractility. Of note, perfringolysin O, a homolog of pneumolysin that is produced by Clostridium perfringens, has been shown to directly suppress cardiac function, although the specific molecular mechanism was unexplored (53).
The pneumococcus also produces profuse amounts of hydrogen peroxide (H2O2) using an enzyme known as pyruvate oxidase (54). Whereas catalase in the blood neutralizes H2O2, cardiomyocytes adjacent to pneumococci within microlesions are likely exposed to high levels of this damaging reactive oxygen species that is also capable of causing cell membrane damage (55). A role for H2O2-mediated damage during IPD is supported by studies that demonstrate that cell death occurs in cardiomyocytes after their exposure to high levels of H2O2 (56). Moreover, studies of pneumococcal meningitis that demonstrate pneumolysin and H2O2 act synergistically to cause cell death of neurons (51, 57). Importantly, at sublethal concentrations, H2O2 induces cardiomyocyte hypertrophy (56). These enlarged cells showed enhanced actin stress fibers and disrupted myofibrils. Oxidative stress is now recognized to induce cellular senescence in cardiomyocytes, with cardiomyocytes treated with doxorubicin having increased positive staining for senescence-associated β-galactosidase, cyclin-dependent kinase I expression, decreased cardiac troponin I phosphorylation, and decreased telomerase activity observed (58). Thus, H2O2-driven cardiomyocyte senescence may have long-term consequences beyond acute infection.
Cardiac Scarring Is Observed in Hearts after IPD
Hearts from mice that had been rescued from IPD using antimicrobials were marked by considerable collagen deposition in areas of former microlesion sites (Figure 2B). Collagen deposition after extended cardiac ischemia is known to lead to the formation of firm, noncompliant, and contracted scars (59). It is therefore possible that the scars formed after a cardiac microlesion negatively impact contractility thereafter. After a traumatic cardiac damaging event, such as an infarct, transforming growth factor-β is markedly up-regulated, and plays a central role in infarct healing, cardiac repair, and left ventricular remodeling by modulating the fibroblast phenotype and gene expression. This promotes extracellular matrix deposition in the infarct by decreasing matrix degradation through induction of protease inhibitors (60, 61). The role transforming growth factor-β plays in cardiac microlesion repair remains unknown, and is currently under study. Determining the molecular mechanisms underlying the observed collagen deposition may offer an explanation of the greater incidence of mortality in IPD survivors.
Possible Prophylactic and Therapeutic Approaches to Prevent Heart Damage
Immunization of mice with a recombinant protein composed of a pneumolysin toxoid fused to the portion of CbpA that mediates interactions with LR was demonstrated to elicit protective antibodies against the formation of cardiac microlesions (46). Thus, antibody-mediated neutralization of CbpA and pneumolysin are potential prophylactic options for preventing cardiac damage. Statins, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, block the synthesis of cholesterol, yet also exert pleiotropic effects that include a decrease in inflammation and resistance to infection (29, 36, 38, 39). Pretreatment of human brain microvasculature endothelial cells (HBMEC) with simvastatin decreased de novo production of PAFR by 65% after exposure to TNF-α for 2 hours. Likewise, overnight exposure of HBMEC to simvastatin decreased the ability of S. pneumoniae to adhere to these cells (62). In addition, statins were found to confer resistance to pore formation mediated by pneumolysin and other cholesterol-dependent cytolysins, presumably by inhibiting the formation of lipid rafts (62), of which cholesterol is an integral part. Although it remains unknown if statins are cardioprotective during IPD, the above studies highlight a potential use for antibodies and PAFR antagonists in conferring prophylactic and possibly therapeutic protection against PAFR-mediated invasion of respiratory pathogens.
Other Contributing Factors
Adverse cardiac events after pneumonia are not just part of the host response to infection, but may also arise as a result of usage of medications commonly used to treat pneumonia. For example, some β-lactam antibiotics can complicate management of pre-existing heart failure due to increasing plasma sodium levels, macrolide and fluoroquinolone antibiotics can induce cardiac arrhythmias, and azithromycin has specifically been linked with increased cardiovascular death (12–15, 63, 64). Takotsubo cardiomyopathy, a condition triggered by a significant stressor, such as hospitalization for pneumonia, may also explain some of the increased cardiovascular death associated with this disease (65). The incidence of Takotsubo cardiomyopathy is thought to be around 2% in people with a suspected coronary artery syndrome, and is most commonly seen in postmenopausal women over 65 years of age. Myocardial suppression by lactate is also common in sepsis with concomitant metabolic acidosis. Some studies have suggested that lactate clearance may be important in prognosticating in pneumonia (66).
