Abstract
The primary thrust of tissue engineering is the clinical translation of scaffolds and/or biologics to reconstruct tissue defects. Despite this thrust, clinical translation of tissue engineering therapies from academic research has been minimal in the 27 year history of tissue engineering. Academic research by its nature focuses on, and rewards, initial discovery of new phenomena and technologies in the basic research model, with a view towards generality. Translation, however, by its nature must be directed at specific clinical targets, also denoted as indications, with associated regulatory requirements. These regulatory requirements, especially design control, require that the clinical indication be precisely defined a priori, unlike most academic basic tissue engineering research where the research target is typically open-ended, and furthermore requires that the tissue engineering therapy be constructed according to design inputs that ensure it treats or mitigates the clinical indication. Finally, regulatory approval dictates that the constructed system be verified, i.e., proven that it meets the design inputs, and validated, i.e., that by meeting the design inputs the therapy will address the clinical indication. Satisfying design control requires (1) a system of integrated technologies (scaffolds, materials, biologics), ideally based on a fundamental platform, as compared to focus on a single technology, (2) testing of design hypotheses to validate system performance as opposed to mechanistic hypotheses of natural phenomena, and (3) sequential testing using in vitro, in vivo, large preclinical and eventually clinical tests against competing therapies, as compared to single experiments to test new technologies or test mechanistic hypotheses. Our goal in this paper is to illustrate how design control may be implemented in academic translation of scaffold based tissue engineering therapies. Specifically, we propose to (1) demonstrate a modular platform approach founded on 3D printing for developing tissue engineering therapies and (2) illustrate the design control process for modular implementation of two scaffold based tissue engineering therapies: airway reconstruction and bone tissue engineering based spine fusion.
Keywords: Additive manufacturing, Image-based design, Tissue engineering, Regulatory, Translation, Tracheobronchial malacia, Spine fusion
INTRODUCTION
Tissue engineering as a discipline has existed for at least 27 years, since the term was first coined in 1987. Since this time, however, tissue engineering therapies, scaffold based or otherwise, have had difficulty impacting clinical practice. Although some recent high profile technologies have been used in clinical treatment, 5,12,17,23,28,29 including bone regeneration,23,28 cartilage repair5 and tracheal reconstruction,17 sustained, consistent clinical translation of tissue engineering therapies has been difficult to achieve. Part of the difficulty is due to the milieu in which tissue engineering operates, spread across academic research laboratories, which, when they develop technology, seek to license this technology to start up or large device companies. Large companies, with the infrastructure to develop technologies and obtain regulatory approval, must understandably pursue those therapies which can recoup costs and make profits. This leaves clinical translation for smaller market indications dependent primarily on academic research institutions or small start up companies that have limited resources for translation.
Academic research institutions and laboratories, however, face numerous difficulties in achieving clinical translation. Among the most challenging is funding and infrastructure. However, equally challenging is that the reward structure in academics is primarily geared towards basic research and discovery, not the coupled clinical problem definition, technology integration and development for regulatory approval needed for clinical translation. Development for regulatory approval is especially critical for clinical translation. The difficulty, however, is that development for regulatory approval must be ingrained at the beginning of the scaffold/tissue engineering process.
Fortunately, a very detailed design/development process is outlined by the FDA for regulatory approval as part of the quality systems regulation (QSR). This design/development process is known as Design Control. Any tissue engineering therapy that will be tested in clinical trials on the translational path to clinical use must be manufactured under FDA quality systems requirements (QSR), the basis of which is Design Control (Fig. 1).6,31
FIGURE 1.
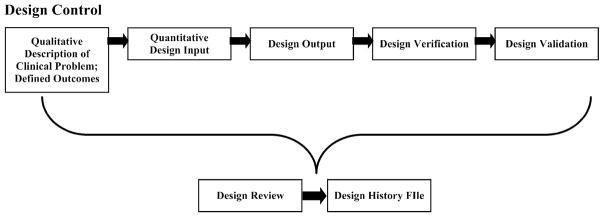
Schematic of the design control process. The process begins with definition of the specific clinical problem through quantitative design inputs. Design outputs are tests developed to characterize specific aspects of the engineered therapy. Design verification is the process of showing design outputs = design inputs, i.e., that the engineered therapy produced is equivalent to the one designed. Finally, design hypotheses concerning how the engineered system affects tissue response in bench, pre-clinical animal models and clinical trials are tested in design validation. The whole process is continually developed and assessed in design review, and all discussions and outcomes are documented in the Design History File (DHF).
Design Control requires that the clinical target, or indication, be precisely defined prior to developing the scaffolds and/or biologics that will address that indication. This step is defined as Design Input. At this point, of course, a number of assumptions, or design hypotheses, are made about how design choices affect tissue response and clinical outcome. Bench testing, pre-clinical animal models, and eventually clinical trials are used to test these design hypotheses and determine if the design parameters believed to influence outcome actually do and in what manner. Design hypothesis testing is known within the Design Control process as Design Validation
However, prior to testing an engineered therapy system in a pre-clinical model or human being, we must be sure that the engineered system matches our design inputs. Otherwise, it cannot be known with certainty whether the way in which the engineered system affects tissue response is due to the choice of design parameter we know, or if the system is different than we assume and this difference is responsible for the tissue response. In Design Control, this is a critical part of the process known as Design Verification. Indeed, Design Verification requires rigorous definition of Design Outputs, which are tests specifically developed to prove that the final engineered therapy that we produce meets the Design Inputs. The whole Design Control process is continuously documented through Design Review meetings at each stage. The final document is known as the Design History File, basically a “book” about how you designed the engineered therapy, why you made the design choices you did, and if you had to make any corrections. The purpose of this paper is to illustrate the implementation of modular scaffold engineering based on 3D printing for clinical translation within the Design Control framework.
METHODS
Our goal in this “Methods” section is to not just describe the methods we use to design, fabricate and test scaffolds, but to fit these methods within the context of FDA design control. Therefore, we will step through our understanding of the design control process for modular scaffolds using two scaffold examples: (1) a bioresorbable tracheobronchial splint for airway reconstruction and (2) a 3D printed bioresorbable spinal cage for spinal fusion.
