Abstract
Neonates have little immunological memory and a developing immune system, which increases their vulnerability to infectious agents. Recent advances in understanding of neonatal immunity indicate that both innate and adaptive responses are dependent on precursor frequency of lymphocytes, antigenic dose and mode of exposure. Studies in neonatal mouse models and human umbilical cord blood cells demonstrate the capability of neonatal immune cells to produce immune responses similar to adults in some aspects but not others. This review focuses mainly on the developmental and functional mechanisms of the human neonatal immune system. In particular, the mechanism of innate and adaptive immunity and the role of neutrophils, antigen presenting cells, differences in subclasses of T lymphocytes (Th1, Th2, Tregs) and B cells are discussed. In addition, we have included the recent developments in neonatal mouse immune system. Understanding neonatal immunity is essential to development of therapeutic vaccines to combat newly emerging infectious agents.
Keywords: Adaptive immunity, innate immunity, T-cells, B-cells, neutrophils, NK-cells, dendritic cells, γδ-T cells, chemokines, Toll-like receptors, TCR, BCR, neonates, cord blood, CD71 erythroid cells, IL-27 macrophages, CD103 dendritic cells, B-reg cells, Treg cells, T follicular cells, Th 17, CD5 cells, RTEs
Introduction
Neonates possess a developing immune system, which is different from adults as a result of initially living in a semi-allogeneic sterile environment to then being exposed to a microbial-rich surrounding, rendering newborns highly susceptible to infections. It is estimated that 40% of the annual 3 million worldwide neonatal deaths are the result from infections [1]. Transplacentally transferred maternal antibodies contribute to early defense against pathogenic organisms in neonates. However, this passive protection is short lived and decays by the time a child is about 6 months of age. For example, the estimated duration of protection by maternal antibodies among infants is 3.3 months for measles, 2.7 months for mumps, 3.9 months for rubella, and 3.4 months for varicella [2]. In neonates, the cellular immune system matures rapidly in the first three months of life. Multiple factors influence this maturation process [3,4]. Over the last few decades extensive study of the development of immunity in neonates has occurred. This review will summarize several aspects of the development of cellular innate and adaptive immunity in human neonates and discuss recent developments in mouse neonatal immunity.
Development of cellular immunity in neonates
The development of human immune system starts at an early embryonic stages. Initially the fetal liver followed by hematopoietic stem cell progenitors (HSC) in the bone marrow gives rise to lymphocytes and polymorphonuclear cells - neutrophils, eosinophils and mast cells. After 7 weeks of gestation T-cell progenitor cells expressing CD34 receptors migrate to the thymus and differentiate into mature subsets with CD4, CD8 and αβ T- cell receptors (TCRs) [9]. Minor portions of T-cell progenitors in the fetal liver possess γ/δ TCRs by 6–8 weeks of gestation and do not migrate to the thymus for maturation. Studies using human cord blood show that multi-potent lymphoid progenitors that are CD34+CD7+ and CD34+CD10+CD19+ differentiate to become B cells [5]. The maturation and differentiation of fetal B-cells involves activation of transcriptional factors in a stepwise manner and somatic recombination of V, D, J and H exons of immunoglobulin genes leading to accumulation of IgD and IgM molecules on the B cell surface [6].
The development and maturation of neonatal lymphoid progenitors is highly regulated by multiple factors including cytokines, stromal cells, transcription factors and extracellular matrix components. For example the differentiation of granulocyte progenitors is regulated by cytokines IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF along with IL-4 and tumor necrosis factor (TNF-α) induces the expression of CD11b, CD11c and CD86 receptors on dendritic cells [7]. Further, exposure to an altered intrauterine environment to allergens, microbial infections or maternal immune-mediated disease can also have an impact on immunity at birth and on immune maturation during the early life of children. For example, a study using human cord blood cells and PBMCs from 2-year-olds born to allergic mothers show reduced CD14+ monocyte p38-MAPK phosphorylation and IL-6 secretion compared with age-matched infants with non-allergic mothers. As a consequence reduced anti-microbial responses of CD14+ monocytes were measured in children born to allergic mothers [8].
Earlier studies also demonstrated that maternal infections occurring in the third trimester are strongly associated with an increased susceptibility both to parasitemia and to malaria attacks during infancy [9]. Placental infection with Plasmodium falciparum (causative agent of malaria) or parasite-derived antigens in utero activate cord blood antigen presenting cells (APC) and modulates cord blood cytokine responses to TLR ligation [10]. P. falciparum infections at delivery are associated with higher TLR3-mediated IL-6 and IL-10 responses in the child in the first 3 months of life and significantly higher TLR3-, TLR7/8-, and TLR9-mediated TNF-α responses between 6 to 12 months of age [10]. Parasite antigen-specific immune responses of neonates born to helminth-infected mothers display a highly skewed Th2-type cytokine pattern, with a prominent role for the regulatory cytokine interleukin IL-10 that inhibits both APC HLA expression and Th1-type T-cell responses [11]. P. falciparum infection of the placenta has also been shown to have a long lasting effect on a child’s CD4 T-cell response to tuberculin PPD 12 months after BCG vaccination [12]. Maternal filarial infections also influence neonatal immune development and imbalanced cytokine levels in the plasma [13].
Several studies have shown that mother’s nutritional imbalance, both deficiency and excess, can have a considerable effect on neonatal immunity at birth and immune maturation in early life [14,15] Nutritional stress in mothers results in elevated levels of hypothalamic-pituitary-adrenal hormone (HPA) and fetal exposure to high HPA results in a reduction in thymic weight, a decreased cortical lymphocyte count and activation of an endogenous endonuclease, which in turn results in thymocyte apoptosis and immature B and T cell development [15,16]. Perturbations to the developing immune system in neonates resulting from maternal nutritional imbalance may result in susceptibility to infections at early birth or later-life risk of immune-mediated or inflammatory diseases.
Studies using human milk have shown that it contains immunomodulatory cells and cytokines that protect newborns and infants from respiratory infections such as respiratory syncytial virus (RSV) bronchiolitis, as well as allergy [17]. Human milk also contains lactoferrin, an iron-binding glycoprotein that is important for innate immune host defenses at birth because it exhibits broad-spectrum antimicrobial activity and prevents invasive fungal infections [18,19].
The complement system, which accounts for ∼5% of the total globulin fraction of serum, includes over 30 proteins, protein fragments, serum proteins, and cell membrane receptors. They induce chemotaxis of inflammatory cells and generate proteolytic fragments that help in phagocytosis by neutrophils and monocytes. The components of the complement system (C proteins) are expressed initially in the fetus during pregnancy and increase to adult levels by the first 12–18 months of life. The C proteins are found in the fetus under physiologic conditions including cytokine stimuli and play a critical role in enhancing neutralizing antibody activity and protect the fetus from the maternal immune system [20]. Neonates express C3, C4, and total hemolytic complement (CH50). Deficiency of these factors results in enhanced susceptibility to pre- or perinatal infections.[21].
Phenotypic and functional characteristics of human neonatal innate immunity
The innate immune system consists of granulocytes (mainly neutrophils), antigen presenting cells (APCs), natural killer (NK) cells and γδ-T cells [22]. These cells are immediately available to efficiently kill a broad range of pathogens. Given the limited exposure to antigens in utero and the suboptimal neonatal adaptive immune response, newborns rely heavily on their innate immune response for protection against infection [23].
Neonatal neutrophils
Neutrophils are a major component of the innate immune system and are responsible for engulfing and killing pathogens during infection. The majority of cells in human blood are neutrophils (70–75%) [24]. However, neonatal neutrophils have both quantitative and qualitative deficiencies. At birth, the number of neutrophils ranges from 1.5–28 × 109 cells/L blood, compared to steady state levels of 4.4 × 109/L in adults [25]. Both neutrophil storage pools as well as production of neutrophil progenitor cells in neonates are less than those of adults leading to diminished neutrophil responses to infection [26,27]. Besides the quantitative deficiencies, neonatal neutrophils have lower surface expression levels of TLR4 but similar levels of expression of TLR2 compared to adults [25]. However, the down-stream signaling through MyD88 and p38 pathways are defective in neonates following TLR2 and TLR4 stimulation [28]. This diminished response is attributed to high levels of adenosine in neonatal blood, which increases cyclic AMP (cAMP) levels, leading to protein kinase A (PKA) dependent or independent inhibition of TLR stimulated TNF-α secretion [23]. Once a neutrophil senses a pathogen it adheres to the vascular endothelium and migrates along a chemotactic gradient towards the site of infection to engulf and destroy the pathogen [29]. Neutrophils then undergo apoptosis to avoid excessive inflammation. Neonatal neutrophils express low levels of cell surface L-selectin and Mac-1 (CD11b/CD18) which mediate the initial loose adhesion and subsequent tight binding of neutrophils to vascular endothelium [30,31]. This loose adhesion leads to a 50% reduction in transmigration of neonatal neutrophils to sites of infection. Newborn neutrophils also exhibit a 60% reduction in chemotaxis in response to chemotactic factors such as colchicine and N-formyl-methionyl-leucyl-phenylalanine (fMLP) [32]. This impaired chemotactic response is due to blunted intracellular calcium influx and altered actin polymerization, limiting the ability of neutrophils to deform and penetrate the vascular endothelial lining [33]. Compared to adult neutrophils, human cord blood neutrophils do not respond efficiently to Fas-mediated apoptosis [33]. This reduced responsiveness to apoptotic stimuli in cord blood neutrophils is attributed to low surface expression of Fas receptor, reduced intracellular pro-apoptotic caspase-3 and decreased activity of pro-apoptotic proteins such as Siglec-9 and SHP-1 [34,35]. Moreover, neonatal neutrophils are defective in making Neutrophil Extracellular Traps (NETs), composed of granules and nuclear constituents used by neutrophils to kill extracellular bacteria [36]. Neonatal neutrophils have reduced capacity to phagocytize pathogens and the ability to degrade the ingested intracellular pathogens is impaired [37]. The NADPH oxidase system and ability to generate hydroxyl radicals is defective in neonatal granulocytes as a result of reduced lactoferrin and myeloperoxidase granules [38]. Recently, an increased number of myeloid derived suppressor cells with neutrophilic/granulocytic phenotype (Gr-MDSC) has been shown to suppress T-cell proliferation, Th1, Th2 and Th17 cytokine secretion and NK cell cytotoxicity in neonates compared to children and adults [39]. Overall, defects in neutrophil amplification, mobilization and function make neonates particularly susceptible to sepsis.
