Abstract
Bone metastasis is a frequent finding in the natural history of several types of cancers. However, its anticipated risk, diagnosis and response to therapy are still challenging to assess in clinical practice. Markers of bone metabolism are biochemical by-products that provide insight into the tumor–bone interaction, with potential to enhance the clinical management of patients with bone metastases. In fact, these markers had a cornerstone role in the development of bone-targeted agents; however, its translation to routine practice is still unclear, as reflected by current international guidelines. In this review, we aimed to capture several of the research and clinical translational challenges regarding the use of bone metabolism markers that we consider relevant for future research in bone metastasis.
Introduction
Bone metastasis (BM) is not only a major topic in the clinical management of cancer because of its prevalence but is also a unique subject of cancer research because of the proven relationship between cancer cells and the stromal cells in bone. Indeed, BM is the first good example of the importance of the microenvironment in the metastatic tissue to unravel new therapeutic targets—the bone-targeted agents (BTAs).
Because of this strong evidence, it was thoughtful to investigate where we could use bone remodeling biomarkers to understand the clinical behavior of cancer in bone. Furthermore, because it is very difficult to undertake useful objective evaluation of BM by analyzing serial X-rays from bone surveys or bone scans (BS), the bone remodeling markers appeared as a logical option to investigate in this context.
Despite the accomplishment of several studies focused to understand the role of bone remodeling markers to evaluate BM status,1,2 to monitor treatment response to BTAs and to anticancer therapy,1,3,4 as well as to predict bone complications,1 the most adopted guidelines do not recognize a definitive role for the use of bone remodeling markers to interpret clinical outcomes and treatment response in patients with BM.5,6
However, it is intriguing to see that the major clinical trials with BTAs in phase 2 studies had the bone remodeling markers as the primary end point. In consequence, several new drugs were not pursued to phase 3 trials because they did not show superiority in the bone remodeling marker response. In addition, the clinical trials with new drugs to treat metastatic prostate cancer (which often presents with BM) included, as secondary end point, bone remodeling markers response.7
Finally, because bone remodeling markers can reflect multiple interactions of cancer cells with osteoclasts and osteoblasts, they can have a role also in the understanding of the mechanistic pathways involved in cancer progression in bone, either in preclinical or in clinical research.
In this review, we aimed to capture several of these aspects that we consider relevant for future research in BM.
Biochemical markers of bone turnover
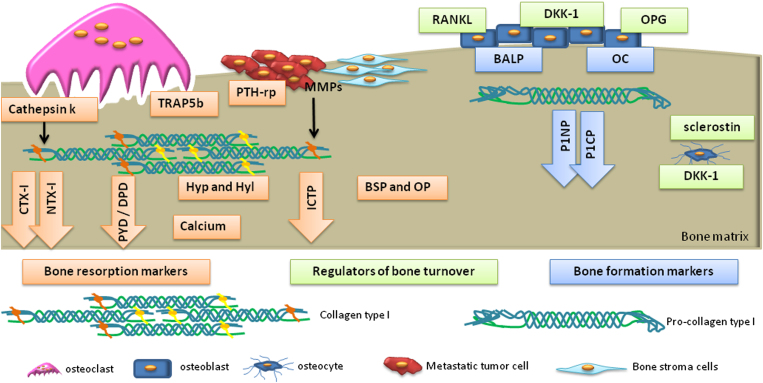
Biochemical markers of bone turnover are generally categorized into bone formation and bone resorption markers.8,9 Bone formation markers include osteoblastic enzymes or by-products of active osteoblasts during osteoblastogenesis. The majority of bone resorption markers are by-products of type I collagen degradation, noncollagenous bone matrix proteins or osteoclastic enzymes. In addition, several regulators of bone cells' activity and thereby bone turnover may also be used as biomarkers. The biochemical markers of bone turnover are represented in Figure 1 and summarized in Table 1.
Figure 1.
Biochemical markers of bone turnover. Blue boxes/arrows represent bone formation markers: bone-specific alkaline phosphatase (BALP); osteocalcin (OC); propeptides of type I procollagen (P1NP and P1CP). Orange boxes/arrows represent bone resorption markers: pyridinoline (PYD); deoxypyridoline (DPD); carboxy-terminal crosslinked telopeptide of collagen type I (CTX-I); amino-terminal crosslinked telopeptide of type I collagen (NTX-I); Hydroxyproline (Hyp); hydroxylysine (Hyl); bone sialoprotein (BSP); osteopontin (OP); tartrate-resistant acid phosphatase 5b (TRAP5b); cathepsin K. Green boxes represent regulators of bone turnover: receptor activator of NF-κB ligand (RANKL), osteoprotegerin (OPG), dickkopf-1 (DDK-1) and sclerostin. During the process of bone remodeling, osteoblasts produce RANKL and OPG that regulate the differentiation and maturation of osteoclasts. Osteoclastic activity is measured by the quantification of its lysosomal enzymes: cathepsin K and TRAP5b. The degradation of bone collagen type-I releases CTX-I and NTX-I (following degradation by cathepsin K), PYD, DPD and Hyp/Hyl. During the bone resorption process, calcium and enzymes from the bone matrix such as BSP and OP are released. Bone formation is a process associated with the release of BALP and OC-specific osteoblast enzymes. Osteoblasts secrete to the extracellular space collagen type I as a procollagen type-I molecule; afterward, its terminals are cleaved releasing P1NP and P1CP. In the presence of DDK-1 and sclerostin, the Wnt pathway is downregulated and consequently osteoblastic differentiation is inhibited. In bone metastasis (BM), matrix metalloptoteinases (MMPs) are produced by bone stromal cells and bone metastatic cells. These proteases are able to degrade collagen type-I originating carboxy-terminal crosslinked telopeptide of type I collagen (ICTP).