The first step in design control is defining design inputs, which include the clinical indication, the patient population and market, the surgical clinical requirements, and the functional (biocompatability, biologic regeneration, mechanical, and mass transport) requirements that must be satisfied to treat and/or mitigate the clinical condition. It is important to note that the FDA categorizes medical devices into three classes: Class I (Exempt), Class II (premarket notification, 510 k pathway), and Class III (high risk, Premarket Approval pathway). The tracheobronchial splint is a class III device. In general, spinal cages are class II devices (except for cages that use biologics like BMP2, which are still class III, and possibly cages made from resorbable materials). For class II devices, the FDApublishes special controls guidance documents which lay out many design inputs and design outputs specific to the given device.
Design inputs are often initially defined as qualitative requirements to address the specific clinical indication. For example, the splint treats tracheobronchomalacia (TBM), a condition of excessive dynamic collapse of the tracheal and/or bronchial airways during respiration which results in reduced cross-sectional area of the affected airway segment by more than 50%.25 For the splint, such qualitative requirements (denoting these as “S” for “splint”) were initially defined by Zopf et al.35,36 as:
-
S1
The splint should provide radial compressive mechanical support to keep the trachea/bronchus open and patent.
-
S2
The splint should provide this radial mechanical support for a period of 24– 30 months to allow tracheal or bronchial remodeling and development.
-
S3
The splint should allow transverse and bending displacement, not interfering with cervical motion.
-
S4
The splint should allow growth and expansion of the tracheobronchial complex during this 24–30 month period.
-
S5
The splint should not cause adverse tissue reaction or remodeling.
-
S6
The splint should not interfere with the mucociliary architecture of the tracheal or bronchial lumen; it should therefore be placed externally.
-
S7
It is desirable that a second surgical procedure should be avoided to remove the splint; the splint should therefore be bioresorbable.
-
S8
Surgical placement of the splint and attachment of the trachea or bronchus into the splint should be straightforward.
These qualitative requirements may be broadly classified into mechanical/mass transport (S1–S4), biomaterial (S4–S6), and surgical (S7–S8) requirements. Our tracheobronchial splint design hypothesis is that scaffolds meeting design inputs S1–S8 will successfully mitigate TBM and reverse respiratory distress. For a class III device this design hypothesis must be tested both in pre-clinical animal models and ultimately clinical trials, although bench testing will also be required. However, a critical question is how confident are we that the device we produce meets requirements S1–S8? This question is the purpose of design verification. For verification, we must define quantitative embodiments of qualitative design inputs that can be objectively tested. These objective tests are denoted in design control as Design Outputs. The quantitative embodiments of design inputs for the splint (which we denote as SQ) are:
Mechanical (SQ1–SQ4): Splint displaces less than 10% under 20 N compression (S1); Splint displaces at least 20% under 15 N opening load (S4); Splint allows at least 20% displacement under 20 N 3 point bending load (S3).
Biomaterial (SQ4–SQ6): Splint Material as Manufactured should pass International Standards Organization (ISO) 10993 standard “Biological Evaluation of Medical Devices”; No adverse tissue reaction in preclinical animal model.
Surgical (SQ7–SQ8): Splint designed with opening to allow placement around trachea/bronchus; Periodically placed holes in splint allow suturing of collapsed airway.
The specific design outputs for the splint (denoted as “SO”) are defined as:
Mechanical (SO1–SO4): Finite element analysis of splint design; compression, three point bending and opening mechanical tests of manufactured splint.
Biomaterial (SO4–SO6): Splint Material as Manufactured should pass ISO 10993; No adverse tissue reaction in preclinical animal model.
Surgical (SO7–SO8): Splint opens under 15 N load; Suture holes allow surgical needle placement.
The qualitative design inputs for spine fusion also center on mechanical performance, biocompatability, tissue response (obtaining bony fusion), and surgical implementation. The clinical purpose of spine fusion is to distract vertebrae and decompress nerve roots, providing mechanical stability via the cage until bony fusion is achieved.18 The qualitative requirements for a resorbable spine fusion cage may be summarized as (denoting as “C” for cage):
-
C1
The cage should withstand compression, torsion and bending for loads at a given spinal level (lumbar or cervical), for potentially millions of cycles until bony fusion is achieved.
-
C2
The cage should maintain vertebral distraction and nerve decompression, not subsiding or sinking into the inferior vertebrae.
-
C3
The cage should maintain sufficient mechanical support until bony fusion is achieved (typically by 1 year maximum), and then degrade.
-
C4
The cage should not cause adverse bone or soft tissue response.
-
C5
The cage should effectively deliver osteobiologics (e.g., bone graft or BMP) to enhance vertebral fusion as quick as possible without bone growth outside the disc space.
-
C6
The cage should be readily implanted and fixed in the disc space to avoid expulsion.
The qualitative cage design requirements C1–C6 can again be categorized as mechanical (C1–C3), biomaterial/biologic (C3–C5), and surgical (C6). The spinal cage design hypothesis is that cages satisfying design inputs C1–C6 will provide immediate and long term mechanical stability, maintain disc height, and achieve bony fusion at the desired spinal level. As with the splint, the qualitative design cage design inputs C1–C5 must be embodied in quantitative design requirements the can be objectively tested for design verification.
Unlike class III devices which may have unique design inputs and outputs, spinal cages utilized with bone graft are class II devices, for which an FDA special guidance document exists: “Guidance for Industry and FDA Staff–Class II Special Controls Guidance Document: Intervertebral Body Fusion Device”.2 Within this guidance document are specific tests, or Design Outputs, recommended by the FDA. Since cages with graft are a class II device, fulfillment of these design outputs for design verification, along with successful completion of a pre-clinical animal study in comparison to a predicate device for design validation, constitute evidence that the cage is safe and effective via the 510(k) premarket notification pathway.
The specific design outputs (denoted as “CO”) from the intervertebral body fusion device guidance document categorized into mechanical, biomaterial, and surgical are:
Mechanical (CO1): Static and Dynamic (Fatigue, up to 5 million cycles) mechanical testing in Compression, Shear, and Torsion of the cage according to the American Society for Testing and Materials (ASTM) F 2077-03 Standard “Test Methods for Intervertebral Body Fusion Devices”. Maintain these sufficient fatigue properties through degradation.
Mechanical (CO2): Compression Subsidence Testing according to ASTM F 2264-04 “Standard Test Method for Inducing Load Induced Subsidence under Static Axial Compression”.