Neonatal APCs
Neonatal APCs include mainly monocytes and dendritic cells. Neonatal circulating monocytes express reduced levels of MHC class II molecules contributing to impaired APC activity [40]. DCs can be separated into two main groups: conventional DCs (cDC) and plasmacytoid DCs (pDCs) [24]. MHC class II expressing DC-like cells can be detected in the human fetal thymus and liver and are also identified in mesenteric lymph node and thymus around 12 weeks of gestation [41]. Fetal skin and tonsils are populated by DC-like cells by around 23 weeks of gestation [42,43]. DCs in human blood are mainly CD11c+ cDC and CD123+ pDCs, and are considered immature DCs migrating from bone marrow to peripheral tissue [24]. Based on the expression of CD1c (BDCA-1) and CD141 (BDCA-3), cDCs can be subdivided in to cDC1 (CD1c+ CD141-) and cDC2 (CD1c− CD141+) [44]. Most of the information regarding human neonatal DCs comes from cord blood studies. In cord blood, the pDC:cDC ratio is 3:1 compared to a 1:3 pDC-cDC ratio in adults [45]. In newborns, DC populations and monocytes are low in numbers and are found to express lower MHC-II, CD80 and CD86 compared to adult cells indicating their inability to fully activate antigen specific T and B cell responses [24,46,47].
TLRs are important pathogen recognition receptors (PRR) expressed on DCs, monocytes and other immune cells and TLR activation is highly relevant to neonatal responses to life threatening infections [23,48]. Human cDCs express TLRs 1–8 and 10 and pDCs express TLRs 1, 6, 7, 9 and 10 [24]. Basal TLR expression levels of full-term neonatal APCs are similar to that of adults [23]; however, maturational and functional responses (cytokines) to neonatal TLR stimulation are very different compared to adults [49]. For example, TLR stimulation of whole blood from preterm infants induces a dominant anti-inflammatory innate cytokine, IL-10 compared to term infant DCs that produce elevated levels of IL-10, IL-6 and Th17 inducing IL-23 [50]. This enhanced secretion of IL-10, IL-6 and IL-23 declines over the first year of life with a parallel increase in pro-inflammatory cytokines such as IL-1β and TNF-α. TLRs 1–9 induced TNF and IFN-γ responses increase from birth to 1 month [51] whereas LPS induced IL-12p70 reach adult levels only by 9 months [52]. Population (North American vs. Australasian or South African) based differences also exist in innate immune response to various TLRs and vaccine adjuvants [53,54].
Human cDCs and pDCs are the major producers of IL-12p70 and IFN-α/β, respectively [24]. IFN-α/β plays a crucial role in anti-viral immunity and assists the Th1 type immune response [55]. Neonatal pDCs exhibit severe defects in IFN-α/β production upon TLR7 or TLR9 ligation despite comparable levels of TLR expression in adults and neonates [56]. IL-12 plays a major function in co-stimulating Th1 immunity. However, IL-12p70 (consists of IL-12p40 and IL-12p35 subunits) is one of the last cytokines to reach adult levels upon TLR stimulation [57]. This reduced IL-12p70 secretion is attributed to defective transcription of the IL-12p35 subunit in neonates while IL-12p40 transcription is preserved [58]. Addition of recombinant IFN-γ to LPS stimulated monocyte-derived neonatal DCs in vitro restores IL-12p70 secretion to adult levels [59]. Synergistic stimulation with TLR4/8 or TLR3/8 appears to up regulate cord blood DC IL-12p70 secretion but not to the extent of adult DCs [60]. Altogether, neonatal monocytes, cDCs and pDCs exhibit an altered profile with low expression levels of MHC-II, CD80, CD86, CD40 and ICAM-1 (Table 1) and a bias against Th1 cell polarizing cytokines which leaves them susceptible to microbial infections and impaired immune responses to most vaccines.
Table 1.
Comparison of activation and cytokine responses* of human neonatal cord blood derived monocytes and dendritic cells compared to adults.
| Surface molecules/ cytokines |
Relative expression in newborns |
Function |
|---|---|---|
| HLA-DR | ↓ | MHC class II cell surface receptor for antigen presentation |
| CD80 | ↓ | Costimulatory signaling molecule for T cell activation |
| CD86 | ↓ | Costimulatory signaling molecule for T cell activation |
| CD40 | ↓ | Costimulatory signaling molecule for T cell activation |
| TNF-α | ↓ | Proinflammatory cytokine which activates neutrophils and T helper cells |
| IFN-α | ↓ | Antiviral cytokine important for MHC class I expression |
| IFN-γ | ↓ | Important Th1 cytokine against antiviral and intracellular pathogens. |
| IL-12 | ↓ | Cytokine produced by dendritic cells inducing Th1 type immunity |
| IL-1β | ↓ | Proinflammatory cytokine secreted in response to infection and causes fever |
| IL-6 | ↑ | Proinflammatory cytokine. |
| IL-10 | ↑ | Antiinflammatory cytokine involved in downregulation of Th1 response. |
| IL-23 | ↑ | IL-17 functions to regulate Th17 function and proliferation |
Relative expression levels of cytokines upon stimulation of APCs with TLR ligands.
Studies in neonatal mice demonstrated that immune suppressive cytokine IL-27, heterodimeric cytokine from IL-12 family, was up regulated. An elevated level of IL-27 gene expression was observed in cord blood derived macrophages compared to adults affecting CD4-Th1 response [61]. Neonatal CD103+ and CD11b+ populations of CD11c+ MHC-II hi DCs carry antigens to mediastinal lymph node from lung. The neonatal lungs contained fewer conventional DCs, with a lower ratio of CD103+ to CD11b+ DCs, and a much lower number of plasmacytoid DCs in comparison with adult lungs [62]. Recently, studies in a neonatal mouse model have shown that CD103+ DCs were functionally-limited in neonates, while CD11b+ DCs were diminished in both number and function compared to adults impacting antigen cross presentation and CD8 T-cell response [62]. Murine neonatal lung immune responses also show a shift in cytokine and transcription factors towards Th2 response [63]. Recently mice models demonstrated that lack of intestinal CD103+ DCs in neonates made them susceptible to Cryptosporidium parvum infections [64]. Intestinal epithelial TLR3 expression was also show to be limited in suckling mice contributing to enhanced rotavirus susceptibility. Human studies also demonstrated an age dependent increase in TLR3 expression in small intestine [65]. CD103+ DCs in the gut are thought to be also involved in oral tolerance development, as they convert naïve T-cells into FoxP3+ regulatory T-cells (Treg) and prevent food allergies [66].
Neonatal natural killer (NK) cells
NK cell blood counts are higher in newborns than in adults, with an increased expression of inhibitory receptor CD94/NKG2A [67]. NK cells are rapidly activated upon cell-to-cell contact with DCs, monocyte/macrophages and by cytokines [68]. Activated NK cells mediate protection by cytolytic ability and by secreting large amounts of the Th1 cytokine, IFN-γ [69]. There are two functionally distinct mature NK cell subsets. The CD56bright CD16dim/neg subset secretes high amounts of cytokines, is poorly cytotoxic and preferentially homes to lymph nodes [70]. The CD56dim CD16+ subset is highly cytotoxic and found in inflamed tissues. The CD56bright subset is slightly higher in number in human newborns compared to adults. NK cell function is tightly regulated by the presence of activating and inhibitory receptors on the cell surface. For instance, the CD94/NKG2A receptor is an inhibitory receptor whereas CD94/NKG2C is an activating receptor both binding the same human leukocyte antigen-E (HLA-E) molecule [71]. Human cord blood NK cells express higher inhibitory NKG2A receptors and lower leukocyte immunoglobulin-like receptor (LIR)-1, which binds to classical MHC-I [72]. Natural killer cytotoxic capacity of human cord blood NK cells, i.e. their capacity to quickly lyse cognate targets without undergoing differentiation, is at least 3-fold lower than in adults [73]. In addition, human cord blood NK cells show decreased cytoplasmic granules and poor degranulation ability [70]. Overall, the capacity to produce IL-15, IL-12 and IFN-γ in response to pathogens is reduced in neonates [74]. However, human cord blood NK cells express similar levels of surface CD16 (Fc γRIII) compared to adult cells and are able to perform antibody-dependent cellular cytotoxicity (ADCC) similar to adult cells [75]. Cord blood NK cells show low TLR3 mRNA expression and lack TLR3 protein expression. Cord NK cells do not produce IFN-γ in response to polyinosinic-polycytidylic acid [poly(I:C)], compared to a strong IFN-γ response from poly(I:C)-stimulated adult NK cells [76]. Additionally, there is enhanced expression of TGF-β in fetal lymph nodes and in a mouse model it has been shown that fetal NK cells are highly susceptible to TGF-β mediated suppression compared to adult NK cells [77]. The presence of prostaglandins and other soluble factors in human cord blood as well as elevated numbers of Treg- cells may contribute to the inherent defects in human cord blood NK cell function [77]. NK cells play a major role in the resolution of severe acute respiratory viral infections caused by influenza or respiratory syncytial virus. Although information on NK cells in bacterial infections in children is limited it has been shown that young children with recurrent otitis media and sinusitis have NK cell genetic defects [67,78].