Table 1. Biochemical markers of bone turnover.
| Bone formation markers | Bone resorption markers | Regulators of bone turnover |
|---|---|---|
| BALPOCP1NP and P1CP | PYDDPDICTP or CTX-MMPCTX-INTX-IHyp, HylNoncollagenous bone matrix proteins: BSP and OPOsteoclast-derived enzymes: ) TRAP5b and cathepsin k and LCalciumVitamin DPTH-rp | RANKLOPGSclerostinDKK-1 |
Abbreviations: BALP, bone-specific alkaline phosphatase; BSP, bone sialoprotein; CTX-I, carboxy-terminal crosslinked telopeptide of collagen type I; DKK-1, dickkopf-1; DPD, deoxypyridoline; Hyl, hydroxylysine; Hyp, hydroxyproline; ICTP or CTX-MMP, carboxy-terminal crosslinked telopeptide of type I collagen; NF-κB, nuclear factor-κB; NTX-1, amino-terminal crosslinked telopeptide of type I collagen; OC, osteocalcin; OP, osteopontin; OPG, osteoprotegerin; P1NP and P1CP, propeptides of type I procollagen; PTH-rp, parathyroid hormone-related peptide; PYD, pyridinoline; RANKL, receptor activator of NF-κB ligand; TRAP5b, tartrate-resistant acid phosphatase 5b.
Bone turnover biomarkers used in preclinical and clinical research of BM include bone-specific alkaline phosphatase (BALP), osteocalcin (OC), procollagen type I N propeptide (P1NP), pyridinoline (PYD), deoxypyridoline (DPD), amino-terminal crosslinked telopeptide of type I collagen (NTX-I) and carboxy-terminal crosslinked telopeptides of collagen type I (CTX-I and ICTP).
BALP is an enzyme specifically produced by osteoblasts that is involved in mineralization. Its release into circulation corresponds predominantly to the matrix maturation phase of bone formation, an intermediate phase of osteoblast activity.9
OC is a 5.8-kDa noncollagenous protein synthetized by osteoblasts that binds to hydroxyapatite and is involved in calcium binding. Glutamic acid residues in OC are converted to γ-carboxyglutamic acid by vitamin K posttranslational carboxylation, and they are responsible for the calcium binding. OC is considered a specific marker of osteoblast function.10,11
P1NP is derived from extracellular processing of procollagen type I molecule. Procollagen contains amino- and carboxy-terminal extensions that are enzymatically cleaved upon procollagen secretion, P1NP and P1CP.10
PYD and DPD are two nonreducible pyridinium crosslinks that are present in bone collagen, which are released into the circulation upon collagen breakdown either in a free state or bound to a peptide. These pyridinium crosslinks result from the posttranslational modification of lysine and hydrolysine in bone and represent fragments of crosslinking amino-acid derivatives that stabilize the collagen type I fibrils in bone.12
The crosslinked telopeptides that are released from type I collagen degradation by proteases during bone resorption generate neoepitopes (CTX-I, ICTP, NTX-I). The C-terminal telopeptide, CTX-I, as well the N-terminal telopeptide, NTX-I, when compared with ICTP, reflect different enzymatic pathways of bone breakdown, by cathepsin K or matrix metalloptoteinase-1 (MMP-1) cleavage of collagen type I, respectively.13
Inhibitors of Wnt signaling, namely sclerostin and dickkopf-1 (DKK-1), are also considered bone remodeling markers. Sclerostin is produced by osteocytes, whereas DKK-1 is produced by osteoblasts and by a variety of different cells in several tissues, including cancer cells. Both sclerostin and DKK-1 are secreted into circulation, and serum levels reflect inhibition of bone formation.14,15
State-of-the-art high-throughput techniques are being used for the identification of new key biological and molecular pathways involved in the regulation of bone metabolism.16 Therefore, new bone remodeling markers may emerge in the future.
Laboratory determination of biochemical markers of bone turnover
Bone remodeling markers reflect the metabolic activities of osteoclasts (resorption) and osteoblasts (formation) and can be measured in serum or urine. Available analytical methods for the determination of bone remodeling markers include enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA) and electrochemical luminescence.
Preanalytical and analytical variability in the quantification of bone remodeling markers represents a major problem in its use for clinical purposes. In this way, the identification of the variability sources and the strategies to go beyond this should be designed. There are two main sources of preanalytical variability: (1) technical sources, which include sample collection, sample handling and storage, thermodegradation, photolysis and timing of sample collection (diurnal variation); and (2) biological sources, such as age, gender ethnicity, diet, exercise, among others.11,17 To overcome preanalytical variability, laboratories must establish their own reference ranges using gender- and age-specific reference values,11 and address intralaboratory reproducibility both in manual and automated assays.18 The International Osteoporosis Foundation (IOF) and International Federation of Clinical Chemistry (IFCC), IOF/IFF working group, have published recommendations to minimize variability in the determination of bone remodeling markers.19,20 One bone resorption marker, serum CTX-I, and one bone formation marker, serum P1NP, are considered as reference markers and measured by standardized assays in observational and intervention studies. This, along with the consequent adoption of international reference standards, allows improving the use of these clinical markers.21 To ensure the integrity of results, laboratories can participate in external quality assurance schemes. For example, in the United Kingdom, the External Quality Assessment Service now includes bone turnover markers in their portfolio.22
Over the past decade, most of the immunoassays that were used have been automated, contributing to the decrease of technical variability.18 However, there are specific aspects of each remodeling biomarker that should be taken into account to control variability in their quantification.
