Highlight
Local root abscisic acid (ABA) accumulation depends on the spatial distribution of soil moisture in potato. Implications for ABA signalling under heterogeneous soil drying are discussed.
Key words: Partial root-zone drying, root ABA, root-to-shoot signalling, root water potential, root water uptake, stomatal conductance, water-saving irrigation.
Abstract
Patterns of root abscisic acid (ABA) accumulation ([ABA]root), root water potential (Ψroot), and root water uptake (RWU), and their impact on xylem sap ABA concentration ([X-ABA]) were measured under vertical partial root-zone drying (VPRD, upper compartment dry, lower compartment wet) and horizontal partial root-zone drying (HPRD, two lateral compartments: one dry, the other wet) of potato (Solanum tuberosum L.). When water was withheld from the dry compartment for 0–10 d, RWU and Ψroot were similarly lower in the dry compartment when soil volumetric water content dropped below 0.22cm3 cm–3 for both spatial distributions of soil moisture. However, [ABA]root increased in response to decreasing Ψroot in the dry compartment only for HPRD, resulting in much higher ABA accumulation than in VPRD. The position of the sampled roots (~4cm closer to the surface in the dry compartment of VPRD than in HPRD) might account for this difference, since older (upper) roots may accumulate less ABA in response to decreased Ψroot than younger (deeper) roots. This would explain differences in root ABA accumulation patterns under vertical and horizontal soil moisture gradients reported in the literature. In our experiment, these differences in root ABA accumulation did not influence [X-ABA], since the RWU fraction (and thus ABA export to shoots) from the dry compartment dramatically decreased simultaneously with any increase in [ABA]root. Thus, HPRD might better trigger a long-distance ABA signal than VPRD under conditions allowing simultaneous high [ABA]root and relatively high RWU fraction.
Introduction
Root-sourced abscisic acid (ABA) is considered a long-distance chemical signal that triggers physiological responses enhancing plant water economy (stomatal closure, decrease in leaf growth) in response to soil drying (Tardieu et al., 1996; Zhang and Davies, 1990). When water is withheld, upper soil layers usually dry faster since they are exposed to evaporation and contain the highest root density, and thus soil moisture increases progressively with depth (e.g. Zhang and Davies, 1989; Beff et al., 2013; Puértolas et al., 2014). Moreover, some irrigation techniques, such as partial root-zone drying (PRD), intend to induce lateral soil moisture heterogeneity by applying irrigation only to part of the root system (Stoll et al., 2000). Modelling of ABA signalling under heterogeneous soil moisture is essential to understand plant responses to soil drying under field conditions. This knowledge could inspire more efficient irrigation scheduling to maximize water-use efficiency (Dodd et al., 2008a, b ; Liu et al., 2008).
Studies on ‘one shoot–two root’ grafted plants with only one of the root systems subjected to soil drying have shown that root xylem sap ABA concentration coming from the ‘dry’ root increased compared with the ‘wet’ root, initially increasing xylem sap ABA concentration above the graft union. Later, as water uptake from the dry side decreased, xylem sap ABA concentration ([X-ABA]) subsequently decreased (Dodd et al., 2008a ), similar to observations in some PRD experiments (Khalil and Grace, 1993; Dry et al., 2000). However, the response of [X-ABA] to PRD is not uniform, as some studies report no increase at all in ABA concentration in the xylem sap or in the leaf (Blackman and Davies, 1985; Wakrim et al., 2005; Jensen et al., 2009; Einhorn et al., 2012). Further understanding of the factors causing these different responses to different soil moisture gradients is essential for modelling ABA signalling in response to soil drying.
Although 25–30% of the ABA in xylem sap might come from shoots due to recirculation of basipetally transported ABA in the phloem (Liang et al., 1997), the initial increase in xylem ABA concentration in response to water stress is believed to be triggered mainly by increased root ABA accumulation (Zhang and Davies, 1990; Neales and McLeod, 1991). Therefore, understanding ABA accumulation patterns within the root system is essential to predict long-distance ABA signalling responses to heterogeneous soil drying. Root ABA concentration increases in response to soil drying due to an increase in ABA biosynthesis by roots and ABA recirculation from shoots via phloem transport (Sauter et al., 2001). However, there are few studies on the effect of soil moisture heterogeneity on the spatial distribution of root ABA accumulation. Local soil moisture and root ABA concentration were tightly correlated under PRD in a split-root experiment in sycamore (Khalil and Grace, 1993). When maize was grown in drying soil columns, root ABA concentrations increased in drying soil layers, but continued soil drying later decreased root ABA concentrations (Zhang and Davies, 1989). In contrast, there was no clear relationship between root ABA concentration and local soil moisture in bean growing in drying soil columns (Trejo and Davies, 1991; Puértolas et al., 2013). Understanding the origin of these discrepancies is essential to predict xylem ABA concentration based on differences in local soil moisture.
ABA synthesis in root cells increases as root water potential (Ψroot) decreases (Zhang and Davies, 1987; Simonneau et al., 1998). Therefore, heterogeneity in Ψroot throughout the root system might be the main factor explaining local differences in root ABA accumulation when soil moisture is distributed heterogeneously. Thus, for the same level of soil drying, ABA export from root to shoots could differ according to how soil moisture distribution affects Ψroot within the root zone.
If this hypothesis is confirmed, understanding how Ψroot is distributed across the root zone in response to soil moisture heterogeneity is essential to understand ABA signalling responses to soil drying. Some studies have shown differences in Ψroot across the root zone when soil moisture is heterogeneous (Dodd et al., 2008a; Simonneau and Habib, 1994), but other authors have also reported that, because of this water potential gradient, water can be redistributed within the plant in general and the root system in particular (de Kroon et al., 1996; Stoll et al., 2000). Therefore, the existence and extent of Ψroot heterogeneity across the root zone could be modulated by water redistribution and differences in hydraulic resistance within the root zone. Although water movement within the root system depends on water and osmotic potential gradients (Mao et al., 2009), it is plausible that vertical water recirculation might be more effective than lateral recirculation, as water transport through the vessels via the xylem might be more effective than cell-to-cell pathways (Steudle and Peterson, 1998). This could explain the observed homogeneity of Ψroot under vertical soil moisture gradients (Puértolas et al., 2013), unlike in split-root experiments subjected to lateral gradients (Simonneau and Habib, 1994; Dodd et al., 2008a ). Equilibration of water potential within the root zone required night-time cessation of transpiration (Bauerle et al., 2008). This also suggests the possibility of diurnal fluctuations in water potential heterogeneity, which might influence root ABA accumulation and, in turn, root-to-shoot ABA signalling responses to soil moisture heterogeneity.
