Abstract
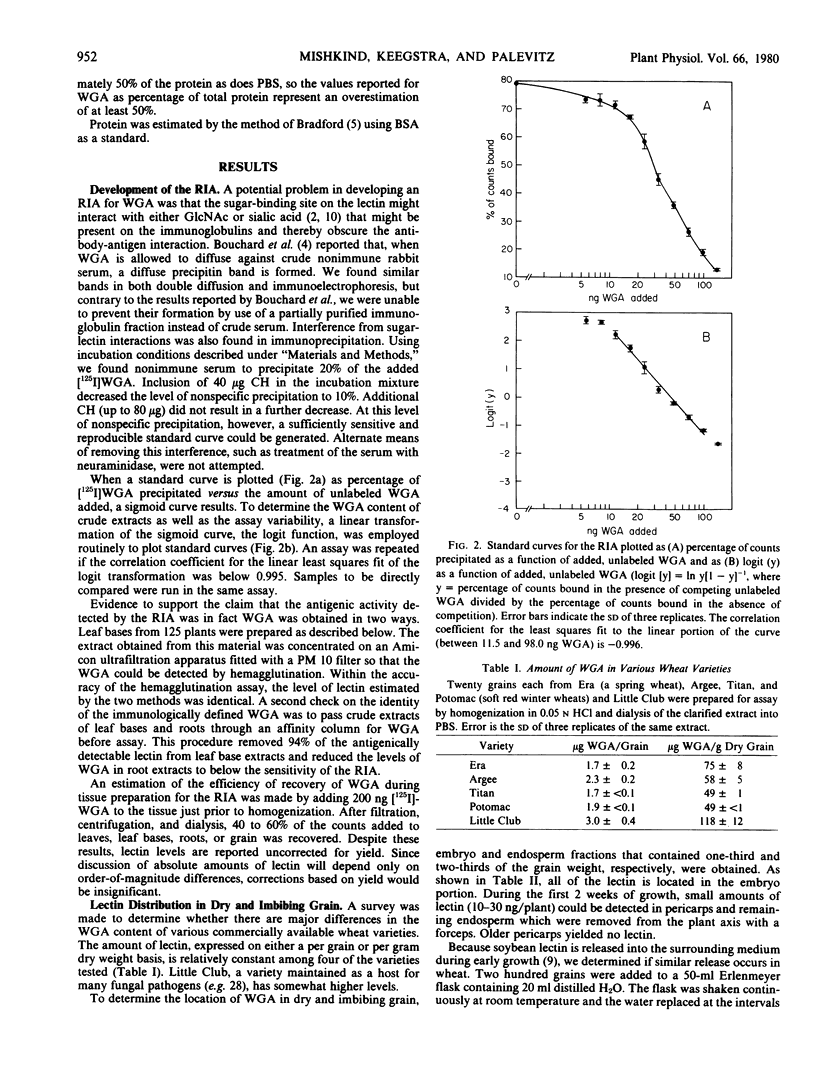
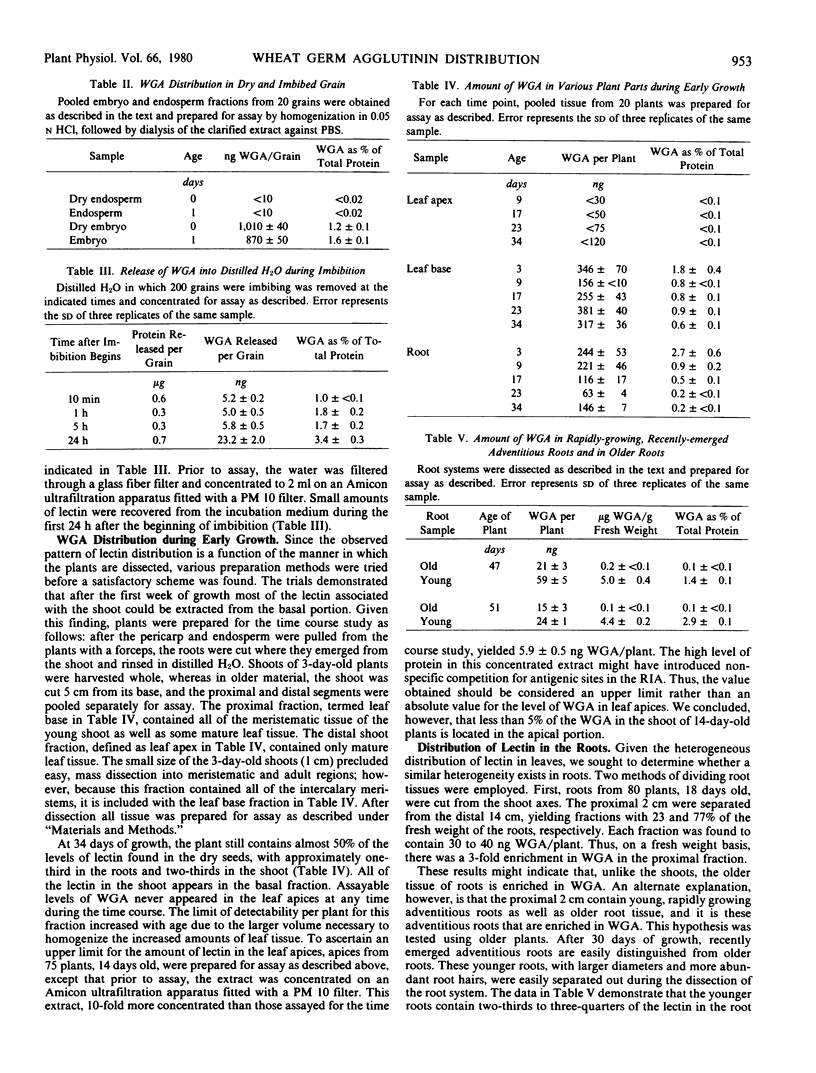
A liquid phase, competition-binding radioimmunoassay for wheat germ agglutinin, with a detection limit of 10 nanograms, was developed in order to determine the distribution of this lectin in young wheat plants. Affinity columns for wheat germ agglutinin removed all antigenically detectable activity from crude extracts of wheat tissue; thus, the antigenic cross-reactivity detected by the assay possesses sugar-binding specificity similar to the wheat germ-derived lectin. The amount of lectin per dry grain is approximately 1 microgram, all associated with the embryo. At 34 days of growth, the level of lectin per plant was reduced by about 50%, with approximately one-third in the roots and two-thirds in the shoot. The data also indicate that actively growing regions of the plant (the bases of the leaves and rapidly growing adventitious roots) contain the highest levels of lectin. Half of the lectin associated with the roots could be solubilized by washing intact roots in buffer containing oligomers of N-acetylglucosamine, whereas the remainder is liberated only upon homogenization of the tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Bloch R., Burger M. M. Purification of wheat germ agglutinin using affinity chromatography on chitin. Biochem Biophys Res Commun. 1974 May 7;58(1):13–19. doi: 10.1016/0006-291x(74)90884-5. [DOI] [PubMed] [Google Scholar]
- Bouchard P., Moroux Y., Tixier R., Privat J. P., Monsigny M. An improved method for purification of wheat germ agglutinin (lectin) by affinity chromatography. Biochimie. 1976;58(10):1247–1253. doi: 10.1016/s0300-9084(76)80124-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Desai N. N., Allen A. K. The purification of potato lectin by affinity chromatography on an N,N',N''-triacetylchitotriose-Sepharose matrix. Anal Biochem. 1979 Feb;93(1):88–90. [PubMed] [Google Scholar]
- Fountain D. W., Foard D. E., Replogle W. D., Yang W. K. Lectin release by soybean seeds. Science. 1977 Sep 16;197(4309):1185–1187. doi: 10.1126/science.197.4309.1185. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Hamblin J., Kent S. P. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat New Biol. 1973 Sep 5;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Hapner K. D., Robbins J. E. Isolation and properties of a lectin from sainfoin (Onobrychis viciifolia, Scop.). Biochim Biophys Acta. 1979 Sep 29;580(1):186–197. doi: 10.1016/0005-2795(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Horton C. B. Studies on the appearance and location of hemagglutinins from a common lentil during the life cycle of the plant. Arch Biochem Biophys. 1972 Mar;149(1):323–326. doi: 10.1016/0003-9861(72)90328-1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. C., Yeoman M. M., Gould A. R. Tissue and subcellular distribution of the lectin from Datura stramonium (thorn apple). Biochem J. 1979 Nov 15;184(2):215–219. doi: 10.1042/bj1840215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Galun E., Sharon N., Lotan R. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975 Jul 31;256(5516):414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Molano J., Polacheck I., Duran A., Cabib E. An endochitinase from wheat germ. Activity on nascent and preformed chitin. J Biol Chem. 1979 Jun 10;254(11):4901–4907. [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Pueppke S. G., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: II. Distribution of Soybean Lectin in Tissues of Glycine max (L.) Merr. Plant Physiol. 1978 May;61(5):779–784. doi: 10.1104/pp.61.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Distribution of Lectins in the Jumbo Virginia and Spanish Varieties of the Peanut, Arachis hypogaea L. Plant Physiol. 1979 Oct;64(4):575–580. doi: 10.1104/pp.64.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Rougé P. Devenir des phytohémagglutinines provenant des diverses parties de la graine dans les jeunes germinations du pois. C R Acad Sci Hebd Seances Acad Sci D. 1975 May 12;280(18):2105–2108. [PubMed] [Google Scholar]
- Talbot C. F., Etzler M. E. Development and Distribution of Dolichos biflorus Lectin as Measured by Radioimmunoassay. Plant Physiol. 1978 May;61(5):847–850. doi: 10.1104/pp.61.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. H., Liener I. E. The use of glutaraldehyde-treated erythrocytes for assaying the agglutinating activity of lectins. Anal Biochem. 1975 Oct;68(2):651–653. doi: 10.1016/0003-2697(75)90663-6. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. 1,4-Butanediol diglycidyl ether coupling of carbohydrates to Sepharose: affinity adsorbents for lectins and glycosidases. Anal Biochem. 1977 Jul;81(1):98–107. doi: 10.1016/0003-2697(77)90602-9. [DOI] [PubMed] [Google Scholar]