BALP
The first assays that were developed for the quantification of BALP were not specific for this isoenzyme; therefore, isoenzymes from other organs such as liver could interfere in the BALP quantification. Since then, different ELISAs have been developed to quantify BALP isoenzyme in serum or heparin plasma.10 Although reproducible and precise, these immunoassays still retain some crossreactivity with the liver isoenzyme: around 15% with the BALP Ostase immunoassay and 3–10% with the Alphase-B enzyme immunoassay. Therefore, BALP results must be carefully interpreted in patients with liver pathology.10 Blood withdrawal for BALP quantification must be performed without ethylenediaminetetraacetic acid (EDTA) or citrate owing to enzyme inhibition. BALP in serum is stable at 2–8 °C for up to 5 days or at −20 °C for longer storage.23
OC
OC can be quantified by RIA, ELISA or chemiluminescense immunoassays. Assays that detect the intact OC or large N-terminal fragments are the most reproducible; however, the use of this marker is limited owing to high variability.10 OC should be analyzed in EDTA-derived plasma obtained by immediate separation after blood withdrawal. OC in plasma is unstable at room temperature (RT) but stable up to 7 days at 4 °C.17
P1NP
The measurement of P1NP in serum may be of diagnostic value.11 There are two commercially available assays: manual Orion RIA detects intact P1NP, whereas the high-throughput automated Roche Elecsys 2010 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan) recognizes both the trimeric and thermal degradation forms of P1NP. The automated method is reported to have better analytical reproducibility.24 P1NP is stable in serum stored up to 48 h at 23–25 °C, up to 76 days at 2–8 °C and up to at least 6 months at −20 to −80 °C.17
PYD and DPD
The measurement of PYD and DPD is not influenced by the degradation of newly synthetized collagen and only reflects the degradation of crosslinked mature collagens.11 DYP and DPD are quantified by reverse-phase high-performance liquid chromatography in the first or second void urine of the morning, collected preferentially before 1000 h owing to the diurnal variation on the concentration of these crosslinks. For longitudinal studies, urine should be collected at the same time point. Creatinine correction of DYP and DPD levels is needed to control for renal function. Urine samples must be collected without preservatives, and DYP and DPD are stable at 2–8 °C up to 7 days or at −20 °C for longer storage.23 The samples should be protected from light, as PYD and DPD are sensitive to UV light, resulting in lower concentrations.10
NTX-I
NTX-I is usually quantified in urine, by ELISA, using a monoclonal antibody against the α-2 isoform (bone-derived peptide). Results must be standardized to creatinine levels, as renal clearance affects NTX-I excretion. Owing to variations with circadian rhythm, urine samples should be collected from the second void of the morning.25 NTX-I is stable in urine collected without preservatives and stored at RT for up to 24 h, at 2–8 °C up to 72 h, 3 months when frozen at −20 °C and for at least 1 year at −80 °C. It should be used in less than three freeze/thaw cycles.23 Urine NTX-I has been the preferred marker in the clinical setting as, in comparison with serum CTX-I, it is less sensitive to the circadian rhythm, it is not affected by food intake and it avoids blood withdrawal.23
CTX-I
With regard to CTX-I, quantification in serum, compared with urine, is preferable owing to a higher precision throughout the range of concentrations. ELISA is performed using a monoclonal antibody against an octapeptide sequence (EKAHD-β-GGR) in the α-1 (I) chain of the β-isoform. CTX-I β-isoform is most predominant in mature collagen from bone, and it results from the isomerization of aspartyl to β-aspartyl residues. This isomerization occurs with the aging of the collagen type I molecule.26 Serum CTX-I is affected by food intake, and blood withdrawal must take place in the fasting state. Food intake substantially decreases the levels of CTX.27 For this assay, serum should be collected within 3 h after blood withdrawal and immediately frozen at −20 °C.28
Quantification of CTX-I and NTX-I by ELISA is currently accurate and reproducible.8 However, for comparison between samples, the assays should be performed in the same laboratory, as results from different laboratories, even with the same test and automated assays, could differ up to 5.6-fold.11,29 Therefore, besides the calibration and the establishment of references for each laboratory, the ideal situation to control technical variability is to centralize the determinations in a unique laboratory.20
ICTP
ICTP, which reflects nonosteoclastic bone resorption mediated by MMPs, is liberated to the bloodstream during pathological conditions. Serum ICTP is quantified by RIA,13 and it is relatively insensitive to changes in bone remodeling mediated by normal osteoclastic activity. This could be explained by the fact that the epitope for ICTP antibody used in its quantification by RIA is cleaved by cathepsin K during normal osteoclastic activity, becoming unavailable for the quantification. Serum must be stored at −20 °C, with a decrease of 12% in ICTP levels if kept at RT for 5 days.11
Preclinical use of bone remodeling markers
Characterization of bone phenotype in preclinical research often includes X-rays, bone densitometry, static and dynamic histomorphometry, biochemical analysis and in vitro studies.
The histomorphometric analysis is a gold-standard approach to address bone formation and resorption, as it allows the quantification and visualization of the mineralizing surface, mineral apposition rate, bone formation rate, among other characteristics. Nevertheless, several preclinical studies focusing on bone remodeling have taken advantage of the measurement of bone remodeling markers, either to assess bone development, bone regeneration or bone pathophysiology. Bone remodeling biomarkers are more sensitive, discriminative between phenotypes and more reproducible than histomorphometric measurements. Biochemical markers of bone remodeling have also been used in preclinical development of antiresorptive agents for bone metastatic disease in cancer xenograft models.30
Measurement of serum CTX-I by ELISA is a convenient and the most commonly used approach when using animal models, when compared with the quantification of NTX-I in urine.
In several studies, the analysis of three-dimensional bone microstructural properties by microcomputed tomography, bone mineral density assessed by X-ray computed tomography, histomorphometric analyses and other biochemical markers such as BALP, OC and tartrate-resistant acid phosphatase 5b (TRAP5b) have shown consistent results when compared with CTX-I determination in the serum of rodents.31,32,33,34,35,36,37,38,39,40,41 In addition, in nonhuman primate models, serum markers of bone resorption were also consistent with the observed phenotype.42,43,44,45,46
Biomarkers of bone remodeling can also be used in in vitro models.47 These cellular models can be reliably used for enhancing preclinical evaluation of pharmacological agents in metastatic bone disease. Nevertheless, in the vast majority of in vitro studies, the determination of osteoclast formation and activity is usually performed by TRAP staining, TRAP activity, pit resorption assays and expression of osteoclast-related markers such as cathepsin K and MMP-9. Osteogenesis is usually assessed by BALP activity and mineralization assays.