This study aimed to determine the relationship of local soil moisture with Ψroot, ABA concentration, and water uptake when soil moisture is heterogeneously distributed, and the influence of these variables on ABA export to the shoot. We assessed the following hypotheses: (i) ABA accumulation is higher in roots growing in drying soil than in wet soil; (ii) differences in Ψroot are associated with differential ABA accumulation within the root system, and are lower under vertical soil moisture gradients than under lateral gradients; and (ii) root water potential and ABA accumulation gradients within the root zone change during the day, with lower heterogeneity during night-time cessation of transpiration. To assess these hypotheses, root ABA concentrations ([ABA]root) and Ψroot were measured in hydraulically isolated soil compartments at progressive degrees of soil drying when soil moisture heterogeneity was imposed laterally or vertically. The experiments used potato, since its ABA signalling has previously been investigated under lateral PRD (Liu et al., 2008), yet there are pronounced vertical gradients in soil moisture under field-grown potato crops even with the application of PRD (Puértolas et al., 2014). Understanding ABA signalling in the field therefore requires knowledge on how vertical and lateral soil moisture gradients affect root ABA accumulation.
Material and methods
Experiment 1: vertical PRD (VPRD) in soil columns
Potato seed tubers (Solanum tuberosum L. cv. ‘Maris Piper’) were sown in pots filled with a fertilized organic loam (John Innes No. 2; J. Arthur Bowers, UK). A moisture release curve for this substrate can be found in Puértolas et al. (2013). Pots were cylinders, 6.5cm in diameter and 23cm in length (0.75cm3 in volume), with stainless steel mesh (0.7mm aperture) at the base to assist drainage, and were designed to fit tightly in a pressure chamber of the same volume (Soil Moisture Equipment Corp., Santa Barbara, CA, USA). A 1.5cm thick quartzite gravel (15–20mm) layer divided the upper from the lower half of the pot, preventing water ascent by capillarity from the lower to the upper part. Before filling, pots were cut in half lengthwise and the two halves were stuck together with duct tape to allow easy extraction of the intact soil column at harvest. Two pairs of 6mm diameter holes (2cm apart) were punched in the wall of the pot, at the midpoint of each pot compartment. Each pair of holes allowed insertion of the two rods of a soil moisture capacitance probe (Model SM-200; Delta-T, Burwell, UK) to estimate root water uptake (RWU) from each soil layer. Plants were placed in a controlled environment room, providing 400–600 μmol m–2 s–1 of irradiance, 14h of photoperiod, and 24/19 °C day/night temperature. Five batches of 11 plants were sown every other day (55 plants in total were measured). All plants in each batch were measured within 2 d.
Plants were harvested 4 weeks after emergence. All plants were watered to field capacity in the morning by completely immersing the pots in water. Plants were randomly assigned to four groups, which were subjected to 0, 1, 2, or 3 d of VPRD. During PRD, only the bottom half of the pot was immersed once a day. Plants with 0 d of VPRD application were fully irrigated until the morning of their measurement date. Each treatment comprised 13–14 plants.
Measurements were taken 4–8h after the lights were switched on in the controlled environment room. Before the measurements, the pot surface and base were covered with duct tape to minimize soil evaporation, weighed on a precision balance to 0.01g (Adventurer Pro AV4102; Ohaus, Thetford, UK) and soil moisture sensors (Model SM200; Delta-T) inserted in each pair of holes (Fig. 1). After 1.5–2h, the sensors were removed, the pot weighed again, and stomatal conductance (g s) was measured in three mature leaflets with a porometer (AP4; Delta-T). Whole-plant water uptake was calculated from the initial and final pot weights. Evaporation was assessed by determining the water loss from a well-watered pot (without a plant) and ignored as negligible (<3% of the water loss of pots containing a plant). Water uptake from each compartment was calculated from soil moisture sensor readings as described by Puértolas et al. (2013) Soil volumetric water content (θ v) in each compartment at the time of measurement was also recorded for each plant. One of the leaves in which stomatal conductance was measured was excised and leaf water potential (Ψleaf) was determined with a pressure chamber. The plant was then de-topped and the pot inserted in a pressure chamber with the stem protruding to determine Ψroot. Since sap flow rate can influence [X-ABA] (Dodd et al., 2008a ), sap was collected at different overpressures above the balance pressure (0.4, 0.6, and 0.8MPa) as described previously (Dodd et al., 2008a ). Sap was collected in pre-weighed Eppendorf vials, frozen in liquid nitrogen immediately after being collected, and stored at –18 °C for ABA determination. Sap flow rate generated at each overpressure was calculated by weighing the vials before freezing them and recording the elapsed collection time. ABA concentration was determined in the sample with the closest sap flow rate to the actual transpirational flow rate (calculated by weighing the pot).
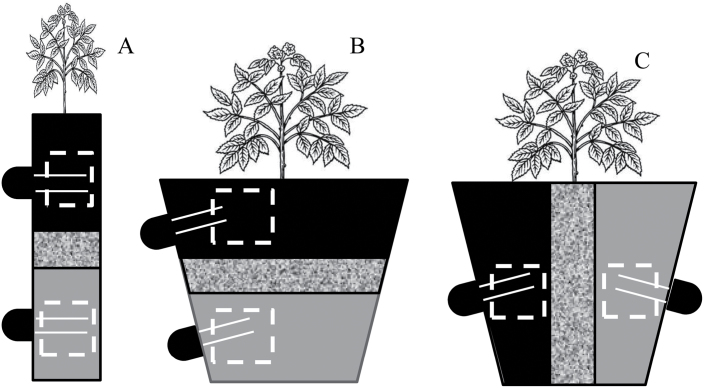
Fig. 1.
Schematic representation of the experimental application of partial root-zone drying in Experiment 1 (A), and Experiment 2 for vertical PRD (B), horizontal PRD (C). Grey areas represent wet compartments and black areas dry compartments. The central grainy stripe represents a gravel layer separating the wet and dry compartments. The locations of SM-200 soil moisture probes (black semi-circles) and the approximate location of root sampling (white dashed square) are also depicted. The dimensions of the two different pots, the sensor rods and the gravel layer are depicted to scale.
Each pot was opened and a root segment (3–6mg in root dry weight) from the interior of each column compartment was excised, briefly (<10 s) washed in tap water to remove adhering soil particles, frozen in liquid nitrogen, and stored at –18 °C for ABA determination. The elapsed time between excision and freezing did not exceed 20 s.
Root samples for ABA determination were freeze dried and finely ground. Deionized water was added at 1:50 weight ratio. Both sap samples and root extracts were analysed by a radioimmunoassay (Quarrie et al., 1988) to obtain root xylem sap ABA concentration ([X-ABA]root) and root ABA concentration ([ABA]root). [X-ABA]root was measured in sap samples, while [ABA]root was measured in the aqueous extract obtained after incubating freeze-dried root tissue samples in a shaker at 4 °C overnight. Xylem sap of S. tuberosum does not present non-specific interference in the assay (Liu et al., 2006). No cross-reaction of the antibody with other compounds in root aqueous extracts was detected, based on a cross-reactivity test (Quarrie et al., 1988). [X-ABA]root decreased as collecting pressure increased (F=30.8, P<0.001).
Roots were extracted, oven dried, and weighed. No differences between the upper and bottom sides in root dry weight were observed (data not shown).