Biomaterial (CO3–CO4): Cage Device as Manufactured should pass ISO 10993; Wear testing of cage; No adverse tissue reaction in preclinical animal model; Degradation should not significantly degrade properties before 1 year. Device sterilization to meet FDA Update Sterility Review Guidance K90-1.32
Surgical (CO4–CO5): Specific Labeling for Surgical Procedure to Implant Cage; Properly defined instrumentation to allow impaction of cage into disc space; Fixation teeth and/or cervical plate to avoid expulsion.
There are two remaining challenges after defining quantitative design inputs and outputs. First, can a scaffold or scaffold/biologic construct (where here biologic is defined as a tissue/bone graft, cells, proteins and/or genes) be designed and manufactured to fulfill the quantitative design inputs as measured by the design outputs (design verification)? Second, are the scaffold construct design hypotheses verified by testing in preclinical animal models (class II) and preclinical animal models and human clinical trials (class III) (design validation)? It is important to note that any combination scaffold/biologic is highly likely to be classified as a class III device and require human clinical trials.
Design verification of course requires methods to design, manufacture, and process what can be very complex scaffold/biologic constructs. Furthermore, as part of QSR, the reproducibility and accuracy of these design (including computational modeling) and manufacturing processes must be rigorously characterized and documented through standard operating procedures (SOPs) in a device master record (DMR). Finally, the more flexible our design and manufacturing processes, in terms of 3D geometry/shape, materials and biologic delivery, the more likely we are to produce constructs that meet our design inputs.
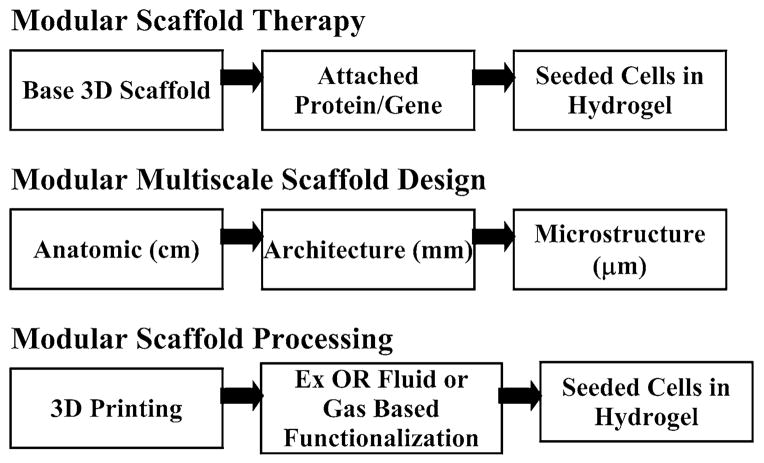
To address these needs, we14 proposed the idea of modular scaffold engineering, that is, modularity in scaffold therapies, design, and manufacturing, based on Arthur.1 Modularity in scaffold therapy implies using the scaffold as the base platform, performing layer based modifications of the base scaffold, and then incorporating biologics. Modularity in scaffold design simply denotes image-based multiscale scaffold design,13 incorporating for example homogenization theory.13,15 Image-based design also allows for patient specific scaffold constructs, using commercial software like MIMICS™ (Materialise, Leuven, Belgium). Modularity therapy processing implies that sequential but loosely connected processes are used to construct scaffold based therapies. For clinical indications requiring anatomic shape and/or defined porous architecture (i.e., non-contained tissue defects), we see 3D Printing in its various forms as the cornerstone of modular therapy processing (Fig. 2).
FIGURE 2.
The concept of modularity in the definition, design and processing of scaffold based tissue engineering therapies. Modular scaffold therapy implies the tissue engineering paradigm combining a base 3D structural scaffold, attached proteins/genes, and cells in hydrogels. Modular scaffold design implies image-based Multiscale design allowing patient specific devices. Modular scaffold processing implies 3D Printing as a platform on which fluid based processing outside the operating room (Ex OR) is used to modify the base scaffold and within the operating room (In OR) cells and other biologics are adsorbed on the scaffold.
There are two advantages of modular scaffold engineering. First, modularity allows a sliding scale of therapy complexity from the simplest scaffold only therapy to the most complex construct integrating scaffolds with surface modification, attached growth factors and seeded cells. It also allows these components to be assembled in the OR if desired. Second, modularity involving separate but sequential processes eases implementation of QSR. Part of QSR requires validation (proof that a process will a priori produce the same results) or verification (sample and test the outcome of each process to assess quality) of a process. Modularity allows a more segmented assessment of process quality, reducing complication in quality assessment.
Our implementation of modular scaffold engineering utilizes multiscale image based design to create the scaffold for patient specific defects. We generate the architecture level design using image-based and topology optimization methods.3,4,7,13,15,19,20,22 Specifically, a custom written MATLAB was developed to generate voxel-based image representations on a 0–255 density scale for a range of custom connected porous architectures, including interconnected cylinders, interconnected spheres, topology optimized microstructures, and triply minimal periodic surface (TPMS) architectures like P, G, and D minimal surfaces.33,34 We use anatomic computer aided design (CAD) methods, especially MIMICS™ to generate the anatomic level design. We can then import a stereolithography (STL) triangle based surface representation of the architecture design into a MIMICS™ STL anatomic defect and use Boolean operations to generate the porous architecture within the anatomic defect shape. Note that this Boolean operation can also be performed entirely within the image dataset, as we have previously described.13,15
Realization of the scaffold design of course requires a manufacturing method. To build these complex designs, we utilize 3D printing, specifically laser sintering as the fundamental modular fabrication process.26,30,33 Additional modular processes include fluid or gas based functionalization, in which gas or fluid is forced through the pores to modify the scaffold surface, for example creating osteoconductive nanocoatings27 or chemical vapor deposition (CVD) coatings to which viruses may be attached for gene therapy.16
For the tracheobronchial splint, we have currently implemented multiscale, patient specific image-based design using a custom written MATLAB™ (The Mathworks) program. This custom MATLAB program generates an image-based representation of a bellowed, open cylinder using a Fourier series to represent the bellow undulation, with periodically space suture hole. In total, the MATLAB program allows the user to specify splint wall thickness, splint length, splint inner diameter, extent of the splint opening angle, splint bellow height, splint bellow period, suture hole length and width. The resulting design is converted to an STL file using MIMICS™, and further volume meshed with tetrahedral elements for finite element (FE) analysis. FE analysis allows quick assessment of design parameter perturbation on the fundamental design inputs S1–S4. From a modular perspective, we adapted the splint design with portals to inject hydrogels with chondrocytes and/or stem cells to create constructs capable of generating cartilage rings.