Neonatal γδ-T cells
γδ-T cells represent 1–5% of the lymphocytes in the peripheral blood of adults and a lower percentage in human cord blood. γδ-T cells are one of the first responders to microbial infections including Mycobacterium tuberculosis, Listeria monocytogenes and Brucella abortus [79]. They release large amounts of IFN-γ and exhibit a cytotoxic function. The major subsets of γδ-T cells in human cord blood are Vδ1 chain paired with Vγ1 (Vγ1Vδ1) (dominant subset) and Vγ2Vδ2 (lower frequency subset) [80]. They are found in the thymus and human cord blood. Human cord blood also contains other gamma-delta chain combinations such as Vγ2Vδ1 or Vγ1Vδ2 subsets, which are infrequent in human adults [81]. Within a few years after birth the Vγ2Vδ2 subset becomes the major subset owing to Vγ2-Jγ1.2 rearrangement, creating a biased adult repertoire [82]. Vγ2Vδ2 T-cells are stimulated by small non-peptidic compounds collectively called phospho-antigens and recognition is T-Cell Receptor (TCR)-dependent [83]. Human cord blood Vγ2Vδ2 T-cells exhibits a naïve phenotype, and poor proliferative and cytokine responses to phospho-antigen stimulation [82]. They express lower levels of CD2, LFA-1, and CD45RO cell surface receptors and have weak cytolytic activity compared to adult cells [80]. The expression of early activation marker CD69 is low on both neonatal and adult cells whereas expression of HLA-DR is low on human cord blood γδ-T cells compared to adults [79]. Human cord blood γδ-T cells also have lower proportions of perforin and granzyme B effector molecules. TLR induced elevated levels of IL-23 produced by neonatal DCs costimulate neonatal γδ-T cells to induce a unique IL-17+/IFN-γ- subset compared to adult γδ-T cells [84].
Phenotypic and functional characteristics of human neonatal adaptive immunity
Experiments in cord blood demonstrate qualitative and quantitative differences in immune responses when compared with adult PBMCs and the impaired immune responses are seen up to 18 months of age after birth [85,86]. However, neonatal naïve T-cells express adult-like phenotypes of CD45RA isoform and the co-stimulatory molecules CD27 and CD28 with diversified T cell receptors. They also exhibit similar or even stronger Th1 immune responses compared to adults to certain vaccines such as the BCG vaccination [87].
Neonatal T-cells
There are two distinct subsets of T-cells expressing the T cell receptors (TCRs) γ/δ and α/β proteins. The cells expressing γ/δ TCRs in the fetal liver do not migrate to the thymus for maturation, but play an important role in protection from microbial infections at an early stage of development. Studies in mice have demonstrated that TCR-γ/δ cells stimulate dendritic cells to produce cytokines and chemokines leading to activation of adaptive immune responses [88]. The α/β T-cells migrate to the thymus for maturation through a series of orchestrated developmental events that result in CD4 or CD8 lineage-committed TCR+ thymocytes and this plays a vital role in antigen recognition and T-cell activation. These T-cells first exit the thymus in a phenotypically and functionally immature state referred to as recent thymic emigrants (RTEs) [89].
Neonatal CD4 T-cells
Studies in mice have shown that CD4+ RTEs are biased towards IL-4, IL-5, and IL-13 production than their mature naïve counterparts [90]. In humans, neonatal CD4+ cord blood (CB) cells are enriched in RTEs and proliferate in response to IL-7 in the absence of TCR stimulation [89,91]. This was shown to be due to faster down-regulation of IL-7Rα on neonatal compared to adult RTEs and higher levels of pSTAT5 activation upon exposure to IL-7 [91]. Further, the promoter regions of Th1 and Th2 cytokine genes of naïve CD4(+) RTEs are characterized by site-specific hyper methylation compared with those of mature naïve (MN) T cells [92].
Experimental studies of neonatal CD4+ T-cells demonstrate polarization towards Th2 responses (IL-4, IL-5, IL-10) with a decrease production of Th1 cytokines (IFN-γ, IL-2, and TNF-α). Suppression of interferon (IFN-γ) secretion by Th1 cells was shown to be due to higher expression and secretion of IL-4 [93]. The Th2 bias of neonatal CD4+ T-cells is reflected at the level of chromatin structure, as the loci of Th2 cytokines is hypo-methylated and favorable for rapid transcription [94]. Recent studies by Yoshimoto et al. show that the epigenetic profile of the Th2 locus at CpG residues undergoes changes in T cell lineage cells beginning in mid-gestation of the fetus and extend throughout the first week of life [95]. Several studies have also shown that the environmental co-stimulatory signals in utero influence Th cell differentiation and establish sub-set specific genome profiles at an early developmental stage of the immune system. Further, studies in RSV infected mice have also demonstrated that dietary supplements, galacto-oligosaccharides and fructo-oligosaccharides, contribute to an accelerated Th1/Th2 shift of the neonatal immune system by reducing RSV-specific Th2 cytokine CD4+ T-cells in the lung while increasing IFN-γ producing CD4+ T-cells [96].
Neonatal Th17 cells
Recent studies have shown that a population of CD161+ CD4+ T-cells preferentially develops into Th17 cells. These Th17 cells play an important role in developing immunity to bacterial and fungal infections at epithelial barriers [97]. Th17 cells express transcription factor RORγT, encoded by transcript variant 2 of the Retinoic Acid-Related Orphan Receptor C gene (RORC) and secrete IL-17A and IL-17F as well as IL-21 and IL-22. Th17 cells have been shown to be important for neonatal immunity to infections from Klebsiella, Citrobacter, Salmonella, and Candida species [98]. Experiments using human cord blood cells have shown that neonates have very a low frequency or complete absence of Th17 cells. Evidence suggests that this might be due to significantly lower levels of RORC mRNA resulting in reduced production of transcription factor RORγT [99]. Recently Santarlasci et al. demonstrated that like adult blood cells, human cord blood shows higher expression of IL-4 induced gene 1 (IL4I1), RORC, IL-17A and IL-17F when cultured in the presence of IL-1β and IL-23 [100]. Black et al. showed that neonatal CD4 T-cells exhibit a significant Th17 bias after TCR stimulation in vitro in the presence of proinflammatory cytokines IL-23, IL-6, IL-1, and TGF-β. This was shown to be due to increased expression of key upstream Th17 signaling components and transcription factors IL-23R, STAT3, RORC, IL6ST(gp130), and TGFβR1 [101]. However after TCR stimulation with anti-CD28 antibodies, in the absence of pro-inflammatory cytokines IL-6, IL-1b and IL-23, unlike adult peripheral blood cells, the human cord blood cells do not show differentiation of Th17 cells and do not produce IL-17, IL-21, IL-22, and GM-CSF. The studies to date confirm that neonatal innate cells differ in their activation-induced cytokine production that up-regulates Th17 defining transcription factor RORγT. The lower expression of RORC in neonatal T-cells may help in overcoming inflammatory responses and adverse immune reactions against microbial commensals during the early stages of T-cell repertoire development [99]. Several studies have shown that T-cell activation in the presence of transforming growth-factor-β (TGF-β) up-regulates RORγT and forkhead box (Foxp) 3 signature lineage transcription factors for Th17 and Treg cells, respectively [102,103]. Th1, Th2 and Th17 cells play an important role in developing immunity to intracellular pathogens and extracellular parasites, whereas Treg cells are essential for the immune tolerance and play a crucial role in the limitation of the excessive immune responses exerted by Th1, Th2 and Th17 cells.