Bone remodeling markers in the clinical setting
Evaluation of bone metastases development risk after adjuvant therapy
Even though the majority of patients with recurrent or metastatic breast and prostate cancers develop BM, a minority does not, and between those developing BM the timing of such event differs; therefore, previous studies addressed predictors of bone involvement. In breast cancer, sub-populations at different risks for BM were identified, as defined by younger age (<35 years), higher tumor staging (tumor size and nodal involvement), lower to intermediate histologic tumor grade, estrogen receptor positivity and previous locoregional or soft tissue recurrence.48,49 More recently, c-Src activation was also identified as a gene-expression signature associated with late-onset BM.50 In prostate cancer, higher PSA levels are also predictors of bone recurrence.51 Similarly, previous studies have found an association between specific tumor markers and bone relapse. Lipton et al.52 retrospectively tested the association between pretreatment serum level of CTX-I and the development of bone-only disease relapse in the cohort of patients enrolled in the NCIC CTG MA.14 study, a phase 3 trial that tested the added benefit of adjuvant octreotide to tamoxifen in 667 women with early breast cancer. At a median follow-up of 7.9 years (123 events of documented disease recurrence, 19 of which involving the bone as an exclusive site of metastasis) and after controlling for other significant clinicopathologic characteristics, elevated CTX-I was associated with a shorter bone-only relapse-free survival (hazard ratio (HR) 3.43, 95% confidence interval (CI): 1.20–9.77; P=0.02); of note, it was not associated with any other type of recurrence. The authors hypothesized that an increased tumor metabolism at baseline may facilitate the development of BM.52,53 In contrast, Coleman et al.54 did not find such a predictive power of high CTX-I levels at baseline (CTX-I ⩾0.3 ng ml−1; HR 1.43, 95% CI: 0.87–2.35; P=0.159) when retrospectively analyzing a cohort of breast cancer patients derived from the AZURE study, a phase 3 trial testing the added benefit of zoledronic acid (ZA) to standard adjuvant treatment in 872 patients with stage II/III breast cancer. Moreover, in this study, P1NP levels at baseline were also not significantly associated with bone relapse. Surprisingly, a normal level of serum vitamin D (>30 ng ml−1) was associated with a lower risk of bone relapse (HR 0.11, 95% CI: 0.02–0.76; P-value=0.0257) and a trend toward a lower risk of any type of relapse (HR 0.56, 95% CI: 0.31–1.01; P-value=0.0519). In a smaller study using prospective data from 388 consecutive patients, Diel et al.55 demonstrated that higher preoperative BSP levels correlate with the development of bone-only metastases, even when controlling for nodal status (HR 93.96, 95% CI: 21.65–408.30; P<0.001). By contrast, from the 14 patients with visceral metastases only, none had increased BSP. The authors noticed, however, the possibility that the high levels of BSP might be the result of tumoral production instead of the result of increased bone turnover.
On the basis of the hypothesis that an increased bone metabolism may facilitate the development of BM, prior studies tested the added benefit of ZA in the adjuvant treatment of early breast cancer. A benefit in terms of relapse-free survival was found in premenopausal women under complete estrogen blockage (goserelin with either tamoxifen or anastrazol; ABCSG-12; Gnant et al.56), in postmenopausal women under aromatase inhibitors (letrozol; ZO-FAST; Coleman et al.57) and in women under systemic adjuvant therapy who had undergone menopause more than 5 years earlier (AZURE; Coleman et al.58). Moreover, an individual patient data meta-analysis condensing information from 41 randomized trials that compared bisphosphonates (BPs) with placebo or no BPs in patients with breast cancer (n=17 016, of which 10 540 were postmenopausal women) further demonstrated a 34% improvement in the rate of bone recurrences (P<0.001) and a novel 17% improvement in the rate of breast cancer death (P=0.004) in postmenopausal women treated with BPs.59 Pre- or perimenopausal women had no benefit. Importantly, the hypothesis of enhanced benefit from BPs in those patients with increased bone metabolism is still lacking support, as suggested by the previously discussed translational studies from the AZURE study, in which increased bone turnover levels at baseline (CTX-I ⩾0.3 ng ml−1) did not predict treatment benefit.54
Diagnosis of bone metastases
BS and conventional radiography (X-ray) are the two most commonly used imaging methods in the diagnosis of BM;60 however, although BS has low specificity, X-ray has low sensitivity.60 Thus, based on the broad picture of bone metabolism provided by bone markers, several authors tested their role as a diagnostic tool. A comprehensive study included postmenopausal patients with BM before and after one administration of different doses of pamidronate (n=19) compared with pre- and postmenopausal healthy women. When compared with postmenopausal healthy women, those with BM had higher levels of urinary calcium, hydroxyproline, PYD, DPD and CTX-I, but only hydroxyproline, PYD and DPD were significant.61 After BP therapy, patients were followed up weekly for 8 weeks (range 4–10). Parameters of bone resorption declined significantly at week 1, especially CTX-I, and for 63 days for urinary calcium (90% CI: 35–70+), 56 days for CTX-I (21–70+), 49 days for hydroxyproline (28–70+), 49 days for DPD (21–70+) and 21 days for PYD (14–28 days); on the other hand, those of bone formation did not change meaningfully, an effect that reflects the capacity of BPs to uncouple bone formation and resorption in favor of bone formation.