The output of the modular splint design process is an STL surface representation we use to fabricate the splint from PCL using laser sintering on an EOS Formiga P100 system (Fig. 3a). We purchase raw PCL powder from Polysciences, Inc (www.polysciences.com), which we subsequently have milled into a target particle size range of between 25 and 125 microns (0.025– 0.125 mm) by Jet Pulverizer (www.jetpulverizer.com), which can be milled under good manufacturing practice (GMP) conditions. We use laser sintering parameters including bed temperature (48–56 °C), laser power (1–5.4 W), laser scan speed (900–1800 mm/s), laser scan spacing (0.07–0.2 mm) that we and others have previously established for PCL laser sintering,.8–11,20,28 Following fabrication, we measure splint dimensions and mechanically test the splint (Fig. 3b) to determine whether the fabricated splint meets the quantitative design outputs SO.
FIGURE 3.
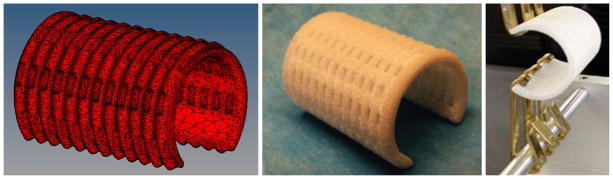
Splint modeling, fabrication and testing. (a) 10-node tetrahedral finite element model of bellowed splint design (b) Example of final laser sintered PCL tracheobronchial splint. (c) Opening mechanical test of splint.
Three different mechanical tests are implemented as design outputs for splint mechanical performance. Compression tests are performed to assess SO1 and SO4, that the splint allows displacement less than 10% of the inner diameter under a 20 N load, both initially and after a 1 year period of resorption. Compression tests are performed on an MTS Alliance RT/30 uniaxial testing machine with a 500 N load cell at a speed of 5 mm/min, with the splint opening opposite of the test platen. Tensile opening tests are performed with metal hooks inserted into the splint opening, which are then pulled apart at a test speed of 5 mm/min. Tests are again performed on an MTS Alliance RT/30 machine with a 500 N load cell. Finally, three point bending tests are performed on the same test set up, with a standard 3 point test set up.
For design validation, we must test verified fabricated splints in large preclinical animal models and eventually phase I clinical trials. Since we project that the splint will be needed for less than 4000 patients per year in the United States, we applied for and received human use designation (HUD) for the splint (HUD designation #12-0288). As such, the regulatory path is through humanitarian device exemption (HDE) rather than Premarket Approval (PMA). The splint has been tested in a 1-month Yorkshire pig model, both using a cartilage ring resection model of TBM36 and on the intact trachea to test our splint design hypotheses concerning the relationship between splint design parameters and tracheal growth. Growth was determined on tracheal models reconstructed from CT scans at 4 and 8 months created in MIMICS using hydraulic diameter and cross-sectional area measurements. Finally, although we haven’t begun clinical trials on the splint, we have successfully implanted splints in human patients to treat TBM under FDA provisions for emergency use.35
For the cervical spinal cage design, multiscale design consists of topology optimization to design the porous architecture and image-based global anatomic design to create a structure to fit the cervical disc space with serrated teeth for fixation to avoid expulsion. Again, this design approach reflects the needs to meet multiple design inputs, including mass transport requirements to enhance bone growth (C4), sufficient mechanical properties to avoid loss of disc space and subsidence (C1, C2), and fixation to avoid expulsion (C5). We utilized an algorithm we previously developed for microstructure optimization19,20,22 that allows us to specify a desired percent of base elastic modulus for load bearing as a target together with a targeted diffusion level for mass transport, with a constraint on the microstructure volume. For this case we chose 15% for elastic modulus, 53% for diffusion, and 30% for volume fraction. Note that the target values for elastic modulus and diffusion are the maximum that can be obtained for 30% volume fraction, based on known mathematical bounds.19
Once designed, we again fabricate the optimized cage structure by laser sintering PCL. The final optimized cage structure was then tested under static and dynamic fatigue compression, compression-shear and torsion as per ASTM F2077-03. Sufficient test results verifying scaffold design together with successful large preclinical animal results would provide sufficient information for regulatory approval under 510(k) premarket notification. However, because the cage in this case is resorbable, additional data may be required. Moreover, for our interests, we have designed the optimized cages to delivery BMP2 for bone fusion. This will automatically make such cages a class III device that must be approved through a PMA pathway. For BMP2 delivery, we absorb BMP2 directly onto the optimized scaffold. We have determined that direct adsorption on laser sintered PCL scaffolds provides for localized, controlled delivery of BMP2 with resultant localized bone formation. Although albeit in a conventional sense, this sequential use of a laser sintered PCL cage with BMP2 again represents a modular scaffold therapy (scaffold plus attached growth factor) construct by modular design (topology optimization of microstructure integrated with global anatomic design of cage shape and fixation), created by modular sequential processes (laser sintering of a scaffold followed by BMP2 adsorption). Finally, although beginning, we are currently testing cervical fusion using these scaffolds in a large preclinical animal model, the 6 month old Yorkshire pig.
RESULTS
Tracheobronchial Splint
Finite element analysis of the tracheobronchial splint demonstrated that splint compliance increased with increasing bellow height and decreasing wall thickness. Comparison between mechanical testing and FE showed that the FE analysis predicted the trends of splint compliance change with wall thickness and bellow height for both compression testing and opening tests (Fig. 4).
FIGURE 4.
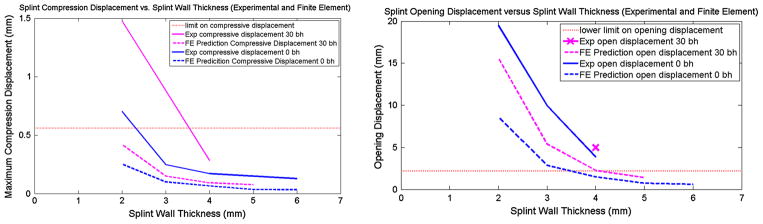
(Left) Effect of wall thickness on compressive displacement predicted by FE (computational) analysis vs. mechanical testing of 3D printed PCL scaffolds. Dotted limit is upper limit on compressive displacement (Right) Effect of wall thickness on opening displacement predicted by FE (computational) analysis vs. mechanical testing of 3D printed PCL scaffolds. Dotted line is lower limit on opening displacement, i.e., opening displacement should be above the dotted line. Results show that wall thickness of 3 mm would provide compressive stiffness and opening stiffness within design inputs, which are denoted by dashed lines for each graph.