TFH cells
A subset of CD4+ T-cells, play an important role in B-cell proliferation, isotype class switching and antibody affinity maturation in the germinal center. CD4+ Follicular Th (TFH) cells produce IL-21, co-stimulatory ICOS and inhibitory PD-1 molecules, and express high levels of CXCL13 chemokine receptor CXCR5. These TFH cellular components provide B-cell help in the development of antibody responses and germinal center reactions [104,105]. Compared to adults, neonates show reduced CD4+CXCR5+PD-1+ TFH-cells in their frequency and secretion of IL-21 [106]. This was shown due the decreased expression of IL-4 which leads to limited localization of TFH-cells in germinal centers of neonatal lymphoid tissues and down regulation of the chemokine receptor CCR7 [106]. Further, studies in mice also show that T-cell intrinsic and extrinsic factors could contribute to the limitations of neonatal TFH-cell expansion and differentiation including reduced strength of BCR signaling and lower levels of co-stimulatory receptors including CD21, CD40, CD80, and CD86 [107].
Neonatal Treg cells
It has also been demonstrated that skewing of naïve T-cells towards Th17 or Treg cell lineage is mutually exclusive and there exists a reciprocal development pathway [103]. Differentiation towards Th17 and Treg phenotypes depends on the local cytokine milieu. Presence of TGF-β along with IL-6, IL-21 exclusively drives naïve T-cells to become Th17, whereas IL-2 induces TGF-β-treated naïve T-cells to differentiate into Foxp3(+) Treg cells [102]. Tregs, express CD4, CD25, and Foxp3, maintain immunologic self-tolerance and negatively regulate various immune responses. Tregs are present in high frequency in human cord blood (∼12% of CD4+ T cells) and neonatal lymph nodes (∼8%) [108,109]. Human fetal Treg cells reflect a greater propensity of naïve fetal T-cells to differentiate into Tregs in response to maternal antigens that cross the placenta [110]. It has been demonstrated that human neonatal non-differentiated naïve T-cells (CD4+CD8−Foxp3−) have an intrinsic default mechanism to become Treg cells in response to TCR stimulation differentiating into CD4+Foxp3+ Treg cells and exerting suppressive functions [111]. Several reports suggest that maternal CD4+CD25+Foxp3+ Treg cells play an important role in controlling maternal alloreactivity to the developing fetus and central tolerance during early fetal thymic development [112,113].
A new subset of T helper cells, Th9, produces interleukin-9 found in the peripheral blood of allergic patients [114]. Adult CD4+ naïve and memory T-cells differentiate into Th9 cells in the presence of TGF-β and IL-4, whereas human cord blood CD4+ naïve T-cells are defective in their ability to differentiate into Th9 unless also supplemented with IL-1β and IL-10. TGF-β can convert Th2 and Th17 cells to produce IL-9 and the role of IL-9 in disease is unclear. Like many of the other cytokines, IL-9 has pleiotropic effects such as induction of inflammation, particularly allergic inflammation, survival of CD4+ T-cells, induction of mast cell growth and survival, eotaxin production, and goblet cell metaplasia [115]. It is still unknown whether Th9 cells are directly involved during infection or act to increase inflammation that may or may not help to eradicate the infection.
Neonatal CD8 T-cells
Human cord blood and neonatal mouse blood studies have demonstrated a deficiency in both magnitude and functionality of the neonatal CD8+ T-cell response [116]. This was shown to be due in-part to impaired production of bioactive IL-12p70 from neonatal APCs compared to adult APCs [57]. Recent studies in mice have shown that neonatal CD8+ T-cell hierarchy is distinct from adults and is influenced by intrinsic T-cell properties in RSV infected mice. In addition to a lower CD8+ T-cell response, neonatal mice also show a differential immunodominant profile than adults following RSV infection [117]. The impairment in neonatal CD8+ T-cell activation is also to limited CD28 mediated co-stimulation as a result of reduced expression of APC CD86 and CD80 receptors as well as differences in the uptake and processing of soluble antigen by neonatal CD103+ DCs [62]. CD103+ DCs derived from skin, lung, and intestine efficiently cross-present exogenous antigens in lymph nodes to specific CD8+ T cells- [118]. CD8+ Cytotoxic T-lymphocytes (CTLs) are important in host defense against intracellular infections and are important effectors of antiviral and antitumor immunity [119]. Earlier studies have demonstrated that human infants mount adult-like protective CD8+ T-cell responses to viral infections and DNA vaccines [120].
Neonatal T-cell signaling pathways
There is mounting evidence to suggest that defective signaling of T-cell activation and proliferation leads to reduced immune responses (Fig. 1). Experiments with human umbilical cord blood demonstrated a defective T-cell receptor signaling pathway characterized by lower expression of Lck, a protein tyrosine kinase. Inefficient phosphorylation of LcK resulted in reduced expression of TCR associated proteins [121]. Studies by Palin et al. showed that impaired human neonatal CD4+ T-cell immunity is due to higher expression of Cb1-b, aE3 ubiquitin ligase inducing anergy and reduced signaling of αβ-TCR/CD3 after stimulation with anti-CD3 and anti-CD28 [122]. Studies in mice showed that microRNA, miR-181a, enhances activation-induced calcium flux resulting in enhanced phosphorylation of downstream signaling molecules and reduced transcription of CD154, IL-2, and IFN-γ genes [122]. CD154 is a CD40 ligand (CD40L) and plays a role as a co-stimulatory molecule by binding to CD40 on antigen presenting cells (APCs). CD154 also regulates B-cell function by engaging CD40 receptors. A defective interaction of CD40-CD154 results in B-cell inability to undergo immunoglobulin class switching and antibody production. A lower level of CD154 on T-cells also results in lower expression of signaling cascade proteins such as nuclear factor of activated T-cells (NFATc2) and lower expression of cytokine genes IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-13 [123]. Understanding the immune mechanisms regulating immune responses will pave the way to develop interventional strategies for enhancing neonatal immune responses.
Figure 1.
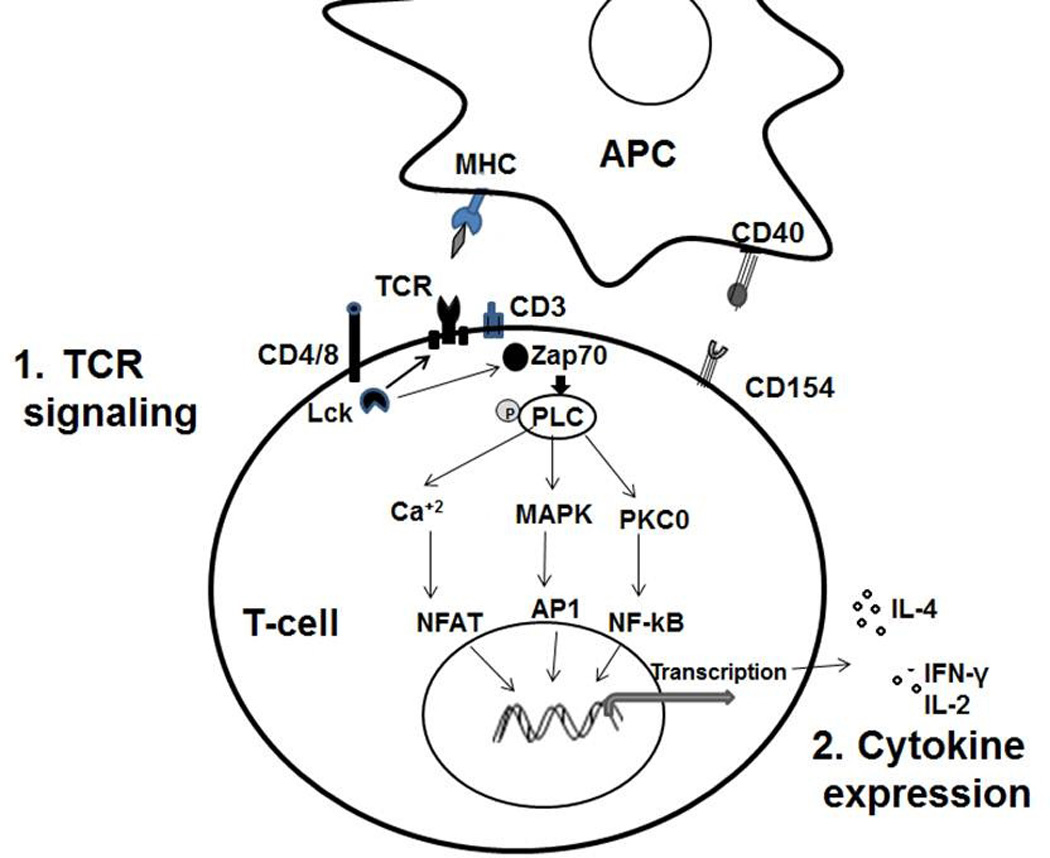
Pathways of neonatal T-cell responses during APC interactions: 1. TCR signaling: Neonatal T-cells have reduced TCR mediate activity due to inefficient phospholipase C (PLC) activation and reduced Lck expression. 2. Cytokine expression: Neonatal T-cells demonstrate polarization towards Th2 responses by producing higher IL-4 and decreased production of multifunctional Th1 cytokines (IFN-γ, TNF-α and IL-2).
