Abstract
Purpose
Idiopathic generalized epilepsies (IGE) comprise a group of clinical syndromes associated with spike wave discharges, putatively linked to alterations in neurotransmission. The purpose of this study was to investigate whether patients with IGE have altered glutamine and γ-aminobutyric acid (GABA) levels indicative of altered excitatory and inhibitory neurotransmission in frontal regions.
Materials and Methods
Single-voxel MEGA-edited PRESS magnetic resonance imaging (MRI) spectra were acquired from a 30-mL voxel in the dorsolateral prefrontal cortex in 13 patients with IGE (8 female) and 16 controls (9 female) at 3T. Metabolite concentrations were derived using LCModel. Differences between groups were investigated using an unpaired t-test.
Results
Patients with IGE were found to have significantly higher glutamine than controls (P = 0.02). GABA levels were also elevated in patients with IGE (P = 0.03).
Conclusion
Patients with IGE have increased frontal glutamine and GABA compared with controls. Since glutamine has been suggested to act as a surrogate for metabolically active glutamate, it may represent a marker for excitatory neurotransmission.
Keywords: MR spectroscopy, GABA, glutamine, idiopathic generalized epilepsy
IDIOPATHIC GENERALIZED EPILEPSIES (IGE) comprise a group of clinical syndromes, which account for 15–20% of all epilepsies (1), and are characterized by generalized spike and wave activity on electroencephalography (EEG). Connections between the cortex and thalamus are thought to be important in the generation of spike-wave discharges (2). Cortico-thalamic and thalamo-cortical projections are excitatory, using glutamate (Glu) as the major neurotransmitter; these connections are modulated by inhibitory interneurons, using γ-aminobutyric acid (GABA) as the major inhibitory neurotransmitter (3).
Previous magnetic resonance spectroscopy (MRS) studies in IGE have reported increased Glx, which represents a combined measure of glutamate (Glu) and glutamine (Gln), in frontal and thalamic regions (4,5), with conflicting reports of both increased and reduced GABA (6,7). Using conventional MRS, Glu and Gln have overlapping peaks that are difficult to separate, and are therefore typically reported as a combined signal of Glx (8). Glutamate is released into the synaptic cleft from neurons and binds to postsynaptic receptors such as AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) and NMDA (N-methyl-D-aspartate) (9). Because of its role in synaptic plasticity, glutamate is involved in cognitive functions like learning and memory in the brain. Its action is terminated by glial uptake. In glial cells it is converted into glutamine, which is then transferred back across the synaptic cleft into the neuron (10). Since there are significant amounts of intracellular glutamate which do not take part in synaptic transmission, it has been suggested that glutamine is a useful surrogate marker for synaptically active glutamate (11). There are more limited data about GABA from MRS studies. Quantification of GABA with MRS is challenging due to its low cerebral concentration and overlap in the spectrum by more intense signals from Glx, N-acetylaspartate (NAA), and creatine (Cr), but recent advances in GABA-editing sequences such as MEGAPRESS have enabled the quantification of GABA concentrations in vivo (12–14). The MEGAPRESS acquisition also allows for detection of glutamate and glutamine with good sensitivity (15). MEGAPRESS may therefore be a useful method to investigate differences in glutamine and GABA in IGE.
The purpose of the current study was to investigate whether subjects with IGE show evidence of cortical hyperexcitability as reflected by increased glutamine and/or altered GABA concentrations, using MEGAPRESS MR spectroscopy.
MATERIALS AND METHODS
Ethical Approval and Participant Consent
This study was approved by the King’s College Hospital Research Ethics Committee (Ethics Ref 08/H0808/157). All participants gave written informed consent.