Based on Fig. 4, compressive stiffness from the computational (FE) analysis for bellowed designs ranges between 47.6 N/mm for 2 mm wall thickness to 263 N/mm for 5 mm wall thickness, while opening stiffness ranged from 0.96 N/mm for 2 mm wall thickness to 10.6 N/mm for 5 mm wall thickness. Experimentally, compressive stiffness was 17.8 ± 0.6 N/mm for 2 mm wall thickness up to 158.1 ± 5.8 N/mm for 4 mm wall thickness with 30% bellow height, while opening stiffness ranged from 0.38 ± 0.02 N/mm for 2 mm wall thickness up to 3.4 ± 0.1 N/mm for 4 mm wall thickness. As shown in Fig. 4 and by the stiffness results, experimentally measured splint stiffness was lower than that predicted by computational (FE) analysis for the same splint designs. However, splints as designed and fabricated met mechanical design requirements SQ1–SQ4. Furthermore, we are beginning to test laser sintered splints under ISO 10993 biocompatibility requirements. These results both suggest that the splints as fabricated would meet design verification.
The next obvious question is whether verified scaffolds would treat and/or mitigate the clinical problems. In other words, do tracheobronchial splints fabricated to meet design inputs mitigate respiratory distress and allow tracheobronchial growth? This is the design validation aspect of design control. Our evidence comes from our previous large preclinical studies on TBM models in Yorkshire pigs, splints on intact tracheas in Yorkshire pigs, and our experience in humans under emergency use.
Our results in a TBM model in 1-month old Yorkshire pigs showed statistically significant greater survival in TBM pigs with implanted splints vs. control TBM pigs that did not have the splint.36 Furthermore, TBM pigs with splints had a normal to moderate Westley croup score, a clinical score of TBM which characterizes TBM severity including cyanosis, wheezing and loss of consciousness due to the inability to breathe. However, this was an extremely severe TBM model (created by the resection of 5 cartilage rings), and all control pigs had to be sacrificed within 24 h. Splint pigs demonstrated infection likely due to small perforations in the very thin mucosal wall remaining after cartilage ring resection. Such a model does not reflect etiology of the human condition, in which the cartilage rings and full tracheobronchial wall is intact, but collapsed due to external compression.
To assess any role the splint material may have played in the infections, the splint implantation was repeated on intact tracheas in two 1-month old Yorkshire pigs, which avoided rents in the tracheal mucosa. The splints were 25 mm long, had an inner diameter of 14 mm, a wall thickness of 2.5 mm and a 30% bellow height. The resulting compression stiffness was 28.2 ± 1.4 N/mm and opening stiffness was 0.43 ± 0.05 N/mm. These pigs were followed for 8 months, showing no infection. Furthermore, the tracheal lumen, including areas in which the splint was attached, grew by 20.3 and 22.5% from 4 to 8 months in hydraulic diameter, and 49.5 and 59% in cross sectional area from 4 to 8 months (Fig. 5), which in within normal growth ranges for pigs.
FIGURE 5.
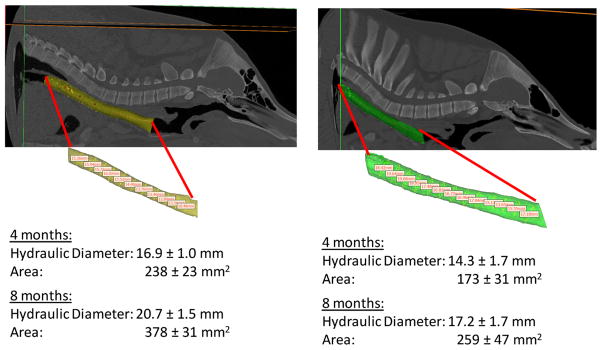
Growth of tracheal lumen in pigs where splint was placed over intact trachea. (a) Pig 1 CT scan and resulting lumen model at 8 months post splint implantation. (b) Pig 2 CT scan and resulting lumen model at 8 months post splint implantation. Resulting measures of hydraulic diameter and cross sectional area along trachea at 4 and 8 months post splint implantation. Note that standard deviations are for measurement along trachea in one pig and not between pigs.
These results support two important aspects of the splint design hypothesis. First, the laser sintered PCL splint itself did not cause adverse tissue reaction. Second, the opening stiffness of 0.4 N/mm allowed normal tracheal lumen growth as measured by CT scans.
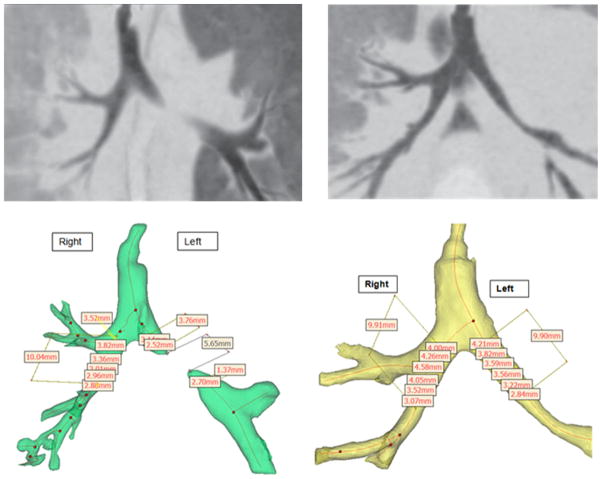
Finally, the splint has been utilized in humans under emergency clearance from the FDA. The first case was performed in February, 2012 on a 3-month child with severe life threatening left mainstem bronchialmalacia. 35 This child was weaned off ventilator support 21 days post-surgery with normal blood gas levels that have been abnormal prior to surgery. Furthermore, follow up on the child at 6 months and 1 year post-surgery using hydraulic diameter and cross sectional area measurements 3D models generated from CT scans showed that the splint treated bronchus grew at the same rate as the contralateral right bronchus (Fig. 6).
FIGURE 6.
CT scan and corresponding digital 3D model with hydraulic diameter measures on exhalation for patient at (a) preop and (b) 1 year post splint implantation. Results show complete discontinuity on exhalation before splint implantation, but hydraulic diameter for splint treated side at 1 year is similar to the treated side.