Neonatal B-cells
Neonatal B-cells are naïve, lack antigenic exposure and have only a partially developed surface immunoglobulin (Ig) repertoire. Based on CD5 expression, B-cells are differentiated into two types, B-1 and B-2. B-1 cells differ functionally from B2 by their natural Ig generation and play an important role in early defense against bacterial and viral infections after birth. Human B-1 cells in cord blood and adult peripheral blood express a novel phenotype of surface markers, CD20+CD27+CD43+ [124]. B-1 cells express CD11b, higher levels of IgM (sIgM), and low levels of secreted IgD (sIgD), CD21, CD23, and CD45R (B220) [125]. Although these B-1 cells express a restricted B-cell repertoire, they are shown to be the primary source of dynamic T-cell independent (TI) antibody production and protect from bacterial infection such as Borrelia hermsii and Streptococcus pneumoniae [126,127].
Neonatal B-cell signaling pathways
The deficiencies observed in neonatal antibody production can be due to various intrinsic features such as B-cell immaturity, poor B-cell repertoire or reduced strength of BCR signaling. Activation of the BCR with T-cell dependent (TD) or T-cell independent (TI) antigens induces cross-linking of surface Ig molecules and binding to transmembrane protein CD79. This is followed by a series of signaling processes involving phosphorylation of Src-related kinases (Lyn, Fyn, fgr, Blk and LCK) and translocation into lipid rafts. Neonatal B-cells express low levels of p59Fyn and p55fgr compared to adults, which leads to differences in antigen internalization outside of lipid rafts [128,129]. Studies with neonatal B-cells indicate that they are functionally deficient to produce antibodies upon TI antigen stimulation with type 4 pneumococcal polysaccharide [130]. The limited ability of neonatal B-cells to respond to antigen stimulation can also be related to higher expression of negative regulators of BCR signaling or cross-linking of antigen to BCR molecules (Fig. 2). The high density of IgM molecules, low expression of complement factors C3d and CD21/CD22 lowers the ability of neonatal B-cells in cross-linking the BCR to bacterial polysaccharides [131,132]. Stimulation of human neonatal B-cells with a TD antigen along with CD40 and IL-4 results in increased expression of BCR negative regulator (CD22) and lowers the threshold of BCR signaling [133]. Further, defects in the nuclear signaling pathway such as NF-κB also causes maturational delay in neonatal B-cells and defects in isotype switching in response to TD antigens [134,135]. All these factors contribute to a defective BCR signaling pathway and deficiency in maturation of B-cell responses in neonates.
Figure 2.
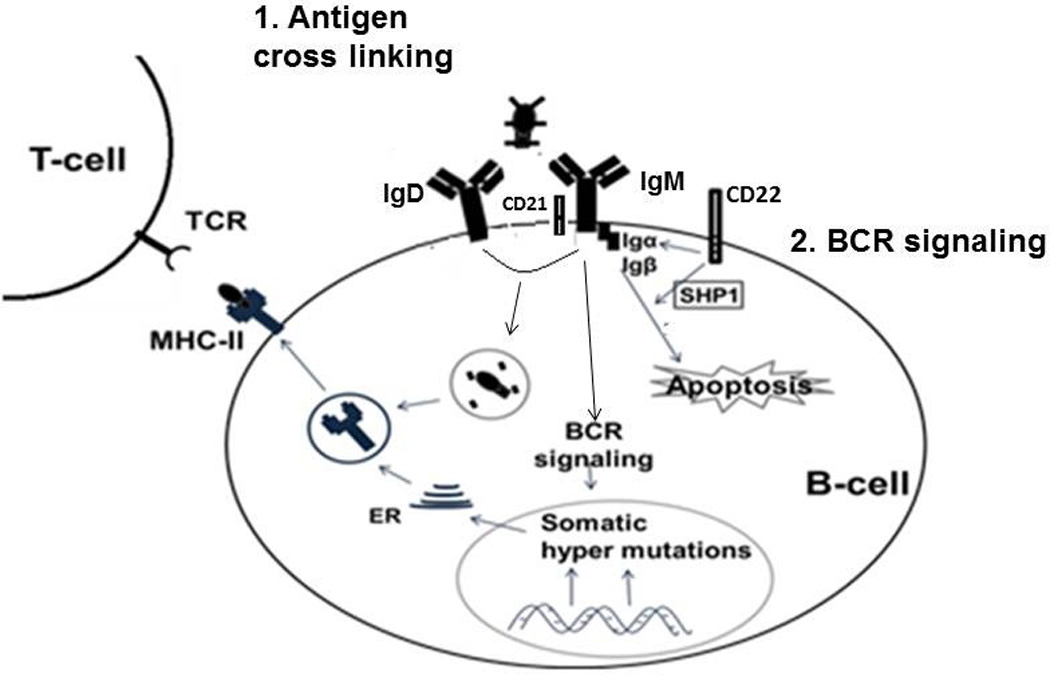
Impairment of neonatal B-cell activation with antigen: 1. Reduced expression of CD21 and increased expression of negative regulators (CD22) lowers the level of BCR signaling and induces apoptosis. 2. Higher density of IgM molecules induces cross-linking with BCR molecules resulting in lower proliferation of B-cells and loss of TCR-MHC II interactions.
Overall, the available data indicate that neonatal naïve B and T lymphocytes are differently programmed in neonates compared to adults. Neonates exhibit an increased susceptibility to infections because of immaturity in their lymphocytes including a high frequency of naïve recent thymic emigrants, low numbers of effector-memory T-cells, impaired Th1 cytokine secretion and reduced strength of B-cell receptor signaling. Several studies suggest that neonates are able to mount adult like T-cell and B-cell responses, but qualitative and quantitative differences have been observed after primary infections with viruses. Further, neonates have underdeveloped germinal centers in lymph nodes and spleen and low expression of B-cell receptors including CD21, CD40, CD80 and CD86 all of which results in low levels of primary IgG responses to infections and vaccines.
Neonatal erythroid cells
Experimental studies using animal models demonstrated that multiple non-immune cells also contribute to the development of immune responses in neonates. Recent experimental findings suggest that erythroid-lineage cells expressing transferrin receptor-CD71,present in neonatal mouse spleen, exert an immunosuppressive role to reduce excessive inflammation resulting in increased susceptibility to pathogens and reduced immune protection [136]. Depletion of CD71+ cells abrogate the suppressive effect of neonatal splenocytes resulting in complete protection against Listeria monocytogenes. Only a small number of CD71+ cells were detected in adult mice, but they did not show immunosuppressive properties suggesting that the immunosuppressive property of this population may be specific to early stage development [136]. Studies by Rimcon et al. demonstrated that the non-lymphoid-erythroid cells bearing the lymphocyte antigen 76 (LY-76, Ter119) from mice (neonatal spleens) have the capacity to modulate the differentiation of CD4+ T-cells into effector cells and provide a bias towards a Th2 type instead of Th1 type cells. These nucleated erythroid cells can produce IL-6 that contribute to IL-4 up regulation in CD4+ T-cells [137].
Role of immune regulatory plasma factors
Plasma is a rich mixture of immune regulatory factors that play an important role in in utero fetal-maternal tolerance and in suppressing pro-inflammatory responses in neonates [22]. Neonatal plasma decreases production of TNF-α induced by most TLR agonists (except TLR8) [23,49]. Also neonatal mononuclear cells suspended in neonatal plasma show decrease TLR induced IL-12p70 production and increase IL-10 compared to cells suspended in adult plasma [138]. Recently Belderbos et al. reported >50 immune suppressive factors present in neonatal plasma [22]. A number of them, IL-4, IL-13, TGF-β, adenosine and prostaglandin E2 (PGE2), are anti-inflammatory cytokines/factors found in higher amounts in neonatal plasma compared to adult plasma. Higher levels of adenosine, a purine metabolite, have been shown to enhance intracellular cyclic AMP concentrations that inhibit Th1-polarizing cytokines such as IFN-α, IFN-γ and IL-12. PGE2 has also been shown to decrease IL-12 and TNF-α production and increase IL-10 [23].
Neonatal chemokine receptors
Chemokine receptors on differentiated T-cells help in selective trafficking to target sites where the T-cells encounter antigen and activate their effector functions. Human neonatal T-cells have a similar phenotype of chemokine receptors as adult naïve T-cells, except CCR1. Experiments in mice show that the CCR1 receptor along with CCL6 plays a critical role in pathogenesis of IL-13 induced inflammation in lung tissue [139]. A chemokine receptor CCR7 is expressed on both naïve and antigen experienced T-cells. The CCR7 receptor along with CD27 and CD28 receptors can be used to differentiate early stage antigenic-specific responses. Our group has shown that predominantly early-differentiated CD4+ T-cells (CCR7+ CD27+) persist in infants during the first year of life [140]. Studies in neonatal mouse model demonstrated that a chemokine receptor CCL19 also plays a major role in emigration of mature T cells out of the thymus [141].
Overall, neonates exhibit a highly regulated and predominantly anti-inflammatory innate immune response characterized by phenotypic and functional alterations in neutrophils, APC, NK and γδ-T cells. Maternal infections during pregnancy and nutritional imbalances in resource poor countries also shape the cellular immunity of newborns along with the various anti-inflammatory plasma factors present in cord blood. As innate immune system instructs adaptive immunity, functional alterations in neonatal innate response contribute to the bias against Th1 adaptive response. Therefore overcoming the innate immune tardiness in neonates can offset the slow maturation of protective Th1 response. Since generation of protective antibodies depends on the activation of memory B-cells and plasma cells which are induced by CD4+ T-cells, intrinsic defects in B- and T-cell development and function also contributes to the diminished immune response in infants. With the development of new technologies, including multicolor flow cytometry, multiplex cytokine analysis and gene expression analyses and by using integrative systems biology approach we can better understand how the infant immune system develops, and how the innate and adaptive immune system interplay to defend the microbial infections at early age.