Other studies tested the accuracy of several bone markers in patients with cancer when comparing specifically those with or without BM. Demers et al.62 tested a panel of seven bone markers (BAP, PYD, DPD, NTX-I, ICTP, PTH-rp and calcium) in patients with various types of solid tumors with or without BM, as defined by BS and/or X-ray bone survey. In this study, urinary NTX-I, its log transformation (log(NTX-I)) and DPD were the markers most strongly associated with BM, but only NTX-I and log(NTX-I) had a relevant discriminating power between those with BM or local disease only (sensitivity of 0.308 and 0.423, respectively, and specificity of 0.902, for both). In this cohort, when comparing markers of bone formation with resorption, those of bone resorption were most different between the groups of patients with BM or local disease; nevertheless, several patients without clinical evidence of BM had also increased tumor marker levels. The authors speculated that this elevation may reflect subclinical bone involvement. Wada et al.63 retrospectively analyzed four bone markers (NTX-I, ICTP, total ALP and TRAP5b) in 156 breast cancer patients, 23 with bone-only metastases, 19 with extraosseous-only metastases and 114 without metastases. Those with BM were further divided by the number of bone lesions. Contrasting with NTX-I and total ALP, the mean values of ICTP and TRAP5b were significantly higher in those patients with BM when compared with those without (visceral metastases or no metastases), as were in those with higher bone tumor burden compared with lower burden and in those with progressive disease despite therapy compared with those with tumor control. Of note, the groups above differed in the median age at the time of marker evaluation, a variable not controlled for in the analysis. On the other hand, Ulrich et al.3 analyzed a set of three bone markers (NTX-I, ICTP and BALP) in 106 patients with breast cancer, either with documented BM (n=19), without BS evidence of BM (n=65) or with pathologic non-malignant alterations in the bone scintigram. Tumor marker levels were higher in the group of patients with BM when compared with the other two groups, whereas those without clinical evidence of BM had similar levels. In this cohort, ICTP was the marker with higher sensitivity (65%), whereas it had similar specificity to BALP (91 vs 92% for BALP). Given the wide range of values and the lower sensitivity, the authors concluded that these markers did not allow for early detection of subclinical bone recurrence and hence the replacement of imaging techniques for this purpose. Moreover, bone markers are neither site-specific nor disease-specific; thus, imaging studies continue to be a key step during the diagnostic process.
A detailed discussion according to the type of primary tumor can be found elsewhere.9,64
Prediction of patients' outcomes
Bone markers have been tested as predictors of skeletal-related events (SRE), bone disease progression and overall survival. The retrospective analysis of large data sets derived from phase 3 clinical trials testing the use of ZA in patients with BM demonstrated that high baseline bone remodeling markers levels are adverse prognostic features. Brown et al.4 retrospectively studied two bone markers (NTX-I and BALP) in patients assigned to placebo (control arm) in two phase 3 trials of ZA. These trials included 441 patients with prostate cancer, non-small-cell lung cancer (NSCLC) or other cancers, all with BM. High baseline and on-study NTX-I levels (⩾100 nmol mmol−1 creatinine) predicted a higher risk of SRE, but also a shorter time to first SRE, bone disease progression and death. Compared with baseline evaluations, on-study measurements were even more strongly correlated with these outcomes. Higher BALP levels (⩾146 IU ml−1) were also correlated with worse outcomes, excluding bone disease progression; furthermore, the effect size was smaller. On the other hand, Coleman et al.65 analyzed the same markers in 1824 BP-treated patients (experimental arm) enrolled in tree phase 3 trials. These trials included patients with several types of primary solid tumors (mostly breast, prostate and NSCLC) and multiple myeloma; moreover, patients received either ZA (n=1462) or pamidronate (n=362). With a median follow-up of 17 months, patients with baseline and on-study urinary NTX-I levels ⩾50 nmol mmol−1 creatinine had a twofold increased risk of developing an SRE or having bone disease progression when compared with those with normal levels (<50 nmol mmol−1 creatinine). The risk of SRE development was numerically more pronounced in breast cancer and lowest in NSCLC, but no interaction was found (P=0.813). Furthermore, NTX-I levels ⩾100 nmol mmol−1 creatinine were associated with a four to six times increased risk of death (vs two to four times for values between 50 and 100 nmol mmol−1 creatinine) when compared with normal levels. Higher levels of BALP (⩾146 U l−1) showed a similar association with worse outcomes but with a heterogeneous correlation by different primary tumors.
Other studies also demonstrated that early (at 3 months) bone marker normalization after introduction of ZA is strongly prognostic. Lipton et al.66 retrospectively analyzed a cohort of patients treated with ZA in the context of a phase 3 trial comparing ZA with pamidronate. In this study, early NTX-I normalization (3 months NTX-I <64 nmol mmol−1 creatinine) was associated with a significant decrease in the risk of first SRE (HR 0.504, 95% CI: 0.318–0.798; P=0.0034) and death (0.454, 95% CI: 0.293–0.704; P<0.001). However, when only considering the group with an early NTX-I normalization, the development of a subsequent SRE or death was not always preceded by an increase in NTX-I levels. Subsequent studies by the same author using independent data sets found similar conclusions.67
More recently, denosumab was compared with ZA in patients with BM from several types of solid tumors and multiple myeloma;1,68,69 in these studies, denosumab was more effective delaying time to first and subsequent SRE, which was correlated with a greater control of bone turnover marker levels (NTX-I and BALP).1,68
Costa et al.12 further studied the role of bone markers in the diagnosis of disease progression. In this prospective cohort study, the authors tested 3 bone markers (NTX-I, ICTP and BALP) in 123 patients with various metastatic cancers, 26 of which were extraosseous only (45 bone-only and 52 bone plus visceral). NTX-I and ICTP, but not BALP, were associated with bone disease progression (mean increase of 52 and 44%, respectively; P<0.001 for both), even after controlling for relevant clinical and demographic characteristics. Moreover, NTX-I had the highest sensitivity (70%), specificity (80%), positive (72%) and negative (79%) predictive values for bone disease progression in the set of markers analyzed (for an increase ⩾30% from baseline). Curiously, when assessing ICTP, not only did it increase in the context of bone and extraskeletal progression, but it also did not decrease with BP therapy. This led the authors to speculate that ICTP could represent a bone collagen product derived from an osteoclast-independent mechanism of bone degradation (MMP-1 action on bone collagen) and therefore not influenced by BP therapy.