Conclusions
It is clear that acute pneumococcal disease is associated with adverse cardiac events. The extent to which these are the direct and cardiotoxic effect of this pathogen remains unclear, but is the focus of further study. At this moment, it is critical to learn the true impact of these interactions on long-term heart function in convalescent individuals. Assuming that S. pneumoniae interactions with cardiomyocytes are detrimental and long lasting, what is the best course of action? Currently, pneumococcal vaccination appears to be the best solution. Since introduction of the seven-valent conjugate vaccine against S. pneumoniae in 2000, it has been estimated that there were 168,000 fewer hospitalizations for pneumonia across all age groups in the United States (5). The 13-valent version of this vaccine has recently been approved for use in adults, and will have expanded coverage, although questions remain regarding its efficacy in reducing adult disease (67, 68). Perhaps a future vaccine strategy includes the inclusion of a conserved protein antigen that elicits antibodies that block the ability of the pneumococcus to target the heart. Such an antigen should simultaneously confer protection against serotypes that are not included as part of the vaccine formulation. Thus, the aforementioned CbpA and pneumolysin toxoid fusion protein is a possible candidate. Alternatively, the use of therapeutic drugs that neutralize pneumolysin or inhibit the bacteria’s interaction with PAFR may be of value in acute disease settings. For example, immediately before treatment with antimicrobials that cause the release of cytotoxic/cardiotoxic bacterial components upon bacterial killing. In summary, there is an increasingly pressing need to address the susceptibility of individuals to severe pneumococcal infections and the associated heart damage, in particular as the number of the elderly, and thus susceptible individuals, continues to climb.
Footnotes
Supported by American Heart Association grant 13IRG14560023 and National Institutes of Health (NIH) grant AI114800 (C.J.O.), and by NIH National Center for Advancing Translational Sciences grants NIH ULTR001120 and F31 A110417701 (A.O.B.).
Originally Published in Press as DOI: 10.1164/rccm.201411-1951PP on January 28, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.(UNICEF) UNCsFCommitting to child survival: a promise renewed. New York: United Nations; 2013. Progress report no. 978-92-806-4706-8
- 2.European Respiratory Society. Sheffield, UK: ERS; 2015. European lung white book. [Google Scholar]
- 3.File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 4.Hoyert DL, Xu JQ. Hyattsville, MD: National Center for Health Statistics; 2012. Deaths: preliminary data for 2011. [PubMed] [Google Scholar]
- 5.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med. 2011;124:171–178.e171. doi: 10.1016/j.amjmed.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan V, Clermont G, Griffin MF, Kasal J, Watson RS, Linde-Zwirble WT, Angus DC. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Griffin AT, Wiemken TL, Arnold FW. Risk factors for cardiovascular events in hospitalized patients with community-acquired pneumonia. Int J Infect Dis. 2013;17:e1125–e1129. doi: 10.1016/j.ijid.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan MS, Bawany FI, Hussham M. Fatal heart rhythms associated with azithromycin. J Pak Med Assoc. 2014;64:238. [PubMed] [Google Scholar]
- 13.Huang BH, Wu CH, Hsia CP, Yin Chen C. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30:1579–1582. doi: 10.1111/j.1540-8159.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen EM, Halm EA, Pugh MJ, Copeland LA, Metersky M, Fine MJ, Johnson CS, Alvarez CA, Frei CR, Good C, et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311:2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha BA. Antibiotic side effects. Med Clin North Am. 2001;85:149–185. doi: 10.1016/s0025-7125(05)70309-6. [DOI] [PubMed] [Google Scholar]
- 16.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 17.Singanayagam A, Singanayagam A, Elder DH, Chalmers JD. Is community-acquired pneumonia an independent risk factor for cardiovascular disease? Eur Respir J. 2012;39:187–196. doi: 10.1183/09031936.00049111. [DOI] [PubMed] [Google Scholar]