Resorbable Cervical Spine Fusion Cage
A new cervical spine cage design was created by modular integration of a topology optimized porous region with a center post for load bearing and serrated teeth for fixation to resist expulsion (Fig. 7). The optimized cages were created by laser sintering of PCL.26,33
FIGURE 7.
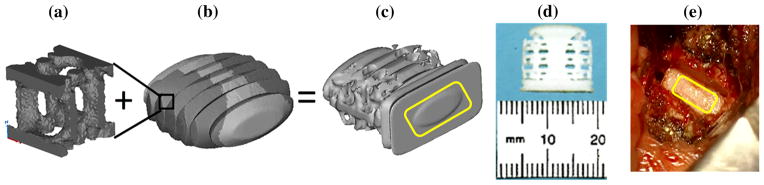
Example of modular design for cervical spinal cage integrating (a) topology optimization of unit cell for porous region with (b) anatomic design of cage layout with darker grey regions for substitution giving (c) the final design together with serrated regions for fixation and a cage front to allow impaction into disc space (d) fabricated laser sintered design using laser sintering.
The fabricated cages have been static and dynamic fatigue tested in compression, compression-shear, and torsion under ASTM F2077-03. The static ultimate loads for the optimized cervical spine cage were 847.0 ± 298.5 Newtons (N) in compression, 397.0 ± 40.9 N in compression-shear, and 0.48 ± 0.03 Newton-meters (Nm) in torsion. For dynamic fatigue testing, the optimized cage device ran for 5 million cycles (limit required by ASTM F2077) in compression at 100% of the ultimate load, in compression-shear at 98% of ultimate load, and at 60% of the ultimate torsion load. It is important to note that all fatigue tests were performed dry and did not account for hydrolytic degradation of the PCL.
We have begun testing the optimized structural cages with BMP2 release for cervical spine fusion in a large preclinical animal model, the Yorkshire pig. It is important to emphasize that biologic release automatically makes the cage a class III device. Prior to our ongoing large animal study, we tested BMP2 release from porous laser sintered PCL scaffolds in mice. The results clearly showed localized bone formation at 8 weeks post-implantation (Fig. 8).
FIGURE 8.
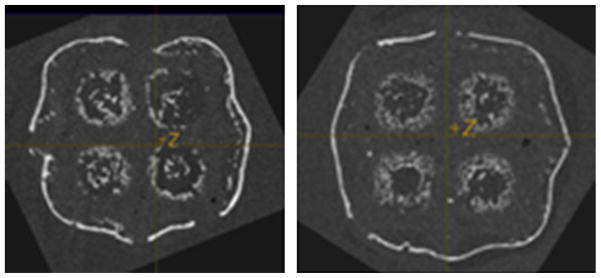
Bone formation surrounding PCL scaffold following BMP2 delivery via PCL adsorption at 65 μg/mL. Results show that on designed PCL scaffold bone formation closely follows scaffold outline and is encased within scaffold pores.
Results for both the tracheobronchial splint and cervical spine cage are summarized with respect to the Design Control framework in Fig. 9.
FIGURE 9.
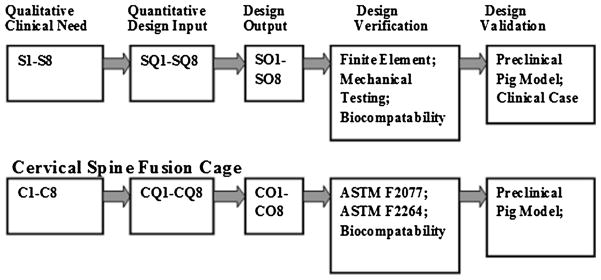
Schematic showing current design inputs and results in framework of design control for tracheobronchial splint and cervical spine cage.
DISCUSSION
The purpose of this paper was to provide an example of design control for scaffold development and translation. Specifically, we sought to illustrate scaffold design control by (1) demonstrating a modular platform approach founded on 3D printing for developing tissue engineering therapies and (2) illustrating the design control process for modular implementation of two scaffold based tissue engineering therapies: airway reconstruction and bone tissue engineering based spine fusion. Design control is fundamental to clinical translation of any tissue engineering technology, as it is the basis for implementation of quality systems regulations (QSR).
Design control, however, is much different than basic hypothesis testing research or technology development done in many academic laboratories. For traditional academic research, a research question is proposed, and in the case of technology development, work is done to develop that single technology. In the case of basic hypothesis testing, an in vitro or sometimes in vivo model is developed to test the hypothesis. Even if a discovery is made that has clinical relevance, the typical path is to patent this discovery, and then license the discovery out to a company who will try to shape it into a product, for which to obtain regulatory approval that company will have to implement design control. Therefore, most academic laboratories have typically not contemplated regulatory requirements and the need to implement design control for QSR.
However, current circumstances regarding translation are pushing academic institutes and their laboratories to become much more engaged in clinical translation and thus regulatory requirements including design control. First, many mature device companies will not invest in therapies until these therapies have reached the clinical trial stage. Reaching this stage and obtaining investigational device exemption (IDE) approval requires therapy production under QSR and thus design control. Second, licensing technology to small spin off companies still requires these companies to burn precious investment money to develop a clinical plan and implement design control. Having such issues at least partially addressed helps increase the chances of success for these spin off companies. Third, there are many clinical indications for which both mature and spin off companies will not develop devices because the market is too small. However, this does not diminish the human need to be treated, and with current technologies like 3D printing it is possible to develop devices for these patients, even down to the level of customized devices. Academic medical centers and engineering schools, along with their associated laboratories, will become more involved in directly translating therapies without companies, thereby requiring academic implementation of design control.
The implementation of design control, however, is a significant challenge in an academic environment. First, it requires an interdisciplinary research team, especially needing clinicians who can clearly define the clinical needs for a specific indication. Engineers and biologists are needed to help convert defined clinical needs into specific, if possible quantitative, engineering and biologic requirements for a therapy. Second, as much if not more effort must be put into developing and writing requirements for design control as often is put into writing manuscripts and grant proposals. It therefore takes more effort and time to explain productivity in writing design control documents as compared to manuscripts to study sections and funding agencies, and, most importantly for faculty, promotion and tenure committees. Third, the facilities needed for testing (design outputs) and the requirements that all testing in addition to preclinical animal testing should be performed under good laboratory practices (GLP) is often not available at academic institutions, requiring great expense to contract outside certified organizations. These issues, for the most part, must be addressed at the institutional level.