Experiment 2: VPRD vs horizontal PRD(HPRD)
The experiment was repeated twice. On each occasion, 26 potato seed tubers (S. tuberosum L. cv. ‘Horizon’) were sown in 0.25 l pots filled with the same soil used in Experiment 1. Plants were transplanted 5–7 d after emergence into 5 l pots (17.5cm in height, 22.5cm in upper diameter) divided into two compartments with equal capacity by a 3–4cm layer of the same gravel type as in Experiment 1 to disrupt water capillarity between compartments. Half of the pots were divided by a vertical gravel layer (producing two lateral compartments of 1800cm3 capacity; HPRD). The rest of the pots were divided by a horizontal layer (producing two vertical compartments of ~1800cm3 capacity; VPRD) (Fig. 1). In HPRD pots, a 5×5cm acetate window was opened in the pot wall at mid-height on each side to allow root sampling, while in VPRD there was a single 14cm high×5cm wide window, which spanned the two vertical sides of the pot. Additionally, a pair of holes was punched in each compartment to insert SM-200 probes. In HPRD, each pair of holes was placed in the middle of the wall beside each opposite window, while in VPRD each pair of holes was located in the middle of each vertical compartment and close to the window. Windows were covered with the same cut piece of pot wall and fixed with duct tape until sampling.
Plants were grown for 5 weeks after emergence, before measurement under the same environmental conditions described in Experiment 1. They were watered to field capacity after transplanting until PRD was applied. For each plant, PRD was applied for a varying period of time before measurement (from 0 to 9 d) to obtain a wide range of soil moisture content on the dry side. During PRD application, plants were watered once a day. In HPRD, pots were inclined 20° and irrigation (to field capacity) was applied to the lower side of the pot to prevent water running through the layer towards the dry side. After 15min, pots were placed back in the normal position. In VPRD, the bottom half of the pot was immersed in water for 5 s as in Experiment 1.
Half of the plants were measured at the end of the night period (pre-dawn measurements), and the rest between 6 and 8h after the beginning of the photoperiod (midday measurements). Two or three plants were measured per day and time of day during a 7 d period. At midday, water uptake from the two compartments was measured as described in Experiment 1.
At both times of the day, a 2cm root segment was excised from the edge of the pot after partially cutting the acetate window as described by Bauerle et al. (2008). Roots were sampled around the centre of each window in HPRD (at ~8.5cm from the soil surface) and at the midpoint in each vertical compartment in VPRD (at ~4cm from the soil surface and the bottom of the pot for upper and lower compartments, respectively). The root segment was tapped to remove adhering soil particles, briefly blotted with absorbent paper, and immediately placed and sealed inside a psychrometric chamber (C52; Wescor, Logan, UT, USA). From excision to sealing inside the chamber, the elapsed time was less than 10 s. Two samples were taken from each plant, one per compartment. The chamber was placed in a room at stable temperature and humidity conditions. Root water potential (Ψroot) was measured after thermal equilibration by dew point psychrometry with a microvoltmeter (HR33-T; Wescor). Preliminary tests determined that thermal equilibration was attained after at least 6h. Each chamber was calibrated previously with NaCl solutions of known water potential. Another root sample was excised through the opening in the wall and processed as in Experiment 1 to determine [ABA]root.
For midday measurements, stomatal conductance was measured in three fully expanded leaves located in the upper third of the canopy. For pre-dawn and midday measurements, Ψleaf and leaf xylem sap ABA concentration ([X-ABA]leaf) were determined as described for Experiment 1.
θ v and RWU were measured at midday with the soil moisture sensors as described in Experiment 1. For both pre-dawn and midday plants, a sample of soil from each compartment was weighed, dried at 80 °C for 4 d and the soil gravimetric water content (θ g) was determined. A tight linear relationship between θ v and θ g was observed in midday plants [θ g (g g–1)=0.94×θv (cm3 cm–3)+0.02; r 2=0.91; data not shown], which was used to convert θ g into θ v for all plants to allow comparison with previous studies (e.g. Dodd et al., 2008a, b ; Liu et al., 2008; Puértolas et al., 2013).
No differences between wet and dry sides in root dry weight were observed in any of the two PRD distributions (data not shown).
Statistical analyses
No statistical differences in any of the studied variables were found between batches of plants (five batches in Experiment 1, two batches in Experiment 2), so all the plants were pooled in both experiments.
Visual inspection of the relationships between soil moisture and different variables, especially [ABA]root, revealed that altered physiological responses were only observed in plants with θ v <0.22cm3 cm–3 in the dry compartment. Plants then were separated into two groups based on θ v in the dry compartment: dry, θ v<0.22cm3 cm–3; wet, θ v>0.22cm3 cm–3. Those groups were used to assess the effect of soil moisture in the dry compartment on [ABA]root, RWU, and Ψroot by repeated-measures analysis of variance (ANOVA) with PRD side (wet or dry) as the within factor (S) and dry compartment soil moisture (D) as the between factor in Experiment 1. For Experiment 2, S was considered the within factor, and D, measurement time of the day (T), and PRD spatial distribution (P – VPRD or HPRD) were the between factors. [X-ABA], Ψleaf, and gs were assessed by ANOVA with D as the fixed factor in Experiment 1. For Experiment 2, T and P were the fixed factors. Relationships between different variables were assessed by linear regression. All statistical analyses were carried out with SPSS 20 (IBM, Armonk, NY, USA).
Results
Local root responses
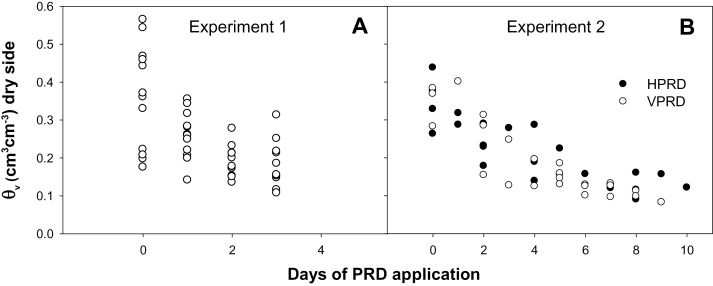
θ v was lower in the dry compartment, and decreased with time of water withholding in both experiments, although it was faster in the small columns of Experiment 1 (Fig. 2). Average soil water content in the dry side decreased with time of PRD application to ~0.10cm3 cm–3 in Experiment 2, but only to 0.19cm3 cm–3 in Experiment 1, although some individuals were close to 0.10cm3 cm–3 (Fig. 2). Daily irrigation of the wet side maintained θ v generally above 0.30cm3 cm–3 (data not shown) for both experiments. In Experiment 2, average (whole-pot) soil moisture (mean of wet and dry sides) was never below 0.16cm3 cm–3. PRD distribution did not influence θ v, as it was similarly lower in the dry side for both HPRD and VPRD.
Fig. 2.