- 18.World Health OrganizationPneumonia2014[accessed 2014 Oct 30]. Available from: http://www.who.int/topics/pneumococcal_infections/en/
- 19.Bruns AHW, Oosterheert JJ, Cucciolillo MC, El Moussaoui R, Groenwold RHH, Prins JM, Hoepelman AIM. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17:763–768. doi: 10.1111/j.1469-0691.2010.03296.x. [DOI] [PubMed] [Google Scholar]
- 20.Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007;45:158–165. doi: 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 21.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 22.Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson GK, Orihuela CJ. Pneumococci: immunology of the innate host response. Respirology. 2010;15:1057–1063. doi: 10.1111/j.1440-1843.2010.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalpke A, Frank J, Peter M, Heeg K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–946. doi: 10.1128/IAI.74.2.940-946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Zou L, Zhang M, Li Y, Chen C, Chao W. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology. 2011;115:555–567. doi: 10.1097/ALN.0b013e31822a22f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 28.Knuefermann P, Sakata Y, Baker JS, Huang CH, Sekiguchi K, Hardarson HS, Takeuchi O, Akira S, Vallejo JG. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation. 2004;110:3693–3698. doi: 10.1161/01.CIR.0000143081.13042.04. [DOI] [PubMed] [Google Scholar]
- 29.Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, El Kasmi KC, Murti G, Kaushal D, Gaber MW, et al. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. 2006;177:6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 30.Janero DR, Burghardt C. Production and release of platelet-activating factor by the injured heart-muscle cell (cardiomyocyte) Res Commun Chem Pathol Pharmacol. 1990;67:201–218. [PubMed] [Google Scholar]
- 31.Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 32.Dagenais P, Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Augmented expression of platelet-activating factor receptor gene by TNF-alpha through transcriptional activation in human monocytes. J Leukoc Biol. 1997;61:106–112. doi: 10.1002/jlb.61.1.106. [DOI] [PubMed] [Google Scholar]
- 33.Cabellos C, MacIntyre DE, Forrest M, Burroughs M, Prasad S, Tuomanen E. Differing roles for platelet-activating factor during inflammation of the lung and subarachnoid space: the special case of Streptococcus pneumoniae. J Clin Invest. 1992;90:612–618. doi: 10.1172/JCI115900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kravchenko VV, Pan Z, Han J, Herbert JM, Ulevitch RJ, Ye RD. Platelet-activating factor induces NF-kappa B activation through a G protein-coupled pathway. J Biol Chem. 1995;270:14928–14934. doi: 10.1074/jbc.270.25.14928. [DOI] [PubMed] [Google Scholar]
- 35.Borthakur A, Bhattacharyya S, Alrefai WA, Tobacman JK, Ramaswamy K, Dudeja PK. Platelet-activating factor-induced NF-kappaB activation and IL-8 production in intestinal epithelial cells are Bcl10-dependent. Inflamm Bowel Dis. 2010;16:593–603. doi: 10.1002/ibd.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu WM, Man RY. Differential actions of platelet-activating factor (PAF) receptor antagonists on the vasodilator and vasoconstrictor effects of PAF in the rat perfused heart. Br J Pharmacol. 1991;104:773–775. doi: 10.1111/j.1476-5381.1991.tb12504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon PK, Ritter AB, Durán WN. Vasoconstrictor effects of platelet-activating factor in the hamster cheek pouch microcirculation: dose-related relations and pathways of action. Circ Res. 1988;62:722–731. doi: 10.1161/01.res.62.4.722. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, Yoshikawa T, Naito Y, Tanigawa T, Yoshida N, Kondo M. Role of platelet-activating factor (PAF) in superoxide production by human polymorphonuclear leukocytes. Lipids. 1991;26:1227–1230. doi: 10.1007/BF02536537. [DOI] [PubMed] [Google Scholar]
- 39.Longphre M, Kleeberger SR. Susceptibility to platelet-activating factor-induced airway hyperreactivity and hyperpermeability: interstrain variation and genetic control. Am J Respir Cell Mol Biol. 1995;13:586–594. doi: 10.1165/ajrcmb.13.5.7576695. [DOI] [PubMed] [Google Scholar]
