Abstract
Poor bioavailability of topically instilled drug is the major concern in the field of ocular drug delivery. Efflux transporters, static and dynamic ocular barriers often possess rate limiting factors for ocular drug therapy. Different formulation strategies like suspension, ointment, gels, nanoparticles, implants, dendrimers and liposomes have been employed in order to improve drug permeation and retention by evading rate limiting factors at the site of absorption. Chemical modification such as prodrug targeting various nutrient transporters (amino acids, peptide and vitamin) has evolved a great deal ofintereSt to improve ocular drug delivery. In this review, we have discussed various prodrug strategies which have been widely applied for enhancing therapeutic efficacy of ophthalmic drugs. The purpose of this review is to provide an update on the utilization of prodrug concept in ocular drug delivery. In addition, this review will highlight ongoing academic and industrial research and development in terms of ocular prodrug design and delivery.
Keywords: Prodrug, transporter, receptor, drug delivery
1. INTRODUCTION
Systemic or local administrations are the most common route to deliver ocular formulations. Topical administration is favored route due to its localized drug action at anterior segment of the eye. However, poor penetration and rapid loss of therapeutics following its topical administration are the major restrictions of the topical route [1, 2]. Several formulation approaches (solutions, ointments, gels, microparticles, nanoparticles, and micelles) have been developed to address issue associated with poor ocular bioavailability at the site of action following topical instillation of therapeutics. Besides these formulation approaches, chemical approach such as prodrug has been utilized to optimize physicochemical and biochemical properties of a drug molecule for increasing its ocular bioavailability [3].
Prodrug design is a chemical approach to deliver parent drug molecule in order to achieve improved drug absorption. It is an effective way to deliver those drug moieties which otherwise do not possess optimal ocular bioavailability due to several physiological (biological barriers) and physicochemical (parent drug solubility) restrictions [2, 3]. The rationale of this review is to provide an update on the utilization of prodrug strategy in ocular drug delivery. In addition, this review will summarize the current academic and industrial research progress in terms of ocular prodrug design and delivery.
2. OCULAR PRODRUG DESIGNED CONSIDERATION
Prodrug concept has tum out to be an important part of the ocular drug design and delivery. Synthesizing prodrugs which accomplish most if not all requirements of an ideal formulation is very challenging. Important considerations while designing ophthalmic prodrugs are:
Parent drug must hold functional group susceptible to chemical derivatization
Chemical modification at the functional group site of parent drug must be reversible.
Parent drug, prodrug, and the pro-moiety attached to parent compound must be safe and non-toxic. Pro-moiety should exert rapid elimination from the body. Generally amino acid, small peptide or vitamins has been used as a pro-moiety which are very safe and easily removable natural body substrates.
In vivo prodrug bioreversion must be governed by functionally active biological enzymes such as esterase and peptidase. The rate of bioreversion should be optimized in order to avoid pro-moiety detachment and parent drug release at non-target site.
Prodrug must possess sufficient shelf life and stability in final formulation.
The majority of ocular preparations are delivered in the form of liquid such as eye drops. Hence, aqueous solubility of prodrug is a critical parameter to consider when parent drug is lipophilic in nature and possess low water solubility.
Prodrug should also possess optimal lipophilicity in order to accomplish higher diffusion across lipophilic ocular barriers (dynamic and static).
Final prodrug should have ability to evade unfavorable physicochemical as well as biopharmaceutical properties of a parent molecule. In addition to resolving formulation issues associated with drug, prodrug should also exhibit high affinity and site specific delivery of parent drug molecule. These characteristics will not only overcome side effects associated with parent molecules but it will also help reducing dose of final formulation [1-3].
3. OCULAR PRODRUG STRATEGIES
3.1. Functional Group Approach
The common functional groups that have been utilized in ophthalmic prodrug design are carboxylic, hydroxyl, amine, and carbonyl groups. Modification of these functional groups which includes esters [4-6], carbamates [7], phosphates [8-11] and oximes [12, 13] results in ophthalmic prodrugs. Table 1 represents such prodrug structures of the most common functionalities.
Table 1.