Subjects
Thirteen participants with IGE (one with juvenile myoclonic epilepsy, two with juvenile absence epilepsy [JAE], one with childhood absence epilepsy, seven with general tonic clonic seizures only (GTCS), one with absences with eyelid myoclonia) were recruited from five hospitals in London and nearby regions. The epilepsy syndrome was determined by an epileptologist with 14 years of specialist experience (M.P.R.) on the basis of clinical history, imaging, and EEG. Inclusion criteria for patients were age ≥18 years old and a diagnosis of IGE. Further clinical details of patients are presented in Table 1. Sixteen healthy participants with no personal or family history of neurological or psychiatric diseases were recruited via a local research volunteer database. Participants were excluded if they had any other neuropsychiatric condition or a full scale IQ (FSIQ) <70. All MRS studies were performed in the morning in order to control for potential diurnal variations.
Table 1.
Clinical Details of Patients
| Gender | Age | Syndrome | Age of onset (yrs) |
Seizures and frequency |
Time since last seizure |
Medications (daily dose mg) |
EEG | MRI |
|---|---|---|---|---|---|---|---|---|
| M | 26 | GTCS | 5 | GTCS 1/month | 2 weeks | Val 1600 Top 200 Lam 100 |
GSW | Normal |
| M | 25 | GTCS | 11 | GTCS 3/month | 3 weeks | Val 300 | GSW | Normal |
| F | 45 | GTCS | 2 | SF | 36 years | (none) | Normal | N/A |
| F | 29 | JAE | 15 | GTCS 2/year Abs 1/month |
1 month | Lam 450 Top 100 |
GSW | Normal |
| F | 36 | GTCS | 21 | GTCS 2/year | 2 months | Lev 1750 | GSW, Ph+ | Normal |
| F | 43 | CAE | 7 | GTCS ×1 Abs (Childhood) |
10 years | (none) | GSW | N/A |
| M | 49 | GTCS | 26 | SF | 1 year | (none) | GSW | Normal |
| F | 21 | JAE | 10 | SF | 4 years | Lam 400 Ethosux 500 |
GSW | Normal |
| F | 20 | JME | 13 | MJ weekly GTCS SF |
1 week | Val 1000 | GSW | Normal |
| M | 30 | Unclass | 11 | SF | 3 years | (none) | Normal | N/A |
| F | 39 | GTCS | 22 | SF | 10 years | Cbz 200 | GSW | Normal |
| F | 21 | Abs with eyelid myoclonia | 6 | Abs daily MJ weekly |
1 day | Lam 500 | PSW | N/A |
| M | 28 | GTCS | 8 | GTCS SF | 1 year | Val 400 | GSW | N/A |
M male, F female, GTCS generalized tonic clonic seizures, JAE juvenile absence epilepsy, CAE childhood absence epilepsy, JME juvenile myoclonic epilepsy, Unclass unclassified, Abs absences, MJ myoclonic jerks, Val valproate, Top topiramate, Lam lamotrigine, Lev levetiracetam, Ethosux ethosuximide, Cbz carbamazepine, EEG electroencephalogram, GSW generalised spike wave, Phþ photosensitive, PSW polyspike wave, MRI magnetic resonance imaging.
MR Data Acquisition
MRI and spectroscopy was performed on a 3T GE HDx system (General Electric, Milwaukee, WI). For each subject, a 3D inversion recovery prepared spoiled gradient-recalled echo (SPGR) scan was obtained in the axial plane (voxel size = 1.1 mm3, field of view = 28 cm2, echo time (TE) = 2.8 msec, repetition time (TR) = 7 msec, inversion time = 450 msec, excitation flip angle = 20°), and was used for localization of the spectroscopy voxels. The SPGR images were also segmented into gray matter, white matter, and cerebrospinal fluid (CSF) maps using statistical parametric mapping (SPM5, Wellcome Dept. of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm) to allow correction for partial volume effects due to CSF in the MRS voxel.