Measurement of θ v in the dry compartment under different lengths of PRD application in Experiment 1 (A) and each PRD distribution (HPRD and VPRD) in Experiment 2 (B). Each point is an individual root system.
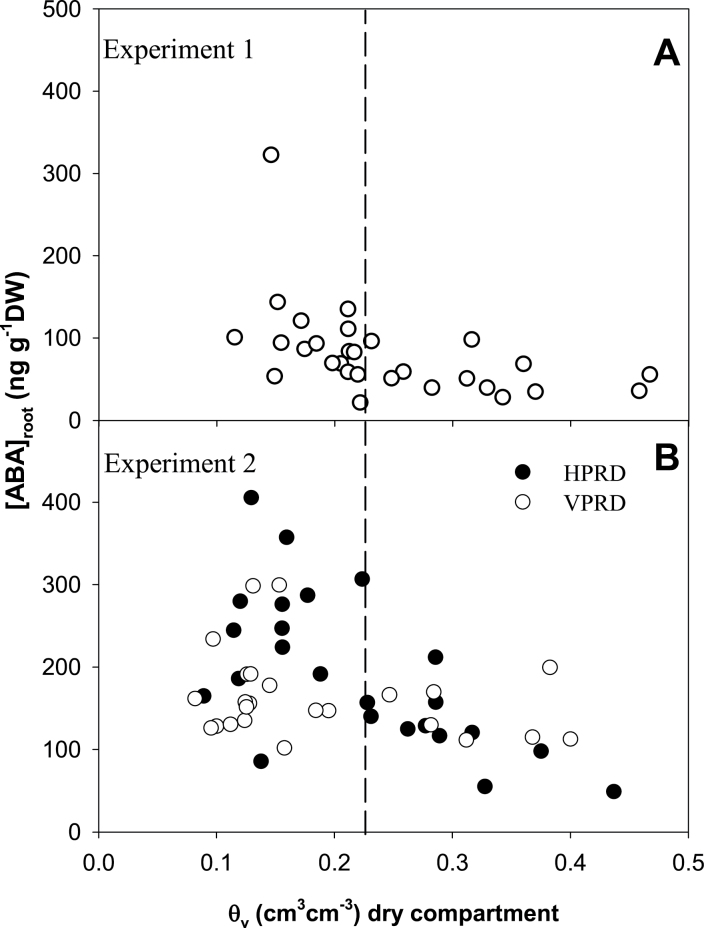
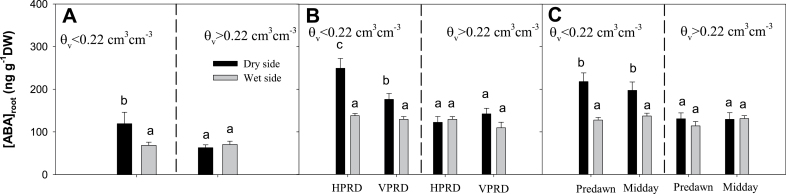
[ABA]root in the dry compartment increased but only when θ v was below 0.22cm3 cm–3 in both experiments (Figs 3 and 4). It did not increase in the wet side regardless of the soil moisture in the dry side (Fig. 4). In Experiment 2, this increase in the dry compartment was higher in HPRD than VPRD, despite statistically similar θ v (Fig. 4, P=0.008 for the compartment×soil moisture level×PRD distribution interaction; Table 1). However, no interaction with time of the day (pre-dawn versus midday) was detected (Fig. 4, P=0.78 for the side×soil moisture level×time of the day interaction).
Fig. 3.
Relationship between θv in the dry compartment and [ABA]root for Experiment 1 (A) and each PRD distribution (HPRD and VPRD) in Experiment 2 (B). Since no differences between pre-dawn and midday were found, data was pooled for Experiment 2. The dashed line is drawn at θ v=0.22cm3 cm–3. Each point is an individual root system.
Fig. 4.
[ABA]root [mean±standard error (SE)] in both compartments in plants with soil volumetric moisture in the dry compartment below (left of the dashed line dividing each panel) and above (right) 0.22cm3 cm–3. (A) Experiment 1. (B) Experiment 2: HPRD versus VPRD (both times of the day pooled). (C) Experiment 2: pre-dawn versus midday measurements (both PRD distributions pooled). Different letters denote statistical differences across compartments and groups within the same panel.
Table 1.
Repeated-measures ANOVA of variables measured in the two compartments of the three different experimentsStatistically significant effects are indicated in bold. NA, not applicable.
| [ABA]root | RWU | Ψroot | |||||
|---|---|---|---|---|---|---|---|
| D.f. | F | P | F | P | F | P | |
| Experiment 1 | |||||||
| Compartment (C) | 1 | 0.4 | 0.56 | 35 | <0.001 | NA | NA |
| Dry compartment moisture (D) | 1 | 2.3 | 0.14 | 17 | <0.001 | NA | NA |
| C×D | 1 | 6.5 | 0.02 | 17 | <0.001 | NA | NA |
| Experiment 2 | |||||||
| Compartment (C) | 1 | 21 | <0.001 | 27 | <0.001 | 12 | <0.001 |
| PRD distribution (P) | 1 | 2.0 | 0.18 | 2.0 | 0.18 | 2.7 | 0.11 |
| Time of the day (T) | 1 | 0.0 | 0.97 | NA | NA | 0.3 | 0.60 |
| Dry compartment moisture (D) | 1 | 13 | <0.001 | 3.1 | 0.09 | 10 | 0.004 |
| C×P | 1 | 0.3 | 0.62 | 0.4 | 0.53 | 0.1 | 0.81 |
| C×T | 1 | 0.9 | 0.36 | NA | NA | 0.5 | 0.50 |
| C×D | 1 | 8.2 | 0.006 | 24 | <0.001 | 13 | <0.001 |