A detailed discussion according to the type of primary tumor can be found elsewhere.9,64
Dosing of BTAs
The role of bone remodeling biomarkers is mostly acknowledged in the clinical development of BTAs. In phase 2 studies when comparing denosumab with BPs, the first end point was the rate of normalization of NTX-I,2,70 as defined as either the proportion of patients with urine NTX-I lower than 50 nmol l−1 per mM creatinine or the percentage of change in urine NTX-I corrected for urine creatinine from baseline, both at week 13.2,70 These phase 2 studies were crucial to select the treatment schedule of denosumab in the three pivotal phase 3 trials that led to the approval of this new BTA.1,68,69
Despite the evidence that BTAs provide a significant clinical benefit for patients with BM, these drugs are also responsible for relevant adverse events, such as hypocalcemia or osteonecrosis of the jaw.71 Moreover, the rate of adverse events associated with BP treatment increases with time,71 and only limited evidence assessed long-term use of BPs.12,71 Given the prompt response of bone markers to treatment with BTAs and their use as predictors of BTAs efficacy (particularly with ZA), some authors hypothesized that the serial measurement of bone markers could be a strategy to tailor therapy regimen. Actually, the serial measurements of these markers could allow changing treatment frequency (for example, every 3 months) and even theoretically allow removing therapy for periods in the context of optimal bone metabolism control. Currently, treatment guidelines recommend continuing therapy until there is a substantial decline of patients' general status6 or even indefinitely and throughout the course of the disease;5 however, a bone marker directed strategy of therapy could maximize benefits, while decreasing risks and costs. This strategy was tested in a phase III noninferiority study referred to as the BISMARK trial,72 in which 289 patients with bone metastases from breast cancer were randomized to ZA, either as a standard fixed schedule or under an NTX-I-directed schedule. Even though the recruitment of patients was lower than planned (compromising study power), the NTX-I-directed schedule group had a higher proportion of patients presenting with an SRE (primary end point HR 1.41, 90% CI: 0.98–2.02; P=0.12), thus not demonstrating noninferiority of this novel approach. Of note, patients in the standard schedule had more than double of ZA administrations and lower median NTX-I values at all evaluations. Even though underpowered, this study suggested that an NTX-I-directed schedule may be an unfavorable strategy. In contrast, two small feasibility studies selected low-risk patients (as defined by baseline CTX<600 ng l−1 after ⩾3 months of BPs) to receive pamidronate either every 4 or every 12 weeks until CTX failure (>600 ng l−1) or SRE development. Both trials reported a noninferiority of the 12-week schedule in this low-risk group.73,74
Two other studies tested the reduction of therapy frequency from every 4 weeks to every 12 weeks after a treatment period of ∼1 year with ZA every 4 weeks.75,76 Both studies reported a noninferiority of an every 12-week therapy schedule after at least 1 year of every 4-week ZA in terms of SREs; however, a significant increase in NTX-I levels from baseline in the every 12-week group was found. The meaning of this increase in the context of the longer survival of patients with bone metastases should be further studied. CALGB-70604 and Z-MARK studies, which are still running, will add further evidence to this topic. These treatment properties might, however, be specific of BPs, as a result of its bone matrix accumulation. On the contrary, denosumab pharmacokinetics may limit this approach.77 It is noteworthy that an exploratory study comparing several doses and schedules of denosumab (namely 180 mg every 12 weeks) with every 4-week BPs showed no difference in the control of NTX-I or occurrence of SREs.
Conclusion
Bone remodeling markers have been perceived as a valuable tool to the clinical development of BTAs. The use of bone remodeling markers in preclinical research is mainly complementary but, in human studies, they have a key role to decide whether the new BTA is considered a superior alternative to pursue to a phase 3 level. Further research to determine better the correlation of different biomarkers with different patterns of bone degradation observed in the context of BM is advisable to increase the accuracy of early-phase clinical trials with new BTAs. In routine clinical practice, bone remodeling markers can be helpful to define better the prognosis and the risk for bone complications in patients with BM. However, they are not ready to be considered as a standard approach in the clinical setting yet, as shown by the levels of evidence in Table 2. Therefore, they are not recommended as part of the guidelines.5,6,78
Table 2. Levels of evidence for the use of NTX-I in several settings78.
| Indication | Level of evidence |
|---|---|
| Preclinical and early clinical development of bone-directed therapies | Level I |
| Predicting bone disease progression, ongoing risk of SREs and mortality | Level IIa |
| Early changes in bone marker levels during bisphosphonate treatment may predict long-term benefits | Level II |
| Detection of bone metastases on an individual basis | Level III |
| Comparing the efficacy of different bone-directed therapies | Level III |
| Guiding the dosing and frequency of bisphosphonate therapy in patients with malignant bone disease | Level III |
Abbreviations: BALP, bone-specific alkaline phosphatase; CRPC, castration-resistant prostate cancer; NTX-1, amino-terminal crosslinked telopeptide of type I collagen; SRE, skeletal-related event.
Level of evidence grading system, as discussed in previous consensus guidelines.80
aNTX-I in multiple myeloma and breast cancer and BALP in CRPC.
Among many other questions to answer, we believe that more prospective studies should address the question of whether the bone remodeling markers could be helpful to decide when to perform objective evaluation of BM status with BS, bone survey or even the new imaging tools such as the axial skeleton MRI. This is probably the most frequent challenge to the clinicians: BM changes are difficult to evaluate in routine clinical practice. Indeed, by the RECIST criteria, BM is usually not a measurable disease, unless there is a soft tissue mass component evaluable by CT scan or MRI.79
There is also a need for a more adequate assessment of the ongoing risk for bone complications after 2 years of BTAs in long-term survivors. In this setting, together with the clinical information, the level of bone remodeling markers could add significant input to decide whether there is a need to continue BTAs, change the treatment regimen or simply stop the BTAs until new relevant information appears.
The research in this field should continue, and with the advent of new treatments affecting the bone microenvironment, such as radium-223 dichloride (Ra-223) and cabozantinib (a c-met inhibitor),7,79 a new effort should be taken to define the role of bone remodeling markers in the assessment of these new therapies.
Footnotes
The authors declare no conflict of interest.