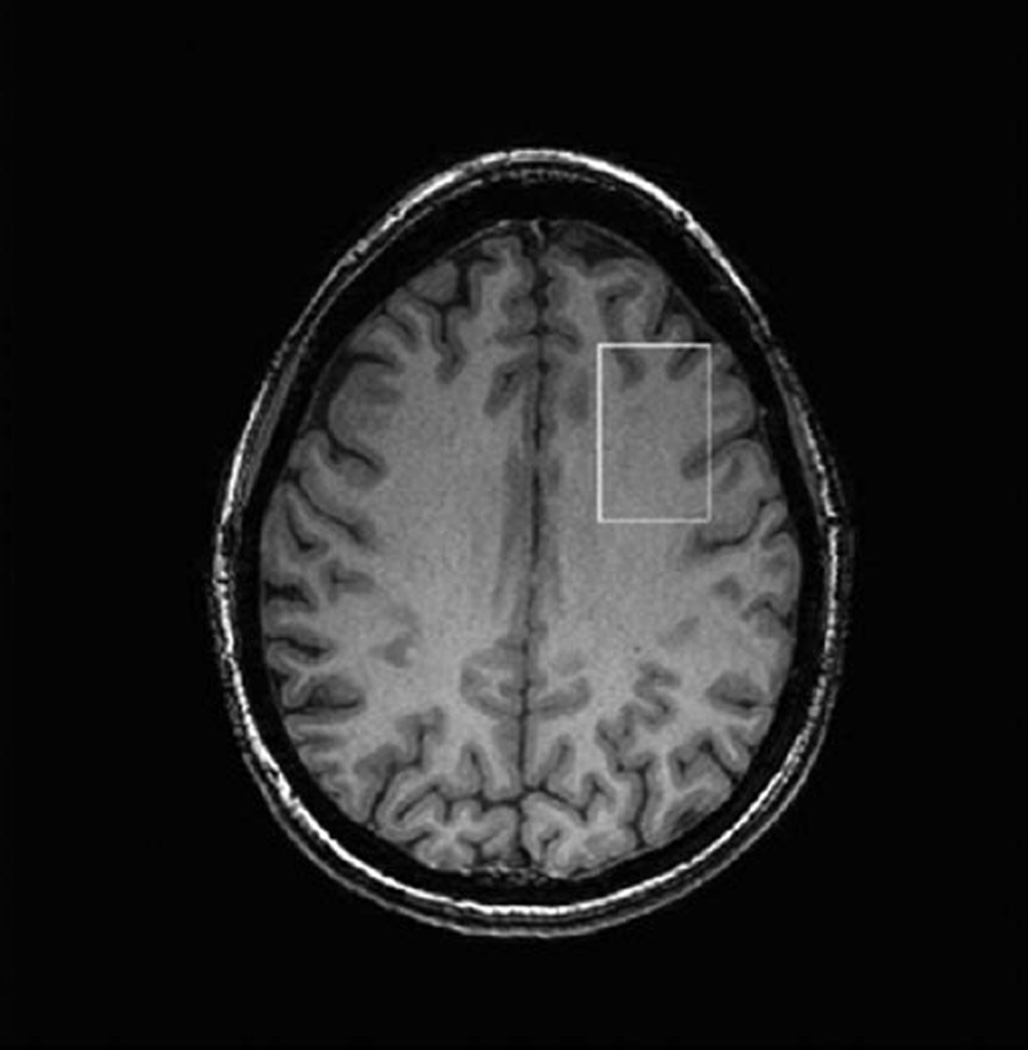
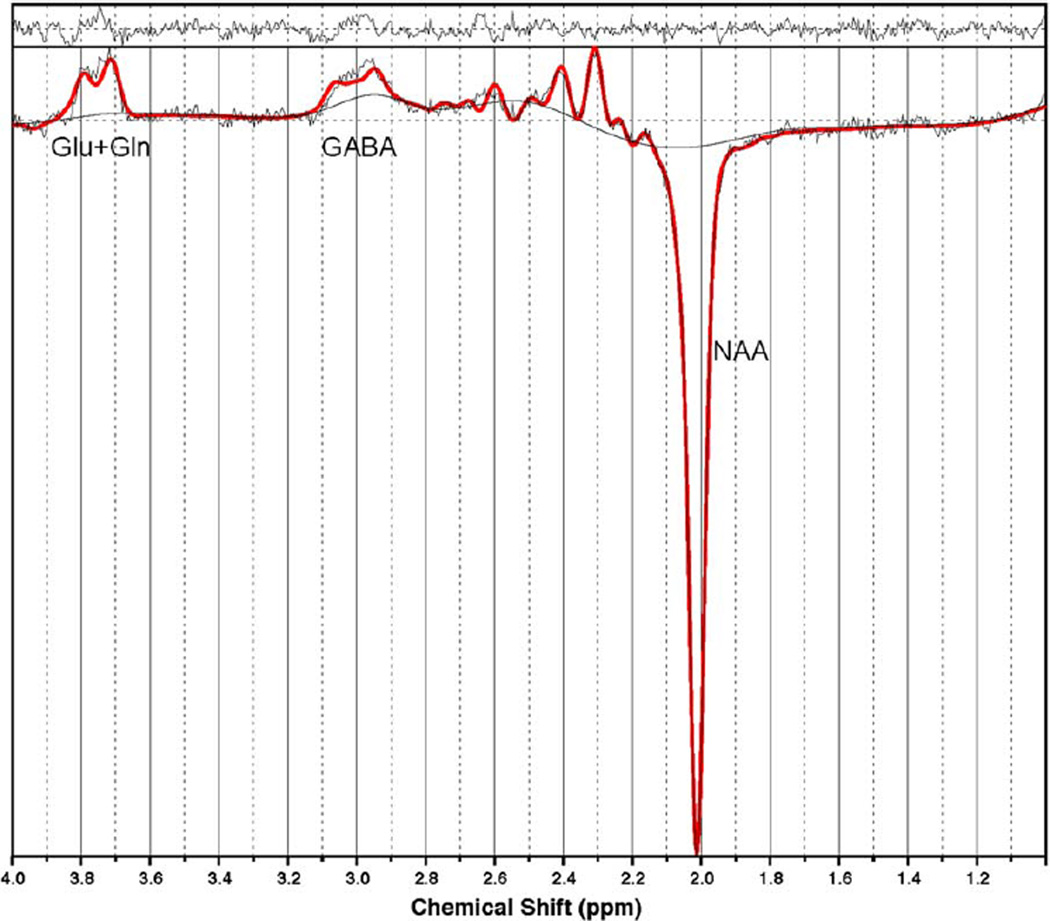
Single-voxel edited 1H MR spectra were acquired from a 25 × 40 × 30 mm voxel of interest positioned in the left dorsolateral prefrontal cortex (DLPFC) using the MEGAPRESS method (16). The DLPFC was selected as the target voxel on the basis of previous reports of elevated glutamate and glutamine (Glx) levels in the frontal lobe of patients with IGE (4). To achieve a consistent MRS voxel position between subjects, the voxel was prescribed, using a carefully defined protocol, on an imaging slice 1.5 mm above the superior margin of the lateral ventricles. The length of the midline of the brain was measured on this slice and the center of the voxel in the AC/PC direction was defined as one-third of this length from the anterior margin of the brain; the voxel was positioned in the left hemisphere, with its center in the left/right direction defined as half the distance between the midline and the left lateral border of the brain, on a line perpendicular to the midline (14). Figure 1 shows a screenshot of the DLPFC voxel. A total of 320 spectral averages were acquired for each spectrum with a TR of 1800 msec, an echo time of 68 msec, and an eight-step phase cycle (ie, with each of the three PRESS pulses independently cycled between 0 and 180°) resulting in an acquisition time of ~10 minutes. MEGA-editing was achieved with 16-msec Gaussian editing pulses applied at 1.9 ppm and 7.5 ppm in alternate spectral lines. For each metabolite spectrum, 16 water reference lines were also acquired as part of the standard PROBE acquisition. Figure 2 shows a screenshot of a representative spectrum.
Figure 1.
Screenshot from MRI scanner showing placement of the MEGAPRESS DLPFC voxel from a control subject selected at the midpoint of the voxel in craniocaudal direction.
Figure 2.