| P×T | 1 | 0.2 | 0.64 | NA | NA | 0.1 | 0.74 |
| P×D | 1 | 5.6 | 0.02 | 3.8 | 0.07 | 0.0 | 0.98 |
| T×D | 1 | 0.1 | 0.77 | NA | NA | 2.6 | 0.12 |
| C×P×T | 1 | 0.1 | 0.78 | NA | NA | 0.0 | 0.94 |
| C×P×D | 1 | 7.6 | 0.008 | 1.5 | 0.24 | 0.0 | 0.94 |
| P×T×D | 1 | 0.4 | 0.55 | NA | NA | 0.1 | 0.80 |
| C×T×D | 1 | 0.1 | 0.79 | NA | NA | 0.3 | 0.58 |
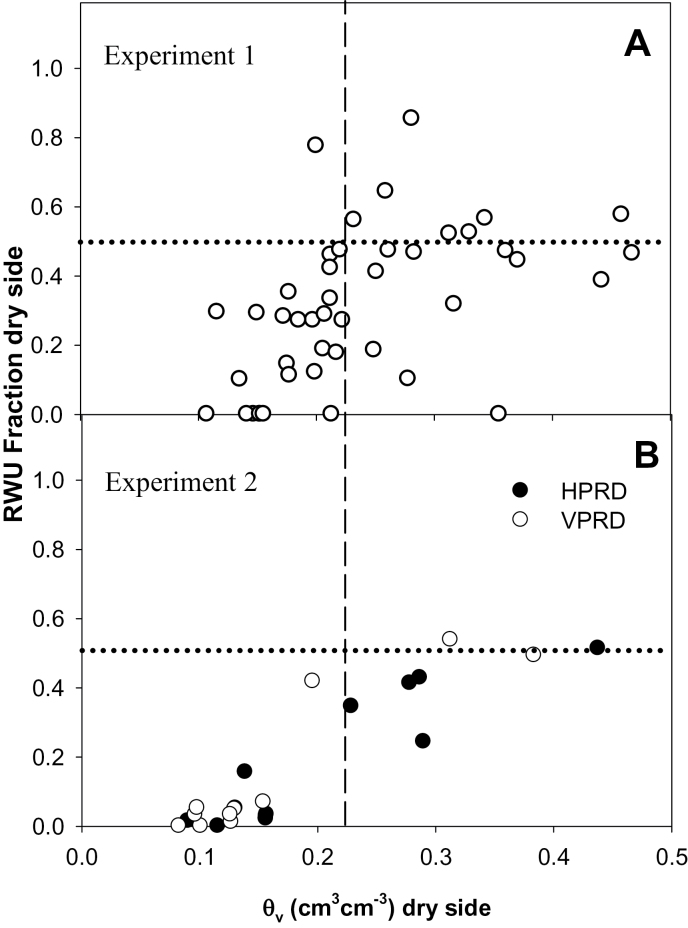
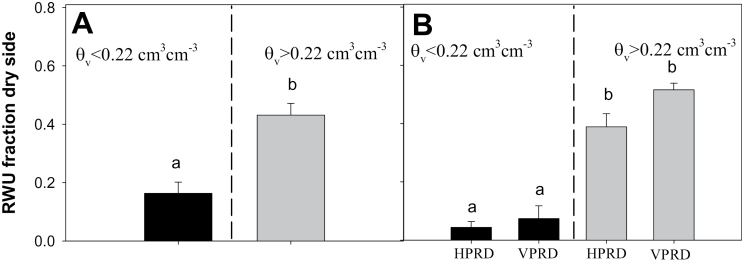
RWU was much higher in the wet compartment (Table 1). The fraction of RWU (RWUF) from the dry compartment to total uptake was positively related to soil moisture in the dry side in both experiments (Fig. 5), but in Experiment 2 it was much lower in plants when the dry compartment had lower soil moisture (θ v <0.22cm3 cm–3; Fig. 6).
Fig. 5.
Relationship between θ v in the dry compartment and RWUF for Experiment 1 (A) and each PRD distribution (HPRD and VPRD) in Experiment 2 (B). The dashed vertical line is drawn at θ v=0.22cm3 cm–3; the dotted horizontal line is at RWUF=0.5. Each point is an individual root system.
Fig. 6.
RWUF in the dry compartment (mean±SE) in plants with soil volumetric moisture in the dry compartment below (left of the dashed line dividing each panel) and above (right) 0.22cm3 cm–3. (A) Experiment 1. (B) Experiment 2: HPRD versus VPRD. Different letters denote statistical differences across compartments and groups within the same panel.
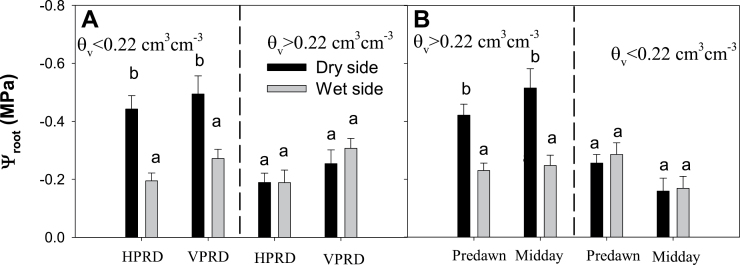
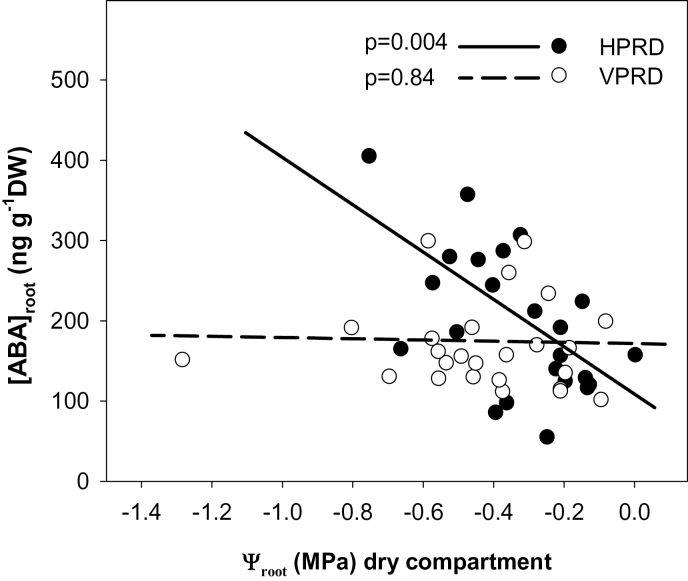
Ψroot in Experiment 2 was significantly lower in the dry side but only when soil moisture decreased below 0.22cm3 cm–3 (Table 1, Fig. 7). No effects of PRD distribution or time of the day were found. [ABA]root in the dry side significantly increased as Ψroot decreased in HPRD but not in VPRD (Fig. 8).
Fig. 7.
Ψroot (mean±SE) in both compartments in plants from Experiment 2 with soil volumetric moisture in the dry compartment below (left of the dashed line dividing each panel) and above (right) 0.22cm3 cm–3. (A) HPRD versus VPRD (times of the day pooled). (b) Pre-dawn versus midday measurements (PRD distributions pooled). Different letters denote statistical differences across compartments and groups within the same panel.
Fig. 8.
Relationship between Ψroot and [ABA]root in Experiment 2 for both PRD distributions. Regression lines and P values are shown for each PRD distribution. Each point is an individual root system.
Whole-plant responses
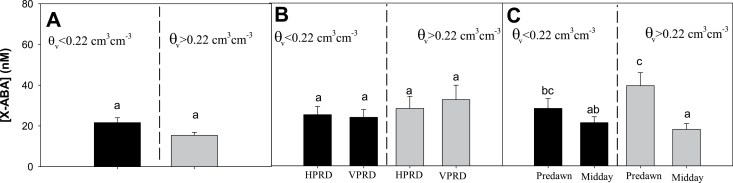
[X-ABA]root did not change with soil drying in Experiment 1 (Fig. 9). In Experiment 2, [X-ABA]leaf was higher at pre-dawn, but VPRD and HPRD distributions had similar effects on [X-ABA]leaf.
Fig. 9.