Conclusion
The development of neonatal immunity is influenced by multiple factors including maternal cytokines, antigen exposure and precursor frequency of lymphocytes and antigen presenting cells. Neonatal immunity shows an inherent bias towards Th2-cell polarizing cytokines and suboptimal Th1 responses and B-cell differentiation, which makes them more vulnerable to acute respiratory and diarrheal diseases. Several experimental data showed that mature adult like cellular immune responses can be developed under appropriate conditions of stimulation of neonatal T lymphocytes. Currently infants are the target age group for many of the vaccines including TB, malaria and HIV vaccines. Therefore it is important to understand more about the intrinsic factors influencing maturation and development of neonatal immune system, which paves the ways to the development of these new vaccines eliciting efficient and safe protective responses against these agents.
Expert commentary
Extensive studies in mice and human neonates have allowed an improved understanding of neonatal immunity but we still have much to learn. Studies using cord blood have demonstrated the immaturity of the neonatal immune system at birth to respond bacterial and viral antigens. However, as discussed in this review, under proper conditions of antigen presentation and cytokine environment, neonates can exhibit adult-like adaptive immune responses. Our group recently discovered a group of infants and young children (6–36 months) who display a Prolonged Neonatal-Like Immune Profile (PNIP) [142]. In the U.S. alone each year, there may be 1–1.2 million children with PNIP. We have determined that PNIP children fail to generate protective immunity to T-cell dependent antigens such as diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertussis filamentous hemagglutinin, polio vaccine, hepatitis B vaccine, and some components of Streptococcus pneumonia polysaccharide-conjugate vaccines [142]. Since maternal antibody levels wane over the first 6 months of life [2], study of PNIP children beginning at 6 months through 18 months old should allow evaluation of the neonatal adaptive response without the influence of maternal antibody as a variable.
Five-year view
Future research to dissect the intrinsic defects in neonatal cells involved in the adaptive and innate immune response represents the path forward in this field of immunology. Our group and others are studying differences in neonates and infants compared to older children and adults regarding antigen processing and presentation by APCs, TCR signaling in T-cells and the interactions between Ig molecules with BCR in B-cells. Within 5 years, the inadequate responsiveness to infections and vaccination of neonates and infants should be more completely understood. Neonates can respond to some antigens very well and the features of those antigens that permit protective responses to pathogen exposure and adequate responses to vaccines (e.g. tetanus toxoid and hepatitis B vaccine) will be evaluated. The use of novel adjuvants to facilitate improved APC processing and presentation of antigen is actively being investigated and an achievable goal in the near future.
Utilizing the advanced study of multiple immune cells with multicolor flow cytometry, multiplexed cytometric assays and systems biology tools will lead to a better understanding of pathogen and vaccine-inducible responses at the single-cell level. Application of these newer technologies will also permit characterization of the regulatory mechanisms involved in generating secondary immune responses. Systems biology will be used in the future to determine molecular signatures of transcriptomes regulating pathogen-specific and vaccine-specific responses in order to predict improved methods to facilitate protective immunity [143].
Our group intends to identify the mechanisms at play with respect to limited vaccine-induced immune memory in neonates and PNIP infants. We will focus on further determining the contributing factors to immune maturational delays with respect to the quality and quantity of the adaptive CD4+ T-cell vaccine-specific recall response, of systemic and mucosal antibody and memory B-cell generation and persistence and of APC/CD4 T-cell interactions following routine U.S. ACIP-recommended vaccinations. Moreover, we will seek to understand the contributing immune mechanisms that cause divergence in vaccine responses of neonates and infants with PNIP compared to older children and adults that could be overcome by a more rational approach to modifications in pediatric vaccines such as the addition of novel adjuvants.
Key Issues.
The immune system of the neonate makes them highly vulnerable to viral, bacterial, fungal and parasitic diseases, often associated with a high mortality. Understanding the molecular regulation of innate and adaptive immune system as well as their molecular signatures is a key issue.
Maternal infections (HIV, malaria, TB) during pregnancy shape neonatal immune development and it is important to consider maternal vaccinations that can generate protective immunity in neonates.
The low precursor cell frequency and quantity, and quality of innate immune response have been shown to impede a stronger adaptive immune response in neonates. Future efforts to improve APC response by using new generation adjuvants/TLR agonists are a key priority.
Multiple factors influence the neonatal adaptive immune responses such as proliferation of T lymphocytes, T-cell help to B-cell immunity and B-cell antibody production. All of these should be considered in enhancing vaccine efficacy.
Other factors that affect neonatal immunity include maternal nutrition, allergens, infections, maternal antibodies and plasma factors.
Neonates and infants receive multiple priming doses of vaccines to achieve immunity. Yet, 19.3 million neonates and infants throughout the world do not receive the multiple recommended doses of vaccines required to achieve optimal immunity (UNICEF). These data strongly argue for additional research to better understand exploitable mechanisms to achieve more robust and prolonged immunity with fewer primary and booster vaccinations.
Acknowledgments
This work was supported by the U.S. NIH NIDCD RO1 08671. Authors would like to thank Dr. Robert Zagursky for his critical reading and valuable discussions.
References
- 1.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Waaijenborg S, Hahne SJ, Mollema L, et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. The Journal of infectious diseases. 2013;208(1):10–16. doi: 10.1093/infdis/jit143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgins DC, Shewen PE. Vaccination of neonates: problem and issues. Vaccine. 2012;30(9):1541–1559. doi: 10.1016/j.vaccine.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Siegrist CA. The challenges of vaccine responses in early life: selected examples. Journal of comparative pathology. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Sanz E, Munoz AN, Monserrat J, et al. Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(13):5925–5930. doi: 10.1073/pnas.0907942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad R, Guardiola P, Izac B, et al. Molecular characterization of early human T/NK and B-lymphoid progenitor cells in umbilical cord blood. Blood. 2004;104(13):3918–3926. doi: 10.1182/blood-2004-05-1845. [DOI] [PubMed] [Google Scholar]
- 7.Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunological reviews. 2010;238(1):37–46. doi: 10.1111/j.1600-065X.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saghafian-Hedengren S, Holmlund U, Amoudruz P, Nilsson C, Sverremark-Ekstrom E. Maternal allergy influences p38-mitogen-activated protein kinase activity upon microbial challenge in CD14+ monocytes from 2-year-old children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38(3):449–457. doi: 10.1111/j.1365-2222.2007.02917.x. [DOI] [PubMed] [Google Scholar]
- 9.Borgella S, Fievet N, Huynh BT, et al. Impact of pregnancy-associated malaria on infant malaria infection in southern Benin. PloS one. 2013;8(11):e80624. doi: 10.1371/journal.pone.0080624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gbedande K, Varani S, Ibitokou S, et al. Malaria modifies neonatal and early-life toll-like receptor cytokine responses. Infection and immunity. 2013;81(8):2686–2696. doi: 10.1128/IAI.00237-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broen K, Brustoski K, Engelmann I, Luty AJ. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Molecular and biochemical parasitology. 2007;151(1):1–8. doi: 10.1016/j.molbiopara.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Walther B, Miles DJ, Waight P, et al. Placental malaria is associated with attenuated CD4 T-cell responses to tuberculin PPD 12 months after BCG vaccination. BMC infectious diseases. 2012;12:6. doi: 10.1186/1471-2334-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achary KG, Mandal NN, Mishra S, et al. Maternal filarial infection: association of anti-sheath antibody responses with plasma levels of IFN-gamma and IL-10. Parasitology. 2013;140(5):598–603. doi: 10.1017/S0031182012002144. [DOI] [PubMed] [Google Scholar]
- 14.Fisher RE, Steele M, Karrow NA. Fetal programming of the neuroendocrine-immune system and metabolic disease. Journal of pregnancy. 2012;2012:792934. doi: 10.1155/2012/792934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer AC. Nutritionally mediated programming of the developing immune system. Advances in nutrition. 2011;2(5):377–395. doi: 10.3945/an.111.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foss B, Dyrstad SM. Stress in obesity: cause or consequence? Medical hypotheses. 2011;77(1):7–10. doi: 10.1016/j.mehy.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Bryan DL, Hart PH, Forsyth KD, Gibson RA. Immunomodulatory constituents of human milk change in response to infant bronchiolitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2007;18(6):495–502. doi: 10.1111/j.1399-3038.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 18.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA : the journal of the American Medical Association. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 19.Manzoni P, Stolfi I, Messner H, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129(1):116–123. doi: 10.1542/peds.2011-0279. [DOI] [PubMed] [Google Scholar]
- 20.Lollgen RM, Lu VH, Middlebrook L, Gunja N, McCaskill M. Party bubbles: Friend or foe? A conjunctival burn in a paediatric emergency department. Emergency medicine Australasia : EMA. 2014 doi: 10.1111/1742-6723.12228. [DOI] [PubMed] [Google Scholar]
- 21.Cedzynski M, Swierzko AS, Kilpatrick DC. Factors of the lectin pathway of complement activation and their clinical associations in neonates. Journal of biomedicine & biotechnology. 2012;2012:363246. doi: 10.1155/2012/363246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belderbos ME, Levy O, Meyaard L, Bont L. Plasma-mediated immune suppression: a neonatal perspective. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2013;24(2):102–113. doi: 10.1111/pai.12023. [DOI] [PubMed] [Google Scholar]