Screenshot from LCModel showing example of the spectra acquired from a control subject. Glu, glutamate; Gln, glutamine; GABA γ-aminobutyric acid; NAA, N-acetylaspartate.
MRS Data Analysis
The spectra were coil combined with weighting factors derived from the first point of the free induction decay signal from the nonwater-suppressed acquisitions from each coil. Water-scaled metabolite concentrations were derived from the edited spectra with LCModel v. 6.3-1 (17) using a simulated basis set including basis spectra for GABA, Gln, Glu, N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), and glutathione (GSH, which also has multiplets at 3 ppm).
For GABA quantification, the control parameter sptype = ‘mega-press-2’ was used for the LCModel analysis (see Section 3.1 of the LCModel manual for more details). For glutamine, quantification was performed using the default settings in LCModel to avoid underestimation of Gln. Factors affecting signal intensity in MEGAPRESS such as J-coupling effects and T2 losses mean that absolute values may have an additional (unknown) scaling factor and should therefore be regarded as institutional units, rather than absolute values (11).
Poorly fitted metabolite peaks (Cramer-Rao minimum variance bounds of more than 30% as reported by LCModel) were excluded from further analysis, resulting in the exclusion of one control subject from the statistical analysis. Metabolite concentrations were corrected for the voxel CSF content, since CSF is considered to provide negligible contribution to the Gln and GABA signals. After segmentation of the SPGR images in SPM (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London) metabolite values were corrected for the presence of CSF in the voxel using the formula Mcorr = M/(WM+GM), where M is the uncorrected metabolite concentration, Mcorr is the corrected concentration, and WM and GM are the white and gray matter fractions (ranging between 0 and 1) within the spectroscopy voxel. Since the metabolite measures were derived with water-scaling, a further correction was applied to correct the estimated water concentration of the voxel for partial volume CSF contamination, assuming a CSF water concentration of 55,556 mmol/L and the LCModel default brain water concentration of 35,880 mmol/L. These two correction factors were combined into an overall correction factor according to:
| [1] |
where the first term represents the water-scaling correction factor and the second term represents the partial volume correction factor. This equation reduces to:
| [2] |
Statistical analysis was performed with SPSS v. 14.0. Group differences in the LCModel-derived metabolite concentrations were tested using a one-tailed unpaired t-test for glutamine (directional hypothesis that glutamine would be increased) and two-tailed unpaired test for GABA. To correct for multiple comparisons (GABA and glutamine), False Discovery Rate correction using the Benjamini and Hochberg method was applied (18). Post-hoc exploratory analyses were carried out: to investigate the relationship between duration of epilepsy, dose of valproate, and metabolite concentrations (Pearson’s correlation); to compare each IGE syndrome with controls; and to compare seizure-free patients with those with ongoing seizures (two-tailed unpaired t-test).