[X-ABA] (mean±SE) in plants with soil volumetric moisture in the dry compartment below (left of the dashed line dividing each panel) and above (right) 0.22cm3 cm–3. (A) Experiment 1. (B) Experiment 2: HPRD versus VPRD (times of the day pooled). (C) Experiment 2: pre-dawn versus midday measurements (PRD distributions pooled). For Experiment 1, [X-ABA] was determined in root xylem sap, while in Experiment 2 it was in the leaf. Different letters denote statistical differences across compartments and groups within the same panel.
Ψleaf did not change with soil drying in any of the experiments (Table 2). It decreased by ~0.2MPa from pre-dawn to midday in Experiment 2 (Table 2; Ψleaf=–0.21±0.01 and –0.45±0.02MPa for pre-dawn and midday, respectively), but there were no differences between PRD treatments. In Experiment 1, root xylem water potential did not differ significantly with different soil moisture in the dry compartment (F=1.2; P=0.25)
Table 2.
ANOVA of variables measured in the whole plant of the three different experimentsSignificant effects are highlighted in bold. NA, not applicable.
| [X-ABA] | Ψleaf | gs | |||||
|---|---|---|---|---|---|---|---|
| d.f. | F | P | F | P | F | P | |
| Experiment 1 | |||||||
| Dry compartment moisture (D) | 1 | 0.3 | 0.75 | 1.9 | 0.16 | 0.1 | 0.95 |
| Experiment 2 | |||||||
| PRD distribution (P) | 1 | 3.4 | 0.07 | 0.3 | 0.60 | 0.0 | 0.96 |
| Time of the day (T) | 1 | 0.4 | 0.56 | 104 | <0.001 | NA | NA |
| Drying compartment moisture (D) | 1 | 8.2 | 0.006 | 2.6 | 0.11 | 0.0 | 0.94 |
| P×T | 1 | 0.7 | 0.42 | 0.2 | 0.69 | NA | NA |
| P×D | 1 | 0.1 | 0.82 | 0.0 | 0.95 | 0.1 | 0.74 |
| T×D | 1 | 5.4 | 0.03 | 0.3 | 0.61 | NA | NA |
| P×T×D | 1 | 0.5 | 0.48 | 0.0 | 0.89 | NA | NA |
The value of g s did not change across treatments or soil moisture levels in either experiment (Table 2).
Discussion
When compared against conventional deficit irrigation where water is applied homogeneously to the entire root zone, agronomical responses to PRD applied under field conditions are far from uniform (Dodd, 2009). Differences in root hormonal responses to soil moisture heterogeneity may partially cause this inconsistency. Our results indicated that the spatial distribution of soil moisture heterogeneity (whether vertical or horizontal) influences the impact of local soil moisture conditions on root ABA accumulation, which can influence not only root-to-shoot ABA signalling but other plant responses like root growth (Sharp and LeNoble, 2002) or root hydraulic conductivity (Hose et al., 2000). Although the roots sampled under VPRD or HPRD treatments were morphologically indistinguishable (despite being sampled at different depths in the soil profile), there were clear differences in their sensitivity of root ABA accumulation to Ψroot (Fig. 8). Nevertheless, these differences in local root ABA accumulation had minimal impacts on root-to-shoot ABA signalling (Fig. 9), since root ABA accumulation was coincident with limited water uptake (and thus sap flow) from roots in drying soil (Fig. 6).
Root ABA accumulation was higher in the dry part of the root system coincident with lower Ψroot in both experiments and PRD distributions (Fig. 4), consistent with the previously reported dependence of ABA synthesis on Ψroot (Simonneau et al., 1998). However, the increase in ABA accumulation with decreasing soil moisture and Ψroot was much higher in HPRD (Figs 3 and 8), which suggests that the capacity of roots to accumulate ABA could depend on their position, as roots in the dry side of VPRD were sampled from a higher position in the soil profile than in HPRD. Greater root maturity in upper soil layers may explain the lower response of [ABA]root to soil water potential observed in VPRD than in HPRD, as it has been reported that maize primary young roots and primary root tips start to accumulate ABA in response to soil drying at higher relative root water content than mature primary roots and secondary roots (Zhang and Tardieu, 1996). This explanation agrees with previous observations of root ABA accumulation in response to soil drying. While root ABA concentration was much greater in the dry side of sycamore seedlings growing in split pots (Khalil and Grace, 1993), it was not clearly higher in the upper layers of drying soil columns in bean (Trejo and Davies, 1991; Puértolas et al., 2013). In an extreme case, root ABA accumulation in the upper soil layers of maize growing in 1 m columns was lower than at intermediate depth, despite higher soil water content in the latter (Zhang and Davies, 1989). All these observations seem to support the hypothesis that the capacity of roots to accumulate ABA in response to soil drying decreases in the upper older roots compared with deeper younger roots. To our knowledge, these results are the first evidence of different sensitivity of root ABA accumulation to changes in Ψroot depending on root position in the root zone. This might explain the discrepancies in local root ABA accumulation responses to soil drying described above. Although the evidence found in this experiment strongly suggest the hypothesis of different sensitivity to water potential according to position within the root, this should be proven in an specific study, preferably in longer pots and with different species.
Equivalent Ψroot in the dry compartment in both HPRD and VPRD treatments (at equivalent θ v) in Experiment 2 (Fig. 7, Table 1) suggests that internal hydraulic redistribution is equally effective, regardless of how soil moisture heterogeneity is imposed. Although hydraulic redistribution should occur via night-time water potential equilibration (Bauerle et al., 2008), differences in Ψroot between dry and wet compartments occurred both pre-dawn and at midday (Fig. 7), indicating that the Ψroot disequilibrium was stable throughout the photoperiod. Therefore, root ABA accumulation seems to be affected only by the degree of soil drying, regardless of the spatial layout of soil moisture heterogeneity, and not by differential internal water redistribution.
The differential root ABA accumulation in drying soil depending on their position (or age) observed in Experiment 2 might affect [X-ABA], with potentially higher export in HPRD than in VPRD. However, no ABA differences between different soil moisture distributions were observed, as soil drying did not significantly affect [X-ABA]leaf (Fig. 9). Increased [X-ABA]leaf, observed only at pre-dawn in plants with high soil moisture (Fig. 9), was not associated with increased [ABA]root or decreased soil moisture in the dry compartment, and probably reflects a concentration effect due to low sap flow observed during the night period, as seen previously (Sobeih et al., 2004).