References
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 2009; 27: 1564–1571. [DOI] [PubMed] [Google Scholar]
- Ulrich U, Rhiem K, Schmolling J, Flaskamp C, Paffenholz I, Salzer H et al. Cross-linked type I collagen C- and N-telopeptides in women with bone metastases from breast cancer. Arch Gynecol Obstet 2001; 264: 186–190. [DOI] [PubMed] [Google Scholar]
- Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 2005; 97: 59–69. [DOI] [PubMed] [Google Scholar]
- Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2014; 25 (Suppl 3): iii124–iii137. [DOI] [PubMed] [Google Scholar]
- Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 2011; 29: 1221–1227. [DOI] [PubMed] [Google Scholar]
- Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol 2005; 2: 504–517 quiz 1p following 33. [DOI] [PubMed] [Google Scholar]
- Jung K, Lein M. Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim Biophys Acta 2014; 1846: 425–438. [DOI] [PubMed] [Google Scholar]
- Hlaing TT, Compston JE. Biochemical markers of bone turnover—uses and limitations. Ann Clin Biochem 2014; 51: 189–202. [DOI] [PubMed] [Google Scholar]
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 2005; 26: 97–122. [PMC free article] [PubMed] [Google Scholar]
- Costa L, Demers LM, Gouveia-Oliveira A, Schaller J, Costa EB, de Moura MC et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol 2002; 20: 850–856. [DOI] [PubMed] [Google Scholar]
- Carl A, Burtis DEB. Tiezt Fundamentals of Clinical Chemistry and Molecular Diagnosis 7th edn Elsevier: Amsterdam, 2014;. [Google Scholar]
- Kyvernitakis I, Rachner TD, Urbschat A, Hars O, Hofbauer LC, Hadji P. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J Cancer Res Clin Oncol 2014; 140: 1671–1680. [DOI] [PubMed] [Google Scholar]
- Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P. Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naive postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab 2013; 98: 3206–3212. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Kiel DP. Clinical review: genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J Clin Endocrinol Metab 2012; 97: E1958–E1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G, Lanteri P, Colombini A, Banfi G. Blood biochemical markers of bone turnover: pre-analytical and technical aspects of sample collection and handling. Clin Chem Lab Med 2012; 50: 771–789. [DOI] [PubMed] [Google Scholar]
- Schafer AL, Vittinghoff E, Ramachandran R, Mahmoudi N, Bauer DC. Laboratory reproducibility of biochemical markers of bone turnover in clinical practice. Osteoporos Int 2010; 21: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P et al. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int 2012; 23: 2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 2011; 49: 1271–1274. [DOI] [PubMed] [Google Scholar]
- Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 2011; 22: 391–420. [DOI] [PubMed] [Google Scholar]
- Naylor K, Eastell R. Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol 2012; 8: 379–389. [DOI] [PubMed] [Google Scholar]
- Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM. The clinical utility of bone marker measurements in osteoporosis. J Transl Med 2013; 11: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem 2008; 54: 188–196. [DOI] [PubMed] [Google Scholar]
- Marshall LA, Cain DF, Dmowski WP, Chesnut CH 3rd. Urinary N-telopeptides to monitor bone resorption while on GnRH agonist therapy. Obstet Gynecol 1996; 87: 350–354. [DOI] [PubMed] [Google Scholar]
- Garnero P, Gineyts E, Riou JP, Delmas PD. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab 1994; 79: 780–785. [DOI] [PubMed] [Google Scholar]
- Cremers S, Sepulveda JL, Turk A. Clinical Chemistry: Endocrine (Thyroid, Pituitary, Adrenal, Bone, Pancreas, Reproductive) and cathecolamines. In: Spitalnik SL, Arinsburg SA, Jhang JS (eds), Clinical pathology- Board Review. Philadelphia, PA, USA, 2015.
- Christgau S. Circadian variation in serum CrossLaps concentration is reduced in fasting individuals. Clin Chem 2000; 46: 431. [PubMed] [Google Scholar]
- Seibel MJ, Lang M, Geilenkeuser WJ. Interlaboratory variation of biochemical markers of bone turnover. Clin Chem 2001; 47: 1443–1450. [PubMed] [Google Scholar]
- Hird A, Chow E, Zhang L, Wong R, Wu J, Sinclair E et al. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three canadian cancer centers. Int J Radiat Oncol Biol Phys 2009; 75: 193–197. [DOI] [PubMed] [Google Scholar]
- Wu K, Lin TH, Liou HC, Lu DH, Chen YR, Fu WM et al. Dextromethorphan inhibits osteoclast differentiation by suppressing RANKL-induced nuclear factor-kappaB activation. Osteoporos Int 2013; 24: 2201–2214. [DOI] [PubMed] [Google Scholar]
- Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G et al. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res 2006; 21: 1276–1287. [DOI] [PubMed] [Google Scholar]
- Fernandez-Murga ML, Vinue A, Caeiro JR, Guede D, Tarin JJ, Andres V et al. Impact of estrogens on atherosclerosis and bone in the apolipoprotein E-deficient mouse model. Menopause 2014; 22: 428–436. [DOI] [PubMed] [Google Scholar]
- Peng J, Lai ZG, Fang ZL, Xing S, Hui K, Hao C et al. Dimethyloxalylglycine prevents bone loss in ovariectomized C57BL/6J mice through enhanced angiogenesis and osteogenesis. PLoS ONE 2014; 9: e112744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualeni B, de Vernejoul MC, Marty-Morieux C, De Leonardis F, Franchi M, Monti L et al. Alteration of proteoglycan sulfation affects bone growth and remodeling. Bone 2013; 54: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014; 9: e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Katsuyama H, Watanabe Y, Okuyama T, Fushimi S, Ishikawa T et al. Does methamphetamine affect bone metabolism? Toxicology 2014; 319: 63–68. [DOI] [PubMed] [Google Scholar]
- Liu S, Song W, Boulanger JH, Tang W, Sabbagh Y, Kelley B et al. Role of TGF-beta in a mouse model of high turnover renal osteodystrophy. J Bone Miner Res 2014; 29: 1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabet Y, Baniwal SK, Leclerc N, Shi Y, Kohn-Gabet AE, Cogan J et al. Krox20/EGR2 deficiency accelerates cell growth and differentiation in the monocytic lineage and decreases bone mass. Blood 2010; 116: 3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiusaroli R, Knobler H, Luxenburg C, Sanjay A, Granot-Attas S, Tiran Z et al. Tyrosine phosphatase epsilon is a positive regulator of osteoclast function in vitro and in vivo. Mol Biol Cell 2004; 15: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorie L, Behets GJ, Baerts L, De Meester I, D'Haese PC, Verhulst A. DPP IV inhibitor treatment attenuates bone loss and improves mechanical bone strength in male diabetic rats. Am J Physiol Endocrinol Metab 2014; 307: E447–E455. [DOI] [PubMed] [Google Scholar]