RESULTS
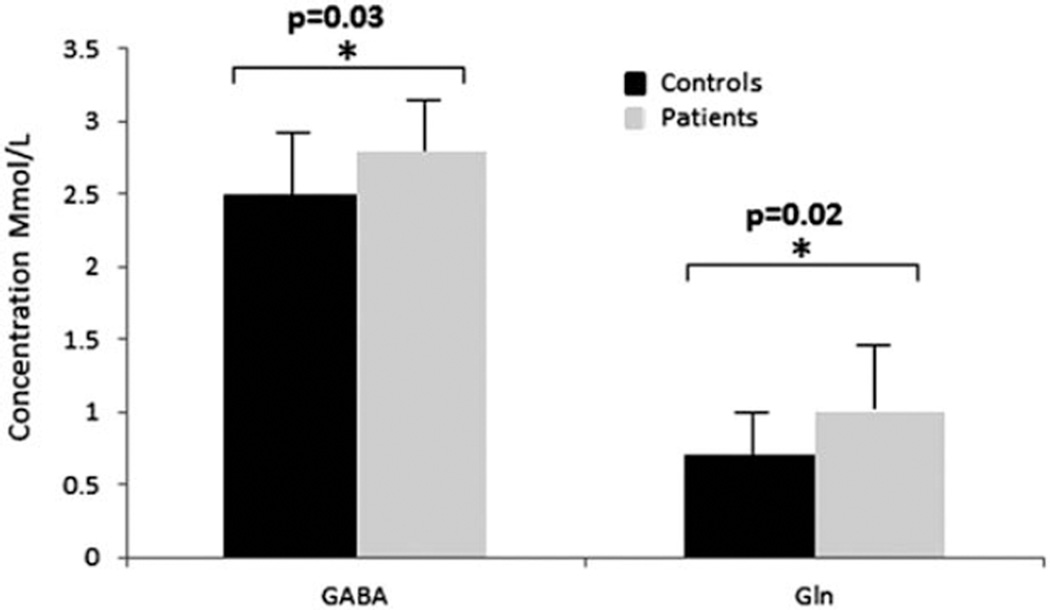
Patients with IGE had significantly higher glutamine concentrations than controls (P = 0.02). GABA levels were also elevated in patients with IGE (P = 0.03), shown in Figure 3. The observed increase in Gln and GABA remained significant after Benjamini and Hochberg correction for multiple comparisons. A post-hoc power calculation for the group difference in GABA and Gln between the IGE and control groups reveals a power of 0.87 for GABA and 0.78 for Gln. For gray matter, white matter and CSF fractions, please refer to Table 2.
Figure 3.
Bar plots showing metabolite values for GABA and glutamine (Gln) for controls (shown in black) and patients (shown in gray). Control values are shown in black and patients in gray. Error bars show standard deviations and significant differences are highlighted with stars.
Table 2.
GABA and Glutamine Concentrations and Gray Matter, White Matter, and CSF Fractions for Each Group
| Control mean ± SD |
IGE Patients mean ± SD |
P value | |
|---|---|---|---|
| GABA (Mmol/l) | 2.5 ± 0.4 | 2.8 ± 0.3 | 0.03* |
| Gln (Mmol/l) | 0.70 ± 0.3 | 1.0 ± 0.6 | 0.02* |
| Gray matter proportion | 0.3 ± 0.05 | 0.3 ± 0.3 | |
| White matter proportion | 0.70 ± 0.07 | 0.70 ± 0.05 | |
| CSF volume proportion | 0.04 ± 0.02 | 0.06 ± 0.02 |
Relationship to Duration of Epilepsy
There was no significant correlation between duration of epilepsy and glutamine or GABA concentrations in DLPFC, although a trend-level correlation between DLPFC GABA and the duration of epilepsy was observed (Pearson’s R = 0.479, P = 0.097).
Relationship to Antiepileptic Therapy
There was no significant correlation between valproate dose and glutamine or GABA concentrations in DLPFC.
Relationship to IGE Subtype
When individual epilepsy subsyndromes were compared with controls for Gln and GABA in DLPFC, juvenile myoclonic epilepsy (JME) patients showed significantly elevated Gln (P = 0.003), while GTCS patients showed elevated GABA (P = 0.047) and a trend toward elevated Gln (P = 0.055).
Relationship to Seizure Freedom
There was no difference in Gln or GABA in DLPFC between patients who had been seizure-free for >1 year and those who had ongoing seizures.
DISCUSSION
The main findings of this study are that patients with IGE showed significantly increased glutamine and GABA in the DLPFC compared with controls. In the subsyndrome analysis, JME and GTCS were associated with elevated Gln, while the elevated GABA was associated with GTCS. Although this study was not specifically designed for subsyndrome analysis, this suggests that there may be differential alteration of neurotransmitter levels in different IGE subsyndromes, which should be investigated further in larger studies. No significant associations were observed between Gln, GABA, and valproate dose, the duration of epilepsy, or seizure freedom.