Difficulty implementing design control in academic development of tissue engineered therapies is further hindered by lack of recognition as a critical and viable research topic. A pubmed search using the terms “FDA Design Control” and “tissue engineering” yielded no references. A pubmed search using the terms “FDA Design Control” and “Medical Device” yielded five references, although only two references for medical devices.21,24 These references, especially Kinsel,21 provide a good review of design control components for medical device design and production. However, no current references that we could find discuss the utilization of design control with tissue engineering, scaffolds, or 3D printed tissue engineered devices.
Given these caveats, we have attempted to illustrate aspects of design control implementation for development of two scaffold based tissue engineering therapies within a modular framework. Indeed, our adoption of a modular implementation built around 3D printing is done to address the issues of expense for verifying and validating production processes. 3D printing, within the limitations of the materials which can be printed, provides us with the flexibility to produce an almost limitless variety of devices and scaffolds for numerous clinical indications, as we have begun to show with both the tracheobronchial splint and cervical spine cage. Thus, once the base material processing parameters for laser sintering have been established, 26,30,33 these same parameters can be used to produce a plethora of devices and scaffolds, where the cost per scaffold is the same if producing 1 or 1000. This enables feasible production of scaffolds and devices to treat small patient markets, for example, tracheobronchial malacia, for which treatment devices/scaffolds were not previously available.
An important point to note in our current implementation of design control is that it is not complete. We have not carried out preclinical studies under GLP, largely due to the expense of such an endeavor, and its lack of availability at the primary authors institution. The issues of GLP, and associated clean room facilities often needed for Good Manufacturing Practices (GMP) implementation into QSR, are two major financial issues facing academic institutions. Finally, we are currently implementing design reviews and design history file documentation.
Another important point to note is that many standards for design output, especially those for mechanical performance, are defined for permanent materials, not the bioresorbable materials that are used for tissue engineering. This is a critical issue for tissue engineering, as there is a complex interplay between material degradation and tissue in growth. It is obvious that the tissue engineering scaffolds must survive until sufficient tissue is regenerated to assume function, especially with regard to mechanical loading and fatigue behavior. However, there almost no papers that discuss or evaluate the fatigue properties of resorbable materials at time 0, let alone how fatigue behavior interacts with degradation and tissue regeneration. We must be able to understand how mechanical and fatigue properties change with degradation, and especially how degradation and tissue in growth interact to affect mechanical performance and especially fatigue behavior of tissue engineered constructs. This is an important area of funding and research that must be better understood to increase confidence in load bearing tissue engineered biologic devices.
The design control process we have implemented based on modularity and 3D printing, however, has allowed us to bring scaffold devices through bench testing, preclinical animal testing, and, in the case of the tracheobronchial splint, into clinical use. We have further demonstrated design verification of our scaffolds, showing that the designed scaffolds meet the design inputs, including mechanical performance. For the tracheobronchial splint, we have shown that when the splint meets the design inputs, especially those inputs for mechanical protection of the collapsed airway while allowing for growth, the splint does mitigate and treat the clinical condition. However, the next step to more rigorously determine that these design parameters lead to successful outcomes is to run a clinical trial. Indeed, a critical question for any device is establishing whether the design inputs postulated to affect clinical outcomes actually do and in what manner. For example, if a cervical cage passes ASTM F2077 and ASTM 2264-04 in addition to ISO 10993, will this case provide consistent fusion? Will this cage provide better pain relief? Can we perform topology optimization of cages specifically to the mechanical standards and have these cages maintain disc space no matter the patient? How do we establish standards for patient specific devices to ensure clinical performance? These are all questions related to how standards specifically and regulation in general relate to clinical outcomes, all of which must be studied in the emerging discipline of regulatory science.
In conclusion, we have begun to implement design control for scaffold based tissue engineering approaches using a modular scaffold engineering approach founded on 3D printing. We have begun with qualitative clinical goals which we converted to quantitative design inputs. We have designed and fabricated tracheobronchial splint and cervical spine fusion scaffolds to meet these quantitative design inputs using image-based topology design techniques followed be laser sintering of PCL. We have verified these fabricated scaffolds meet design inputs by mechanical testing and in vivo biocompatability testing. Finally, we have and are currently validating that these scaffolds mitigate the clinical indication in both preclinical animal models and human clinical use for the tracheal splint and ongoing preclinical animal models for the cervical cage.
Acknowledgments
The authors gratefully acknowledge the financial support of the NIH, R21 HD076370 (to S.J.H., G.E.G. and M.B.W.) for the tracheal splint work and NIH R01 AR 060892 (to S.J.H., M.B.W., E.E. and S.N.S.) for the cervical spine fusion work. In addition, some BMP2 delivery work was supported by NIH R21 DE0224439 (to S.J.H. and M.B.W). We gratefully acknowledge the contributions of Chanaka Rabel, PhD, Department of Animal Sciences, University of Illinois, Urbana-Champaign, Aaron Maki, PhD, Department of Bioengineering, University of Illinois, Urbana-Champaign, Jamey Cooper, University of Illinois, Urbana-Champaign, Anna Ercolin, DVM, Department of Animal Sciences, University of Illinois, Urbana-Champaign, and Kelly Roballo, Department of Animal Sciences, University of Illinois, Urbana-Champaign, for collection of the post-surgical respiratory data and animal monitoring for the tracheal splint; and especially Jonathan F. Mosley, Department of Animal Sciences, University of Illinois, Urbana-Champaign, along with his staff at the Physiology Research Laboratory/Imported Swine Research Laboratory, for the animal management, surgery assistance, and excellent animal care for the tracheal splint and cervical spine fusion work. Finally, we acknowledge the work of Sean L. Borkowski and Ashleen R. Knutsen on the dynamic fatigue testing of the cervical spine cages.
References
- 1.Arthur WB. The Nature of Technology: What it is and How it Evolves. New York: Free Press; 2009. [Google Scholar]
- 2.Class II Special Controls Guidance Document: Intervertebral Body Fusion Device. 2007 http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071408.htm.
- 3.Coelho PG, Fernandes PR, Rodrigues HC, Cardoso JB, Guedes JM. Numerical modeling of bone tissue adaptation—a hierarchical approach for bone apparent density and trabecular structure. J Biomech. 2009;42:830–837. doi: 10.1016/j.jbiomech.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Coelho PG, Fernandes PR, Rodrigues HC. Multiscale modeling of bone tissue with surface and permeability control. J Biomech. 2011;44:321–329. doi: 10.1016/j.jbiomech.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Crawford DC, DeBerardino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94:979–989. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- 6.Design Control Guidance for Medical Device Manufacturers. http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm070627.htm.