- 23.Levy O. Innate immunity of the newborn: basic mechanisms clinical correlates Nature reviews. Immunology. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 24.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. European journal of immunology. 2009;39(1):26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 25.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. International reviews of immunology. 2010;29(3):315–348. doi: 10.3109/08830181003792803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdman SH, Christensen RD, Bradley PP, Rothstein G. Supply release of storage neutrophils A developmental study. Biology of the neonate. 1982;41(3–4):132–137. doi: 10.1159/000241541. [DOI] [PubMed] [Google Scholar]
- 27.Lieschke GJ, Dunn AR. Development of functional macrophages from embryonal stem cells in vitro. Experimental hematology. 1995;23(4):328–334. [PubMed] [Google Scholar]
- 28.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide Clinical and investigative medicine. Medecine clinique et experimentale. 2007;30(2):E44–E53. doi: 10.25011/cim.v30i2.979. [DOI] [PubMed] [Google Scholar]
- 29.Kim SK, Keeney SE, Alpard SK, Schmalstieg FC. Comparison of L-selectin and CD11b on neutrophils of adults and neonates during the first month of life. Pediatric research. 2003;53(1):132–136. doi: 10.1203/00006450-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DC, Abbassi O, Kishimoto TK, Koenig JM, McIntire LV, Smith CW. Diminished lectin-, epidermal growth factor-, complement binding domain-cell adhesion molecule-1 on neonatal neutrophils underlies their impaired CD18-independent adhesion to endothelial cells in vitro. Journal of immunology. 1991;146(10):3372–3379. [PubMed] [Google Scholar]
- 31.Anderson DC, Rothlein R, Marlin SD, Krater SS, Smith CW. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990;76(12):2613–2621. [PubMed] [Google Scholar]
- 32.Weinberger B, Laskin DL, Mariano TM, et al. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. Journal of leukocyte biology. 2001;70(6):969–976. [PMC free article] [PubMed] [Google Scholar]
- 33.Howard TH, Meyer WH. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. The Journal of cell biology. 1984;98(4):1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna N, Vasquez P, Pham P, et al. Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatric research. 2005;57(1):56–62. doi: 10.1203/01.PDR.0000147568.14392.F0. [DOI] [PubMed] [Google Scholar]
- 35.Luo D, Schowengerdt KO, Jr, Stegner JJ, May WS, Jr, Koenig JM. Decreased functional caspase-3 expression in umbilical cord blood neutrophils is linked to delayed apoptosis. Pediatric research. 2003;53(5):859–864. doi: 10.1203/01.PDR.0000059747.52100.2E. [DOI] [PubMed] [Google Scholar]
- 36.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller ME. Phagocyte function in the neonate: selected aspects. Pediatrics. 1979;64(5 Pt) 2 Suppl:709–712. [PubMed] [Google Scholar]
- 38.Rider ED, Christensen RD, Hall DC, Rothstein G. Myeloperoxidase deficiency in neutrophils of neonates. The Journal of pediatrics. 1988;112(4):648–651. doi: 10.1016/s0022-3476(88)80190-2. [DOI] [PubMed] [Google Scholar]
- 39.Rieber N, Gille C, Kostlin N, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clinical and experimental immunology. 2013;174(1):45–52. doi: 10.1111/cei.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. Journal of reproductive immunology. 2002;56(1–2):45–60. doi: 10.1016/s0165-0378(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26(6):741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Hofman FM, Danilovs JA, Taylor CR. HLA-DR (Ia)-positive dendritic-like cells in human fetal nonlymphoid tissues. Transplantation. 1984;37(6):590–594. doi: 10.1097/00007890-198406000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Janossy G, Bofill M, Poulter LW, et al. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. Journal of immunology. 1986;136(12):4354–4361. [PubMed] [Google Scholar]
- 44.Wang JC, Kobie JJ, Zhang L, et al. An 11-color flow cytometric assay for identifying, phenotyping, and assessing endocytic ability of peripheral blood dendritic cell subsets in a single platform. Journal of immunological methods. 2009;341(1–2):106–116. doi: 10.1016/j.jim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borras FE, Matthews NC, Lowdell MW, Navarrete CV. Identification of both myeloid CD11c+ and lymphoid CD11c- dendritic cell subsets in cord blood. British journal of haematology. 2001;113(4):925–931. doi: 10.1046/j.1365-2141.2001.02840.x. [DOI] [PubMed] [Google Scholar]
- 46.De Wit D, Tonon S, Olislagers V, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. Journal of autoimmunity. 2003;21(3):277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clinical and experimental immunology. 2002;128(1):118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends in microbiology. 2000;8(10):452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 49.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. Journal of immunology. 2004;173(7):4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 50.Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatric research. 2006;60(2):205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 51.Burl S, Townend J, Njie-Jobe J, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PloS one. 2011;6(4):e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen M, Leuridan E, Zhang T, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PloS one. 2010;5(4):e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kollmann TR. Variation between Populations in the Innate Immune Response to Vaccine Adjuvants. Frontiers in immunology. 2013;4:81. doi: 10.3389/fimmu.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smolen KK, Ruck CE, Fortuno ES, 3rd, et al. Pattern recognition receptor-mediated cytokine response in infants across 4 continents. The Journal of allergy and clinical immunology. 2014;133(3):818–826. doi: 10.1016/j.jaci.2013.09.038. e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao W, Liu YJ. Innate immune functions of plasmacytoid dendritic cells. Current opinion in immunology. 2007;19(1):24–30. doi: 10.1016/j.coi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.De Wit D, Olislagers V, Goriely S, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103(3):1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 57.Lee HH, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. The Journal of experimental medicine. 2008;205(10):2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. Journal of immunology. 2001;166(3):2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 59.Upham JW, Lee PT, Holt BJ, et al. Development of interleukin-12-producing capacity throughout childhood. Infection and immunity. 2002;70(12):6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Human immunology. 2007;68(10):813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Kraft JD, Horzempa J, Davis C, Jung JY, Pena MM, Robinson CM. Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses. Immunology. 2013;139(4):484–493. doi: 10.1111/imm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS pathogens. 2014;10(2):e1003934. doi: 10.1371/journal.ppat.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roux X, Remot A, Petit-Camurdan A, et al. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. European journal of immunology. 2011;41(10):2852–2861. doi: 10.1002/eji.201041224. [DOI] [PubMed] [Google Scholar]
- 64.Lantier L, Lacroix-Lamande S, Potiron L, et al. Intestinal CD103+ dendritic cells are key players in the innate immune control of Cryptosporidium parvum infection in neonatal mice. PLoS pathogens. 2013;9(12):e1003801. doi: 10.1371/journal.ppat.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pott J, Stockinger S, Torow N, et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS pathogens. 2012;8(5):e1002670. doi: 10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stern A, Wold AE, Ostman S. Neonatal mucosal immune stimulation by microbial superantigen improves the tolerogenic capacity of CD103(+) dendritic cells. PloS one. 2013;8(9):e75594. doi: 10.1371/journal.pone.0075594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. Natural killer cell responses to infections in early life. Journal of innate immunity. 2011;3(3):280–288. doi: 10.1159/000323934. [DOI] [PubMed] [Google Scholar]
- 68.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens Nature reviews. Immunology. 2007;7(4):279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 69.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 70.Le Garff-Tavernier M, Beziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging cell. 2010;9(4):527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 71.Braud VM, Allan DS, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cellular & molecular immunology. 2007;4(5):377–382. [PubMed] [Google Scholar]
- 73.Dalle JH, Menezes J, Wagner E, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatric research. 2005;57(5 Pt 1):649–655. doi: 10.1203/01.PDR.0000156501.55431.20. [DOI] [PubMed] [Google Scholar]
- 74.Lau AS, Sigaroudinia M, Yeung MC, Kohl S. Interleukin-12 induces interferon-gamma expression and natural killer cytotoxicity in cord blood mononuclear cells. Pediatric research. 1996;39(1):150–155. doi: 10.1203/00006450-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 75.Kohl S, Sigouroudinia M, Engleman EG. Adhesion defects of antibody-mediated target cell binding of neonatal natural killer cells. Pediatric research. 1999;46(6):755–759. doi: 10.1203/00006450-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Slavica L, Nordstrom I, Karlsson MN, et al. TLR3 impairment in human newborns. Journal of leukocyte biology. 2013;94(5):1003–1011. doi: 10.1189/jlb.1212617. [DOI] [PubMed] [Google Scholar]
- 77.Ivarsson MA, Loh L, Marquardt N, et al. Differentiation and functional regulation of human fetal NK cells. The Journal of clinical investigation. 2013;123(9):3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes and infection / Institut Pasteur. 2002;4(15):1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 79.Engelmann I, Moeller U, Santamaria A, Kremsner PG, Luty AJ. Differing activation status and immune effector molecule expression profiles of neonatal and maternal lymphocytes in an African population. Immunology. 2006;119(4):515–521. doi: 10.1111/j.1365-2567.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. Journal of immunology. 1994;153(9):3979–3988. [PubMed] [Google Scholar]