The higher glutamine levels seen in the IGE patients in this sample are consistent with reports from previous studies of increased glutamate concentrations in patients with IGE (4), although direct comparisons to previous work are difficult because of heterogeneity in the IGE subsyndromes studied and methodological differences in terms of the positioning of the voxel or MRS acquisition technique (PRESS/STEAM/CSI) (4,5). The Gln values obtained in the study are comparable to those reported in one other study (11), although slightly lower than reported in another study (19). However, in the context of reports of elevated glutamate or Glx concentrations in previous IGE spectroscopy studies, increased cerebral concentrations of Gln or Glu appears to represent a stable and robust marker for IGE.
The increase in GABA in IGE patients is consistent with results of elevated GABA reported in previous studies of IGE patients (4). This apparent increase in GABA may result from compensatory mechanisms. Alternatively, the elevated GABA levels may result from medication effects, since GABA is increased by a number of medications including levetiracetam (20) topiramate (21), gabapentin (22), and vigabatrin (23). In a study using a standard PRESS acquisition technique, sodium valproate has been reported to reduce myoinositol but have no effect on other metabolites (24); this previous study did not, however, examine GABA concentrations. Other drugs are mechanistically thought to affect GABA receptors (phenobarbital, benzodiazepine, felbamate, topiramate, levetiracetam) or GABA turnover (sodium valproate, vigabatrin, gabapentin, tiagabine) (25). Future studies with larger groups, examining specific medications, and using a GABA-edited MEGAPRESS protocol with reduced macromolecular contamination (26–29), may be able to further clarify the spectroscopic differences in GABA and the impact of various treatments.
In addition, while Gln has been proposed as a surrogate marker for metabolically active glutamate, using standard PRESS MRS methods the quantification of glutamine at 3T is challenging due to its low cerebral concentration and overlap with the closely coupled glutamate peaks. The Gln values measured in the present study may therefore include contributions from Glu, but future studies using alternative MRS acquisition sequences like 2D JPRESS or CPRESS may be able to elucidate the specificity of these elevated Gln levels. The low concentration and low spectral signal-to-noise ratio (SNR) of Gln also results in the frequent exclusion of Gln values from PRESS MRS analyses due to unreliable spectral fitting, reflected in high Cramer-Rao lower bounds (CRLB) reported by LCModel. Given the inverse relationship between the metabolite concentration and the CRLB, one potential bias inherent in using a CRLB threshold for low-concentration metabolites like Gln is that the CRLB threshold may result in the systematic exclusion of the lowest values for one group, potentially confounding the assessment of group differences. In the current study, the MEGAPRESS Gln levels quantified with LCModel were within the relatively liberal CRLB cutoff of 30% for all MEGAPRESS subtraction spectra apart from that from one control case, so the CRLB threshold is unlikely to bias the group comparison of Gln between IGE patients and control participants. In contrast, Gln was not detected reliably (CRLB >30%) in over 50% of spectra processed using just the edit OFF (PRESS TE = 68) lines. This is surprising, given that the PRESS TE68 spectrum closely resembles the TE80 spectrum reported to give high reliability for Gln measurements at 3T (11). Therefore, in our sample MEGAPRESS seems to provide improved sensitivity and reliability for Gln fitting relative to standard PRESS, possibly due to the reduced macromolecular overlap in the subtraction spectrum. As mentioned previously, factors affecting the MEGAPRESS signal intensity such as J-coupling effects and T2 signal decay mean that absolute values may have an additional (unknown) scaling factor. However, since these should be constant between the groups, the observed group differences are likely to be biologically meaningful. MEGAPRESS therefore appears to represent a promising method for measuring glutamine.
In conclusion, the data presented here support the hypothesis that patients with IGE have increased frontal glutamine and GABA compared with controls. This is consistent with previous studies that report increased Glx (Gln + Glu) and GABA in similar groups of patients. However, since Gln has been suggested to act as a surrogate for metabolically active glutamate, it may represent a more sensitive measure for excitatory neurotransmission than Glx. The successful spectral fitting of Gln resonances in the present study also highlights the promise of the MEGAPRESS method for the in vivo quantification of glutamine. Better quantification of these metabolites could help us understand better the pathophysiology of epilepsy including differences between the different IGE syndromes, and lead to the development of more targeted drug treatments.