- 7.Dias MR, Guedes JM, Flanagan CL, Hollister SJ, Fernandes PR. Optimization of scaffold design for bone tissue engineering: a computational and experimental study. Med Eng Phys. 2014;36:448–457. doi: 10.1016/j.medengphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Doyle H, Lohfield S, McHugh P. Predicting the elastic properties of selective laser sintered PCL/beta-TCP bone scaffold materials using computational modeling. Ann Biomed Eng. 2014;42:661–677. doi: 10.1007/s10439-013-0913-4. [DOI] [PubMed] [Google Scholar]
- 9.Eosoly S, Vrana NE, Lohfeld S, Hindie M, Looney L. Interaction of cell culture with composition effects on the mechanical properties of polycaprolactone-hydroxyapatite scaffolds fabricated via selected laser sintering (SLS) Mater Sci Eng C. 2012;32:2250–2257. [Google Scholar]
- 10.Eshraghi S, Das S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010;6:2467–2476. doi: 10.1016/j.actbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshraghi S, Das S. Micromechanical finite-element modeling and experimental characterization of the compressive mechanical properties of polycaprolactone-hydroxyapatite composite scaffolds prepared by selective laser sintering for bone tissue engineering. Acta Biomater. 2012;8:3138–3143. doi: 10.1016/j.actbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison RH, St-Pierre JP, Stevens MM. Tissue engineering and regenerative medicine: a year in review. Tissue Eng Part B. 2014;20:1–16. doi: 10.1089/ten.TEB.2013.0668. [DOI] [PubMed] [Google Scholar]
- 13.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 14.Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B. 2011;17:459–474. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollister SJ, Levy RA, Chu TM, Halloran JW, Feinberg SE. An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg. 2000;29:67–71. doi: 10.1034/j.1399-0020.2000.290115.x. [DOI] [PubMed] [Google Scholar]
- 16.Hu WW, Elkasabi Y, Chen HY, Zhang Y, Lahann J, Hollister SJ, Krebsbach PH. The use of reactive polymer coatings to facilitate gene delivery from poly (epsilon-caprolactone) scaffolds. Biomaterials. 2009;30:5785–5792. doi: 10.1016/j.biomaterials.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozoky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, Le Guyader S, Henriksson G, Hermanson O, Juto JE, Leidner B, Lilja T, Liska J, Luedde T, Lundin V, Moll G, Nilsson B, Roderburg C, Stromblad S, Sutlu T, Teixeira AI, Watz E, Seifalian A, Macchiarini P. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 18.Kandziora F, Schollmeier G, Scholz M, Schaefer J, Scholz A, Schmidmaier G, Schroder R, Bail H, Duda G, Mittlmeier T, Hass NP. Influence of cage design on interbody fusion in a sheep cervical spine model. J Neurosurg. 2002;96:321–332. doi: 10.3171/spi.2002.96.3.0321. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Lin CY, Hollister SJ. Topology optimization of three dimensional tissue engineering scaffold architectures for prescribed bulk modulus and diffusivity. Str Multidiscipl Optim. 2010;42:633–644. doi: 10.1007/s00158-010-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang H, Hollister SJ, La Marca F, Park P, Lin CY. Porous biodegradable lumbar interbody fusion cage design and fabrication using integrated global-local topology optimization with laser sintering. J Biomech Eng. 2013;135:101013–101018. doi: 10.1115/1.4025102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsel D. Design control requirements for medical device development. World J Pediatr Congenit Heart Surg. 2012;3:77–81. doi: 10.1177/2150135111422720. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J Biomech. 2004;37:623–636. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Low SW, Ng YJ, Yeo TT, Chou N. Use of Osteoplug polycaprolactone implants as novel burr-hole covers. Singapore Med J. 2009;50:777–780. [PubMed] [Google Scholar]
- 24.May-Newman K, Cornwall GB. Teaching medical device design using design control. Expert Rev Med Devices. 2012;9:7–14. doi: 10.1586/erd.11.63. [DOI] [PubMed] [Google Scholar]
- 25.Murgu S, Colt H. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med. 2013;34:527–555. doi: 10.1016/j.ccm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Partee B, Hollister SJ, Das S. Selective laser sintering process optimization for layered manufacturing of CAPA 6501 polycaprolactone bone tissue engineering scaffolds. ASME J Manuf Sci Eng. 2006;128: 531–540. [Google Scholar]
- 27.Saito E, Suarez-Gonzalez D, Rao RR, Stegemann JP, Murphy WL, Hollister SJ. Use of micro-computed tomography to nondestructively characterize biomineral coatings on solid freeform fabricated poly (L-lactic acid) and poly (epsilon-caprolactone) scaffolds in vitro and in vivo. Tissue Eng Part C. 2013;19:507–517. doi: 10.1089/ten.tec.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schantz JT, Lim TC, Ning C, Teoh SH, Tan KC, Wang SC, Hutmacher DW. Cranioplasty after trephination using a novel biodegradable burr hole cover: technical case report. Neurosurgery. 2006;58:ONS-E176. doi: 10.1227/01.NEU.0000193533.54580.3F. discussion ONS-E176. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 30.Smith MH, Flanagan CL, Kemppainen JM, Sack JA, Chung H, Das S, Hollister SJ, Feinberg SE. Computed tomography-based tissue-engineered scaffolds in craniomaxillofacial surgery. Int J Med Robot. 2007;3:207–216. doi: 10.1002/rcs.143. [DOI] [PubMed] [Google Scholar]
- 31.Teixeira MB, Bradley R. Design Controls for the Medical Device Industry. New York: Marcel Dekker Inc; 2003. [Google Scholar]
- 32.Updated 510(k) Sterility Review Guidance K90-1; Final Guidance for Industry and FDA. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072783.htm.
- 33.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Yoo DJ. Porous scaffold design using the distance field and triply periodic minimal surface models. Biomaterials. 2011;32:7741–7754. doi: 10.1016/j.biomaterials.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368:2043–2045. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 36.Zopf DA, Flanagan CL, Wheeler M, Hollister SJ, Green GE. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol Head Neck Surg. 2014;140:66–71. doi: 10.1001/jamaoto.2013.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]