- 81.Cairo C, Propp N, Auricchio G, et al. Altered cord blood gammadelta T cell repertoire in Nigeria: possible impacts of environmental factors on neonatal immunity. Molecular immunology. 2008;45(11):3190–3197. doi: 10.1016/j.molimm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cairo C, Mancino G, Cappelli G, et al. Vdelta2 T-lymphocyte responses in cord blood samples from Italy and Cote d'Ivoire. Immunology. 2008;124(3):380–387. doi: 10.1111/j.1365-2567.2007.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Experimental hematology. 2004;32(7):622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vgamma9Vdelta2 T cells expressing high levels of cytotoxic mediators and producing IFN-gamma and IL-17. Journal of leukocyte biology. 2011;89(5):743–752. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 85.Vosters O, Lombard C, Andre F, Sana G, Sokal EM, Smets F. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clinical and experimental immunology. 2010;162(3):494–499. doi: 10.1111/j.1365-2249.2010.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(2):160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clinical and experimental immunology. 2005;141(1):10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi C, Sahay B, Russell JQ, et al. Reduced immune response to Borrelia burgdorferi in the absence of gammadelta T cells. Infection and immunity. 2011;79(10):3940–3946. doi: 10.1128/IAI.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fink PJ. The biology of recent thymic emigrants. Annual review of immunology. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 90.Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 2011;117(4):1239–1249. doi: 10.1182/blood-2010-07-299263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants are phenotypically and functionally distinct from adult recent thymic emigrants. Blood. 2009;113(22):5635–5643. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berkley AM, Hendricks DW, Simmons KB, Fink PJ. Recent thymic emigrants and mature naive T cells exhibit differential DNA methylation at key cytokine loci. Journal of immunology. 2013;190(12):6180–6186. doi: 10.4049/jimmunol.1300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends in immunology. 2009;30(12):585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Webster RB, Rodriguez Y, Klimecki WT, Vercelli D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. The Journal of biological chemistry. 2007;282(1):700–709. doi: 10.1074/jbc.M609501200. [DOI] [PubMed] [Google Scholar]
- 95.Yoshimoto M, Yoder MC, Guevara P, Adkins B. The murine Th2 locus undergoes epigenetic modification in the thymus during fetal and postnatal ontogeny. PloS one. 2013;8(1):e51587. doi: 10.1371/journal.pone.0051587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schijf MA, Kruijsen D, Bastiaans J, et al. Specific dietary oligosaccharides increase Th1 responses in a mouse respiratory syncytial virus infection model. Journal of virology. 2012;86(21):11472–11482. doi: 10.1128/JVI.06708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. The Journal of experimental medicine. 2008;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 99.de Roock S, Stoppelenburg AJ, Scholman R, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. The Journal of allergy and clinical immunology. 2013;132(3):754–756. doi: 10.1016/j.jaci.2013.04.014. e753. [DOI] [PubMed] [Google Scholar]
- 100.Santarlasci V, Maggi L, Capone M, et al. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 2012;36(2):201–214. doi: 10.1016/j.immuni.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 101.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. European journal of immunology. 2012;42(2):311–319. doi: 10.1002/eji.201141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng SG. Regulatory T cells vs Th17: differentiation of Th17 versus Treg, are the mutually exclusive? American journal of clinical and experimental immunology. 2013;2(1):94–106. [PMC free article] [PubMed] [Google Scholar]
- 103.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 104.Crotty S. The 1–1–1 fallacy. Immunological reviews. 2012;247(1):133–142. doi: 10.1111/j.1600-065X.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- 105.Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-(+)1CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Debock I, Jaworski K, Chadlaoui H, et al. Neonatal follicular Th cell responses are impaired and modulated by IL-4. Journal of immunology. 2013;191(3):1231–1239. doi: 10.4049/jimmunol.1203288. [DOI] [PubMed] [Google Scholar]
- 107.Mastelic B, Kamath AT, Fontannaz P, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. Journal of immunology. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 108.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. Journal of immunology. 2006;176(10):5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 109.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. American journal of reproductive immunology. 2013;69(4):346–358. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. "Default" generation of neonatal regulatory T cells. Journal of immunology. 2010;185(1):71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 112.Teles A, Zenclussen AC, Schumacher A. Regulatory T cells are baby's best friends. American journal of reproductive immunology. 2013;69(4):331–339. doi: 10.1111/aji.12067. [DOI] [PubMed] [Google Scholar]
- 113.Schumacher A, Zenclussen AC. Regulatory T Cells: Regulators of Life. American journal of reproductive immunology. 2014 doi: 10.1111/aji.12238. [DOI] [PubMed] [Google Scholar]
- 114.Kaplan MH. Th9 cells: differentiation and disease. Immunological reviews. 2013;252(1):104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu J, Harberts E, Tammaro A, et al. IL-9 Regulates Allergen-Specific Th1 Responses in Allergic Contact Dermatitis. The Journal of investigative dermatology. 2014 doi: 10.1038/jid.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCarron MJ, Reen DJ. Neonatal CD8+ T-cell differentiation is dependent on interleukin-12. Human immunology. 2010;71(12):1172–1179. doi: 10.1016/j.humimm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Ruckwardt TJ, Malloy AM, Gostick E, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS pathogens. 2011;7(12):e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunological reviews. 2010;234(1):268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 119.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death Nature reviews. Immunology. 2002;2(6):401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 120.Zhang J, Silvestri N, Whitton JL, Hassett DE. Neonates mount robust and protective adult-like CD8(+)-T-cell responses to DNA vaccines. Journal of virology. 2002;76(23):11911–11919. doi: 10.1128/JVI.76.23.11911-11919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miscia S, Di Baldassarre A, Sabatino G, et al. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. Journal of immunology. 1999;163(5):2416–2424. [PubMed] [Google Scholar]
- 122.Palin AC, Ramachandran V, Acharya S, Lewis DB. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. Journal of immunology. 2013;190(6):2682–2691. doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaminski BA, Kadereit S, Miller RE, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102(13):4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 124.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. The Journal of experimental medicine. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghosn EE, Yang Y, Tung J, Herzenberg LA, Herzenberg LA. CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):5195–5200. doi: 10.1073/pnas.0712350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21(3):379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 127.Haas KM, Poe JC, Steeber DA, Tedder TF. TFB-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23(1):7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 128.Sproul TW, Malapati S, Kim J, Pierce SK. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. Journal of immunology. 2000;165(11):6020–6023. doi: 10.4049/jimmunol.165.11.6020. [DOI] [PubMed] [Google Scholar]
- 129.Hashimoto A, Takeda K, Inaba M, et al. Cutting edge: essential role of phospholipase C-gamma 2 in B cell development and function. Journal of immunology. 2000;165(4):1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 130.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clinical & developmental immunology. 2008;2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Viemann D, Schlenke P, Hammers HJ, Kirchner H, Kruse A. Differential expression of the B cell-restricted molecule CD22 on neonatal B lymphocytes depending upon antigen stimulation. European journal of immunology. 2000;30(2):550–559. doi: 10.1002/1521-4141(200002)30:2<550::AID-IMMU550>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 132.Alper CA, Xu J, Cosmopoulos K, et al. Immunoglobulin deficiencies and susceptibility to infection among homozygotes and heterozygotes for C2 deficiency. Journal of clinical immunology. 2003;23(4):297–305. doi: 10.1023/a:1024540917593. [DOI] [PubMed] [Google Scholar]
- 133.Tian C, Kron GK, Dischert KM, Higginbotham JN, Crowe JE., Jr Low expression of the interleukin (IL)-4 receptor alpha chain and reduced signalling via the IL-4 receptor complex in human neonatal B cells. Immunology. 2006;119(1):54–62. doi: 10.1111/j.1365-2567.2006.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goudeau B, Huetz F, Samson S, et al. IkappaBalpha/IkappaBepsilon deficiency reveals that a critical NF-kappaB dosage is required for lymphocyte survival. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15800–15805. doi: 10.1073/pnas.2535880100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from neonatal B-1 transitional cells exhibits NF-kappaB redundancy. Journal of immunology. 2011;187(11):5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504(7478):158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen. International journal of biological sciences. 2012;8(5):719–730. doi: 10.7150/ijbs.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Belderbos ME, Levy O, Stalpers F, Kimpen JL, Meyaard L, Bont L. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PloS one. 2012;7(3):e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma B, Zhu Z, Homer RJ, Gerard C, Strieter R, Elias JA. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. Journal of immunology. 2004;172(3):1872–1881. doi: 10.4049/jimmunol.172.3.1872. [DOI] [PubMed] [Google Scholar]
- 140.Sharma SK, Pichichero ME. Functional deficits of pertussis-specific CD4+ T cells in infants compared to adults following DTaP vaccination. Clinical and experimental immunology. 2012;169(3):281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ueno T, Hara K, Willis MS, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16(2):205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 142.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. The Pediatric infectious disease journal. 2013;32(11):1163–1168. doi: 10.1097/INF.0b013e31829e887e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nature immunology. 2014;15(2):195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]