Acknowledgments
POTENTIAL CONFLICTS OF INTEREST
FC acknowledges salary support from the Medical Research Council, UK. RLO acknowledges research grant support from the University Research Priority Program “Integrative Human Physiology” at the University of Zurich. LN, RDE, received no funding directly associated with this work. RAEE acknowledges salary support from NIH grant P41 EB015909. GJB received honoraria for teaching from General Electric during the course of this work, and acts as a consultant for IXICO. MPR acknowledges salary support from the Getty Family Foundation, the Medical Research Council UK, and the National Institute for Health Research (NIHR) Biomedical Research Centre in Mental Health at the South London and Maudsley NHS Foundation Trust.
REFERENCES
- 1.Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):10–14. doi: 10.1111/j.1528-1167.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 2.Badawy RA, Harvey AS, Macdonell RA. Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy. Part 1. J Clin Neurosci. 2009;16:355–365. doi: 10.1016/j.jocn.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 4.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MRS reveals frontal lobe metabolite abnormalities in idiopathic generalized epilepsy. Neurology. 2003;61:897–902. doi: 10.1212/01.wnl.0000086903.69738.dc. [DOI] [PubMed] [Google Scholar]
- 5.Helms G, Ciumas C, Kyaga S, Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petroff OA, Hyder F, Rothman DL, Mattson RH. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology. 2001;56:709–715. doi: 10.1212/wnl.56.6.709. [DOI] [PubMed] [Google Scholar]
- 7.Simister RJ, McLean MA, Barker GJ, Duncan JS. A proton magnetic resonance spectroscopy study of metabolites in the occipital lobes in epilepsy. Epilepsia. 2003;44:550–558. doi: 10.1046/j.1528-1157.2003.19102.x. [DOI] [PubMed] [Google Scholar]
- 8.Novotny EJ, Fulbright RK, Pearl PL, Gibson M, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54:S25–S31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- 9.Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 11.Hancu I, Port J. The case of the missing glutamine. NMR Biomed. 2011;24:529–535. doi: 10.1002/nbm.1620. [DOI] [PubMed] [Google Scholar]
- 12.Puts NA, Edden RA. In-vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ. Cardiff Symposium on MRS of GABA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2012;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 2011;33:1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. J Magn Reson. 2011;208:210–213. doi: 10.1016/j.jmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in-vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Provencher SW. Estimation of metabolite concentrations from localized in-vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 19.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Doelken MT, Hammen T, Bogner W, et al. Alterations of intracerebral gamma-aminobutyric acid (GABA) levels by titration with levetiracetam in patients with focal epilepsies. Epilepsia. 2010;51:1477–1482. doi: 10.1111/j.1528-1167.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuzniecky R, Hetherington H, Ho S, et al. Topiramate increases cerebral GABA in healthy humans. Neurology. 1998;51:627–629. doi: 10.1212/wnl.51.2.627. [DOI] [PubMed] [Google Scholar]
- 22.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41:675–680. doi: 10.1111/j.1528-1157.2000.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 23.Mueller SG, Weber OM, Duc CO, et al. Effects of vigabatrin on brain GABA+/CR signals in patients with epilepsy monitored by 1H-NMR-spectroscopy: responder characteristics. Epilepsia. 2001;42:29–40. doi: 10.1046/j.1528-1157.2001.077889.x. [DOI] [PubMed] [Google Scholar]
- 24.Simister McLean MA, Barker GJ, Duncan JS. The effect of sodium valproate on proton MRS visible neurochemical concentrations. Epilepsy Res. 2007;74:215–219. doi: 10.1016/j.eplepsyres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Rugg-Gunn FJ, Sander JW, Smalls JE, editors. Epilepsy 2011 from science to society. 13th ed. 2011. Published by ILAE (UK Chapter) [Google Scholar]
- 26.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45:517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in-vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med. 2012;68:657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dydak U, Jiang YM, Long LL, et al. In-vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]