The effect of ω-3 fatty acid (ω-3FA) supplementation on triglyceride levels was assessed in a retrospective study of patients taking isotretinoin for acne. Oral isotretinoin (13-cis-retinoic acid) is commonly used in the treatment of severe and recalcitrant acne.1–3 This treatment commonly results in complete clearance of acne with long-term remission; however, treatment is associated with a number of adverse effects, including hypertriglyceridemia.4 At high levels of isotretinoin, hypertriglyceridemia may lead to acute pancreatitis.2,3 As many as 44% of patients with baseline triglyceride levels within the reference range who are treated with isotretinoin develop hypertriglyceridemia.4 Elevations in triglyceride levels during isotretinoin treatment can force dosage reductions or discontinuation of treatment. For less marked elevations, dietary modification or therapy to lower lipid levels may be used.
Methods
Patients were recruited from the dermatology clinic at the University of California, Los Angeles, and all study activities were approved by the university’s institutional review board. All the study participants provided written informed consent. Longitudinal survey data were obtained for 39 patients with acne who were treated with isotretinoin for a median of 5.87 months, and triglyceride levels during therapy were reviewed retrospectively. Nineteen patients reported consistent voluntary intake and 20 patients reported no use of ω-3FA supplements. Patients were grouped as follows: triglyceride levels within the reference range at baseline and throughout treatment; triglyceride levels within the reference range at baseline and elevated during treatment; or elevated triglyceride levels at baseline and during treatment. The mean percentage change in triglyceride levels for patients with measurements within the reference range at baseline and during treatment, triglyceride levels within the reference range at baseline and elevated during treatment, and triglyceride levels elevated at baseline and during treatment stratified by use of ω-3FA supplements are presented in the Figure.
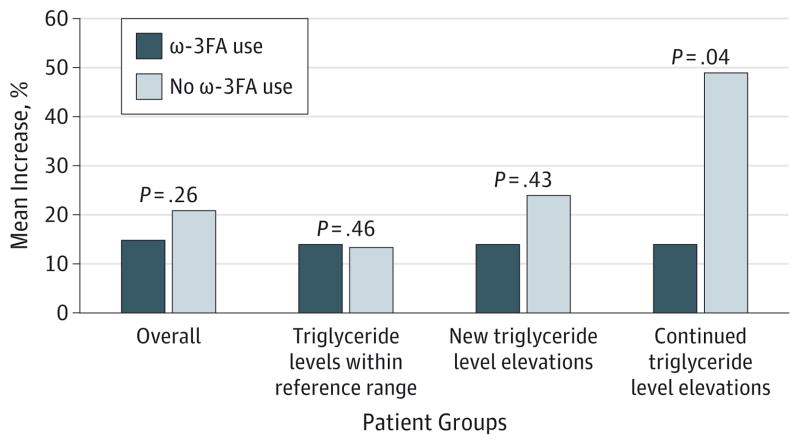
Figure. Mean Increase in Triglyceride Levels in Patients Using Isotretinoin With and Without ω-3 Fatty Acid (ω-3FA) Supplements.
Patients with elevated baseline triglyceride levels who do not take ω-3FA supplements and who start isotretinoin therapy experience a significant increase in triglyceride levels compared with patients receiving isotretinoin therapy and concurrent ω-3FA supplementation.
Results
Comparison of the mean percentage increase in triglyceride levels in patients with and without ω-3FA supplement use and with preexisting elevated triglyceride levels demonstrated that patients not using the supplements had a greater increase in triglyceride levels during treatment from baseline compared with patients using the supplements. In this group, patients who did not use ω-3FA supplements experienced a mean increase of 49% in triglyceride levels during treatment, whereas patients using ω-3FA supplements only experienced a mean increase of 13.91% (P = .04). Thus, ω-3FA supplements stabilized the expected increase in triglyceride levels during isotretinoin therapy in patients with preexisting hypertriglyceridemia. This finding could be clinically significant. Adjunctive treatment with ω-3FA could permit maximal therapeutic dosing of isotretinoin while avoiding the necessity of using an agent to lower lipid levels.
Discussion
Supplements of ω-3FA may be a useful adjunct to the management of lipid levels during isotretinoin therapy. A future prospective, randomized placebo-controlled trial using a standard dose and formulation of ω-3FA is required to confirm this hypothesis.
Footnotes
Conflict of Interest Disclosures: Dr J. Kim is an investigator for and receives research grants from Allergan and Leo Pharma and is a consultant for and receives honoraria from Anacor, Galderma, Herbalife, and Medicis. No other disclosures were reported.
Author Contributions: Drs Krishna and C. Kim had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: J. Kim, C. Kim.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Krishna, Okhovat, J. Kim.
Critical revision of the manuscript for important intellectual content: Okhovat, J. Kim, C. Kim.
Statistical analysis: Krishna.
Administrative, technical, or material support: C. Kim.
Study supervision: Okhovat, C. Kim.
References
- 1.Ellis CN, Krach KJ. Uses and complications of isotretinoin therapy. J Am Acad Dermatol. 2001;45(5):S150–S157. doi: 10.1067/mjd.2001.113717. [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith LA, Bolognia JL, Callen JP, et al. American Academy of Dermatology. American Academy of Dermatology Consensus Conference on the safe and optimal use of isotretinoin: summary and recommendations [published erratum appears in J Am Acad Dermatol. 2004;51(3):348] J Am Acad Dermatol. 2004;50(6):900–906. doi: 10.1016/j.jaad.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Strauss JS, Krowchuk DP, Leyden JJ, et al. American Academy of Dermatology/American Academy of Dermatology Association. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663. doi: 10.1016/j.jaad.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016–1022. doi: 10.1001/archderm.142.8.1016. [DOI] [PubMed] [Google Scholar]