- Stroup GB, Lark MW, Veber DF, Bhattacharyya A, Blake S, Dare LC et al. Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J Bone Miner Res 2001; 16: 1739–1746. [DOI] [PubMed] [Google Scholar]
- Smith SY, Jolette J, Chouinard L, Komm BS. The effects of bazedoxifene in the ovariectomized aged cynomolgus monkey. J Bone Miner Metab 2014; 33: 161–172. [DOI] [PubMed] [Google Scholar]
- Smith SY, Doyle N, Boyer M, Chouinard L, Saito H. Eldecalcitol, a vitamin D analog, reduces bone turnover and increases trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone 2013; 57: 116–122. [DOI] [PubMed] [Google Scholar]
- Smith SY, Recker RR, Hannan M, Muller R, Bauss F. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone 2003; 32: 45–55. [DOI] [PubMed] [Google Scholar]
- Soung do Y, Gentile MA, Duong le T, Drissi H. Effects of pharmacological inhibition of cathepsin K on fracture repair in mice. Bone 2013; 55: 248–255. [DOI] [PubMed] [Google Scholar]
- Papadimitropoulos A, Scherberich A, Guven S, Theilgaard N, Crooijmans HJ, Santini F et al. A 3D in vitro bone organ model using human progenitor cells. Eur Cell Mater 2011; 21: 445–458 discussion 58. [DOI] [PubMed] [Google Scholar]
- Colleoni M, O'Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thurlimann B et al. Identifying breast cancer patients at high risk for bone metastases. J Clin Oncol 2000; 18: 3925–3935. [DOI] [PubMed] [Google Scholar]
- Diel IJ. Prognostic factors for skeletal relapse in breast cancer. Cancer Treat Rev 2001; 27: 153–157 discussion 9–64. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009; 16: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briganti A, Suardi N, Gallina A, Abdollah F, Novara G, Ficarra V et al. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev 2014; 40: 3–11. [DOI] [PubMed] [Google Scholar]
- Lipton A, Chapman JA, Demers L, Shepherd LE, Han L, Wilson CF et al. Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol 2011; 29: 3605–3610. [DOI] [PubMed] [Google Scholar]
- Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 2005; 173: 10–20. [DOI] [PubMed] [Google Scholar]
- Coleman R, Rathbone E, Marshall H, Wilson C, Brown J, Gossiel F et al. Vitamin D, but not bone turnover markers, predict relapse in women with early breast cancer: an AZURE translational study. Cancer Res 2012; 72: S6-4-S6-4. [Google Scholar]
- Diel IJ, Solomayer EF, Seibel MJ, Pfeilschifter J, Maisenbacher H, Gollan C et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res 1999; 5: 3914–3919. [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009; 360: 679–691. [DOI] [PubMed] [Google Scholar]
- Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 2013; 24: 398–405. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011; 365: 1396–1405. [DOI] [PubMed] [Google Scholar]
- Coleman R, Gnant M, Paterson A, Powles T, von Minckwitz G, Pritchard K et al. Abstract S4–07: effects of bisphosphonate treatmenta on recurrence and cause-specific mortality in women with early breast cancer: a meta-analysis of individual patient data from randomised trials. Cancer Res 2014; 73: S4-07-S4-. [Google Scholar]
- Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol 2004; 22: 2942–2953. [DOI] [PubMed] [Google Scholar]
- Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer 1997; 75: 408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers LM, Costa L, Chinchilli VM, Gaydos L, Curley E, Lipton A. Biochemical markers of bone turnover in patients with metastatic bone disease. Clin Chem 1995; 41: 1489–1494. [PubMed] [Google Scholar]
- Wada N, Fujisaki M, Ishii S, Ikeda T, Kitajima M. Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer 2001; 8: 131–137. [DOI] [PubMed] [Google Scholar]
- Joerger M, Huober J. Diagnostic and prognostic use of bone turnover markers. Recent results. Cancer Res 2012; 192: 197–223. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 2005; 23: 4925–4935. [DOI] [PubMed] [Google Scholar]
- Lipton A, Cook RJ, Major P, Smith MR, Coleman RE. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist 2007; 12: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 2008; 113: 193–201. [DOI] [PubMed] [Google Scholar]
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012; 379: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 2007; 25: 4431–4437. [DOI] [PubMed] [Google Scholar]
- Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009; 8: 96–110. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Wright J, Houston S, Agrawal R, Purohit OP-K, Hayward L et al. Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastases from breast cancer. J Clin Oncol 2012; 30: (suppl; abstr 511). [Google Scholar]
- Amir E, Freedman O, Carlsson L, Dranitsaris G, Tomlinson G, Laupacis A et al. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am J Clin Oncol 2013; 36: 436–442. [DOI] [PubMed] [Google Scholar]
- Addison CL, Bouganim N, Hilton J, Vandermeer L, Dent S, Amir E et al. A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events. Breast Cancer Res Treat 2014; 144: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori D, Aglietta M, Alessi B, Gianni L, Ibrahim T, Farina G et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 2013; 14: 663–670. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Lipton A, Chew HK, Gradishar WJ, Sauter NP, Mohanlal RW et al. Efficacy and safety of continued zoledronic acid every 4 weeks versus every 12 weeks in women with bone metastases from breast cancer: results of the OPTIMIZE-2 trial. J Clin Oncol 2014; 32: 5s (suppl; abstr LBA9500̂). [Google Scholar]
- Gibiansky L, Sutjandra L, Doshi S, Zheng J, Sohn W, Peterson MC et al. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin Pharmacokinet 2012; 51: 247–260. [DOI] [PubMed] [Google Scholar]
- Coleman R, Costa L, Saad F, Cook R, Hadji P, Terpos E et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol 2011; 80: 411–432. [DOI] [PubMed] [Google Scholar]
- Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 2013; 31: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DF, Bast RC, Desch CE, Fritsche H Jr., Kemeny NE, Jessup JM et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 1996; 88: 1456–1466. [DOI] [PubMed] [Google Scholar]