This absence of an [X-ABA] response to PRD (Fig. 9) contrasts with results reported elsewhere (Sobeih et al., 2004; Liu et al., 2006; Wang et al., 2010). However, the response of ABA status to PRD is not consistent, as some studies report no increase in xylem sap (Jensen et al., 2009) or leaf (Blackman and Davies, 1985; Wakrim et al., 2005; Einhorn et al., 2012) ABA concentration. The lack of response of [X-ABA] to PRD observed (Fig. 9) seems to agree with a model predicting that [X-ABA] under PRD is determined by the average of root ABA in each side weighted by RWUF (Dodd et al., 2008a ) (Fig. 10A, C). In the two experiments described (Fig. 1), even though ABA accumulates in roots in the dry compartment, [X-ABA] cannot increase in response to PRD since the increase in [ABA]root in the dry compartment is coincident with very low RWUF (<15%, Figs 4 and 6). Nevertheless, [X-ABA] of potato can increase in response to PRD (Liu et al., 2006, 2008). Although these studies did not simultaneously measure RWU and [ABA]root during PRD, [X-ABA] increased (compared with fully irrigated plants) 2–4 d after applying PRD, when RWUF from the dry side exceeded 30% (Liu et al., 2008). When RWU fell below 20% on d 5, [X-ABA] fell accordingly. These observations suggest that when soil moisture in the wet side is close to saturation, ABA signalling can only increase when experimental conditions simultaneously allow appreciable RWUF (at least 20%) and ABA accumulation in the dry compartment.
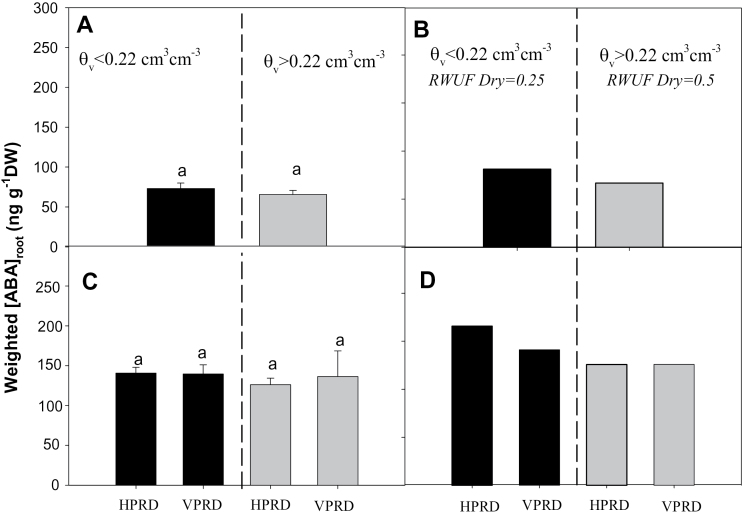
Fig. 10.
(A, C) Average [ABA]root in both compartments weighted by RWUF [weighted [ABA]root=(RWUFdry×[ABA]root dry)+(RWUFwet×[ABA]root wet) (mean±SE)] in plants with soil volumetric moisture in the dry compartment above (left of the dashed line dividing each panel) and below (right) 0.22cm3 cm–3. (A) Experiment 1. (C) HPRD versus VPRD in Experiment 2. Different letters denote statistical differences across compartments and groups within the same panel. (B, D) Predicted weighted [ABA]root assuming the observed [ABA]root average in each side, level of soil moisture, and PRD distribution, but with RWUF in the dry side equal to 0.5 when θ v >0.22, and RWUF in dry side equal to 0.25 when θ v <0.22 in Experiment 1 (B) and for HPRD versus VPRD in Experiment 2 (D). Error bars are not applicable in (B) and (D) as they use modelled data.
From a (PRD) management perspective, it becomes critical to determine experimental conditions that allow simultaneous root ABA accumulation and moderate water uptake from the dry side. The main difference between our study and previous studies in potato (Liu et al., 2006, 2008) is soil texture. Relationships between soil and root water potential, and θ v and either root ABA levels or RWUF from drying roots under PRD conditions differ largely according to soil texture (Dodd et al., 2010). While RWU declines more sensitively with θ v in the organic loam used here than in a sandy soil (see Fig. 3a in Dodd et al., 2010), both substrates showed similar increases in [X-ABA]root as θ v declined. Thus, similar root ABA accumulation would allow greater export to the shoots in a sandy soil than in the soil used in our study, thereby increasing [X-ABA] (Fig. 10). The reasons for higher RWUF from dry sand compartments are not clear but may depend on the soil water potential of the wet side: lower soil matric potential in the wet compartment attenuates the decrease in RWUF from the dry compartment (Dodd et al., 2008b ). Low matric potential in the wet side of sand-grown plants may be related to the difficulty of maintaining high matric potential in the wet compartment, as transpirational flow increases to balance the decrease in transpiration sourced from the dry compartment.
Our findings suggest that VPRD is less likely to increase [X-ABA] than HPRD, as [ABA]root did not respond to decreasing Ψroot in the former (Fig. 8). With a more moderate decrease in RWUF from the dry side with soil moisture than that observed (RWUF=0.25 when θ v <0.22), while maintaining the observed [ABA]root values in each side, the predicted weighted [ABA]root average would only clearly increase in the HPRD treatment of Experiment 2 (Fig. 10B, D). This implies that transient increases in [X-ABA] in response to heterogeneous soil drying would only be significant when soil drying affects parts of the root system that are better able to synthesize ABA in response to decreasing Ψroot.
The relative insensitivity of ABA synthesis to soil drying in some parts of the root system (Fig. 3A) should be considered in designing more efficient water-saving irrigation strategies. For instance, VPRD application could be intentionally achieved using simultaneously subsurface and surface drip irrigation, keeping the lower layers permanently wet while submitting the upper layers to drying and rewetting cycles (Kang et al., 2002). This may be a more efficient technique than HPRD, since the dry part of the root system would be the upper part, which produces less ABA (Fig. 4), maintaining higher stomatal conductance (and photosynthesis) than HPRD for a similar degree of soil drying (and irrigation volume application). Identifying the spatial patterns of local root ABA accumulation in response to soil drying for different crop species may allow specific areas of the root system to be dried with minimal yield penalty.
Acknowledgements
This research was funded by EU project SIRRIMED (FP7- KBBE-2009-3-245159). CB and MRC were PhD visitors at the Lancaster Environment Centre, funded by INIA (Instituto Nacional de Investigaciones Agrarias) and the Spanish Ministry of Education (EST12/00584), respectively.
Glossary
Abbreviations:
- Ψleaf
leaf water potential
- Ψroot
root water potential
- θg
soil gravimetric water content
- θv
soil volumetric water content
- ABA
abscisic acid
- [ABA]root
abscisic acid concentration in root tissue
- ANOVA
analysis of variance
- gs
stomatal conductance to water vapour
- HPRD
horizontal partial root-zone drying
- PRD
partial root-zone drying
- RWU
root water uptake
- RWUF
root water uptake fraction
- SE
standard error
- VPRD
vertical partial root-zone drying
- [X-ABA],
abscisic acid concentration in xylem sap
- [X-ABA]leaf
abscisic acid concentration in leaf xylem sap
- [X-ABA]root
abscisic acid concentration in root xylem sap.