- 40.Sugano T, Narahara H, Nasu K, Arima K, Fujisawa K, Miyakawa I. Effects of platelet-activating factor on cytokine production by human uterine cervical fibroblasts. Mol Hum Reprod. 2001;7:475–481. doi: 10.1093/molehr/7.5.475. [DOI] [PubMed] [Google Scholar]
- 41.Nassif A, Longo WE, Mazuski JE, Vernava AM, Kaminski DL. Role of cytokines and platelet-activating factor in inflammatory bowel disease. Implications for therapy. Dis Colon Rectum. 1996;39:217–223. doi: 10.1007/BF02068079. [DOI] [PubMed] [Google Scholar]
- 42.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 44.Alloatti G, Montrucchio G, Mariano F, Tetta C, De Paulis R, Morea M, Emanuelli G, Camussi G. Effect of platelet-activating factor (PAF) on human cardiac muscle. Int Arch Allergy Appl Immunol. 1986;79:108–112. doi: 10.1159/000233953. [DOI] [PubMed] [Google Scholar]
- 45.Robertson DA, Genovese A, Levi R. Negative inotropic effect of platelet-activating factor on human myocardium: a pharmacological study. J Pharmacol Exp Ther. 1987;243:834–839. [PubMed] [Google Scholar]
- 46.Brown AO, Mann B, Gao G, Hankins JS, Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen EM, et al. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog. 2014;10:e1004383. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolli J, Rosenblatt-Velin N, Li J, Loukili N, Levrand S, Pacher P, Waeber B, Feihl F, Ruchat P, Liaudet L. Bacterial flagellin triggers cardiac innate immune responses and acute contractile dysfunction. PLoS ONE. 2010;5:e12687. doi: 10.1371/journal.pone.0012687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 49.Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala’Aldeen DA, Tuomanen EI. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asmuth DM, Olson RD, Hackett SP, Bryant AE, Tweten RK, Tso JY, Zollman T, Stevens DL. Effects of Clostridium perfringens recombinant and crude phospholipase C and theta-toxin on rabbit hemodynamic parameters. J Infect Dis. 1995;172:1317–1323. doi: 10.1093/infdis/172.5.1317. [DOI] [PubMed] [Google Scholar]
- 54.Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janero DR, Hreniuk D, Sharif HM. Hydrogen peroxide-induced oxidative stress to the mammalian heart-muscle cell (cardiomyocyte): nonperoxidative purine and pyrimidine nucleotide depletion. J Cell Physiol. 1993;155:494–504. doi: 10.1002/jcp.1041550308. [DOI] [PubMed] [Google Scholar]
- 56.Chen QM, Tu VC, Wu Y, Bahl JJ. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch Biochem Biophys. 2000;373:242–248. doi: 10.1006/abbi.1999.1558. [DOI] [PubMed] [Google Scholar]
- 57.Goonetilleke UR, Ward SA, Gordon SB. Could proteomic research deliver the next generation of treatments for pneumococcal meningitis? Interdiscip Perspect Infect Dis. 2009;2009:214216. doi: 10.1155/2009/214216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A, Brunelli C. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol. 2009;297:H2169–H2181. doi: 10.1152/ajpheart.00068.2009. [DOI] [PubMed] [Google Scholar]
- 59.Cleutiens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 60.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, Orihuela CJ, Tuomanen EI. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benson H, Akbarian M, Adler LN, Abelmann WH. Hemodynamic effects of pneumonia. I. Normal and hypodynamic responses. J Clin Invest. 1970;49:791–798. doi: 10.1172/JCI106292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharkey SW, Lesser JR, Maron BJ. Takotsubo (stress) cardiomyopathy. Circulation. 2011;124:e460–e462. doi: 10.1161/CIRCULATIONAHA.111.052662. [DOI] [PubMed] [Google Scholar]
- 66.Attanà P, Lazzeri C, Picariello C, Dini CS, Gensini GF, Valente S. Lactate and lactate clearance in acute cardiac care patients. Eur Heart J Acute Cardiovasc Care. 2012;1:115–121. doi: 10.1177/2048872612451168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu P-J, Nuorti JP. Uptake of pneumococcal polysaccharide vaccination among working-age adults with underlying medical conditions, United States, 2009. Am J Epidemiol. 2012;175:827–837. doi: 10.1093/aje/kwr376. [DOI] [PubMed] [Google Scholar]
- 68.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]