References
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. 2008. Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant, Cell & Environment 31, 177–186. [DOI] [PubMed] [Google Scholar]
- Beff L, Guenther T, Vandoorne B, Couvreur V, Javaux M. 2013. Three-dimensional monitoring of soil water content in a maize field using Electrical Resistivity Tomography. Hydrology and Earth System Sciences 17, 595–609. [Google Scholar]
- Blackman PG, Davies WJ. 1985. Root to shoot communication in maize plants of the effects of soil drying. Journal of Experimental Botany 36, 39–48. [Google Scholar]
- de Kroon H, Fransen B, van Rheenen JWA, van Dijk A, Kreulen R. 1996. High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 106, 73–84. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. 2008a. Abscisic acid signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant, Cell & Environment 31, 1263–1274. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Davies WJ. 2008b. Accounting for sap flow from different parts of the root system improves the prediction of xylem ABA concentration in plants grown with heterogeneous soil moisture. Journal of Experimental Botany 59, 4083–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Egea G, Watts CW, Whalley WR. 2010. Root water potential integrates discrete soil physical properties to influence ABA signalling during partial rootzone drying. Journal of Experimental Botany 61, 3543–3551. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2009. Rhizosphere manipulations to maximize ‘crop per drop’ during deficit irrigation. Journal of Experimental Botany 60, 2454–2459. [DOI] [PubMed] [Google Scholar]
- Dry PR, Loveys BR, During H. 2000. Partial drying of the rootzone of grape. I. Transient changes in shoot growth and gas exchange. Vitis 39, 3–7. [Google Scholar]
- Einhorn TC, Caspari HW, Green S. 2012. Total soil water content accounts for augmented ABA leaf concentration and stomatal regulation of split-rooted apple trees during heterogeneous soil drying. Journal of Experimental Botany 63, 5365–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose E, Steudle E, Hartung W. 2000. Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 211, 874–882. [DOI] [PubMed] [Google Scholar]
- Jensen NL, Jensen CR, Liu FL, Petersen KK. 2009. Water relations and abscisic acid in pot-grown strawberry plants under limited irrigation. Journal of the American Society for Horticultural Science 134, 574–580. [Google Scholar]
- Kang SZ, Shi WJ, Cao HX, Zhang JH. 2002. Alternate watering in soil vertical profile improved water use efficiency of maize (Zea mays). Field Crops Research 77, 31–41. [Google Scholar]
- Khalil AAM, Grace J. 1993. Does xylem sap ABA control the stomatal behavior of water-stressed sycamore (Acer pseudoplatanus L.) seedlings? Journal of Experimental Botany 44, 1127–1134. [Google Scholar]
- Liang JS, Zhang JH, Wong MH. 1997. How do roots control xylem sap ABA concentration in response to soil drying? Plant & Cell Physiology 38, 10–16. [Google Scholar]
- Liu FL, Shahnazari A, Andersen MN, Jacobsen SE, Jensen CR. 2006. Physiological responses of potato (Solanum tuberosum L.) to partial root-zone drying: ABA signalling, leaf gas exchange, and water use efficiency. Journal of Experimental Botany 57, 3727–3735. [DOI] [PubMed] [Google Scholar]
- Liu FL, Song R, Zhang XY, Shahnazari A, Andersen MN, Plauborg F, Jacobsen SE, Jensen CR. 2008. Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environmental & Experimental Botany 63, 385–391. [Google Scholar]
- Mao S-Y, Jiang C-D, Zhang W-H, Shi L, Zhang J-Z, Chow WS, Yang J-C. 2009. Water translocation between ramets of strawberry during soil drying and its effects on photosynthetic performance. Physiologia Plantarum 137, 225–234. [DOI] [PubMed] [Google Scholar]
- Neales TF, McLeod AL. 1991. Do leaves contribute to abscisic acid present in the xylem sap of ‘droughted’ sunflower plants? Plant, Cell & Environment 14, 979–986. [Google Scholar]
- Puértolas J, Alcobendas R, Alarcón JJ, Dodd IC. 2013. Long-distance abscisic acid signalling under different vertical soil moisture gradients depends on bulk root water potential and average soil water content in the root zone. Plant, Cell & Environment 36, 1465–1475. [DOI] [PubMed] [Google Scholar]
- Puértolas J, Ballester C, Elphinstone D, Dodd IC. 2014. Two potato (Solanum tuberosum) varieties differ in drought tolerance due to differences in root growth at depth. Functional Plant Biology 41, 1107–1118. [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. 1988. A monoclonal-antibody to (S)-abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic-acid in crude extracts of cereal and lupin leaves. Planta 173, 330–339. [DOI] [PubMed] [Google Scholar]
- Sauter A, Davies WJ, Hartung W. 2001. The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. Journal of Experimental Botany 52, 1991–1997. [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME. 2002. ABA, ethylene and the control of shoot and root growth under water stress. Journal of Experimental Botany 53, 33–37. [PubMed] [Google Scholar]
- Simonneau T, Barrieu P, Tardieu F. 1998. Accumulation rate of ABA in detached maize roots correlates with root water potential regardless of age and branching order. Plant, Cell & Environment 21, 1113–1122. [Google Scholar]
- Simonneau T, Habib R. 1994. Water uptake regulation in peach trees with split-root systems. Plant, Cell & Environment 17, 379–388. [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. 2004. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. Journal of Experimental Botany 55, 2353–2363. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49, 775–788. [Google Scholar]
- Stoll M, Loveys B, Dry P. 2000. Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany 51, 1627–1634. [DOI] [PubMed] [Google Scholar]
- Tardieu F, Lafarge T, Simonneau T. 1996. Stomatal control by fed or endogenous xylem ABA in sunflower: interpretation of correlations between leaf water potential and stomatal conductance in anisohydric species. Plant, Cell & Environment 19, 75–84. [Google Scholar]
- Trejo CL, Davies WJ. 1991. Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. Journal of Experimental Botany 42, 1507–1515. [Google Scholar]
- Wakrim R, Wahbi S, Tahi H, Aganchich B, Serraj R. 2005. Comparative effects of partial root drying (PRD) and regulated deficit irrigation (RDI) on water relations and water use efficiency in common bean (Phaseolus vulgaris L.). Agriculture Ecosystems & Environment 106, 275–287. [Google Scholar]
- Wang YS, Liu FL, Andersen MN, Jensen CR. 2010. Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Functional Plant Biology 37, 175–182. [Google Scholar]
- Zhang J, Davies WJ. 1987. Increased synthesis of ABA in partially dehydrated root tips and ABA transport from roots to leaves. Journal of Experimental Botany 38, 2015–2023. [Google Scholar]
- Zhang J, Davies WJ. 1989. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant, Cell & Environment 12, 73–81. [Google Scholar]
- Zhang J, Davies WJ. 1990. Changes in the concentration of ABA in xylem sap as a function of changing soil-water status can account for changes in leaf conductance and growth. Plant, Cell & Environment 13, 277–285. [Google Scholar]
- Zhang JH, Tardieu F. 1996. Relative contribution of apices and mature tissues to ABA synthesis in droughted maize root systems. Plant & Cell Physiology 37, 598–605. [Google Scholar]