Ophthalmic Prodrug Design from Most Common Functionalities
| Prodrug | Functional Groups |
Prodrug form | Drugs | Hydrolytic Enzyme |
Comments |
|---|---|---|---|---|---|
| Ester | -OH -COOH |
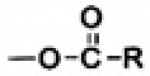
|
Antimetabolites [27] Prostaglandins [26] |
Esterases [30] | Enhanced corneal penetration and IOP reduction [17], chemical instability in aqueous solution [14] |
| Phosphate Ester | -OH |
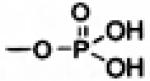
|
Vidarabin[71] Cannabinoids [11] |
Phosphatases [39] | Enhanced aqueous solubility [8], excellent chemical stability and high susceptibility to enzymatic reconversion [40] |
| Carbamate Ester | -NH2 |
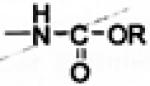
|
Timolol [7] | Esterases [7] | Enhanced corneal penetration [7], high enzymatic stability [14] |
| Oxime |
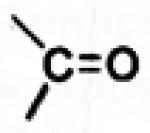
|
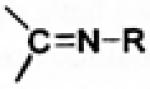
|
β-adrenergic blockers [56] |
Oxime hydrolase, Ketone reductase [56] |
Prolonged IOP reduction and improved therapeutic index [51], site-specific en- zyme activity [56] |
| Oxazolidines |
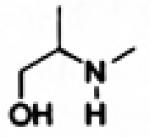
|
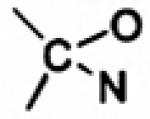
|
Phenylephrine [72] | - | Enhanced corneal penetration and im- proved therapeutic index, poor aqueous stability [72] |
| N-sulfonyl imidates |
—SO2NH2 |
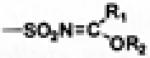
|
Carbonic anhydrase inhibitors [73] |
- | Enhanced aqueous solubility, slow hydro- lytic rate and poor aqueous stability [73] |
3.1.1. Ester Prodrugs
The most common ophthalmic prodrugs developed so far are esters derived from either COOH or OH functional group present in the drug molecules. Usually COOH or OH functional group in drug molecules exist in ionized form under physiological conditions which does not favor drug passage through the lipid membrane, resulting in inadequate drug bioavailability. Appropriate esterification of active molecules with other pro-moieties can generate ester derivatives with desirable hydrophilicity, lipophilicity and in vivo lability [14]. As shown in Fig. (1), ‘A’ type ester prodrugs can be prepared from carboxylic acid drugs and alcohol promoieties, whereas ‘B’ type of ester prodrugs can be derivitized from alcohol drugs and acid promoieties. To synthesize ester prodrugs mostly Steglich esterification reaction conditions have been applied with N,N′-Dicyclohexy-lcarbodiimide (DCC) or Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) as a coupling reagent and 4-Dimethyl-aminopyridine (DMAP) as a catalyst [15].
Fig. (1).
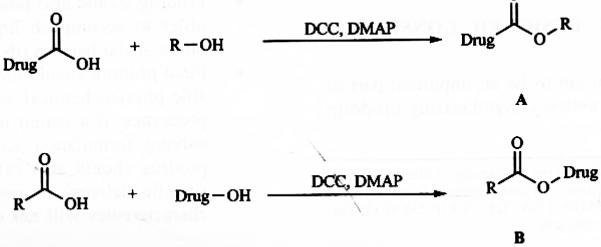
Ester prodrugs from COOH/OH functionalities.
In 1976, Hussain et al. [16] reported the first ophthalmic prodrug dipivefrin, where two hydroxyl functional groups of epinephrine have been esterified to prepare dipivalyl epinephrine. Dipivefrin showed enhanced corneal penetration with I0 times improved therapeutic index compared to epinephrine [17, 18]. Since then, a plethora of ester prodrugs including adrenergic agonists [19, 20], adrenergic antagonists [21, 22], cholinergics [23, 24], carbonic anhydrase inhibitors [25], prostaglandins [5, 26], antimetabolites [6, 27] and steroids [28, 29] have been prepared and their pharmacokinetic behaviors have been thoroughly explored Ester prodrugs can enhance corneal penetration with improved therapeutic index. However, in some instances these components appear to be chemically instable in aqueous eye drop formulations.
Ester prodrugs can be converted back to active parent drugs via esterases present in the eyes. Esterases appear to be to be concentrated in the iris-ciliary body, corneal epithelium, retina and optic nerve. Different classes of esterases i.e., acetylcholine esterase, pseudocholine esterase, butyryl-choline esterase and carboxyl esterases are responsible for facile conversion of the ester prodrugs to parent drugs [30]. Enzyme catalyzed ester hydrolysis is highly dependent on the acyl and the alcohol moieties surrounding the cleavable ester bond. Sterically unhindered straight chain aliphatic esters of timolol i.e o-acetyl/o-propionyl/o-butyryl timolol can be rapidly hydrolyzed both enzymatically and chemically, whereas sterically hindered esters such as the 1′-methylcyclopropanoyl, cyclopropanoyl, 3,3-dimethylbutyryl derivatives are stable enough to generate aqueous solutions with shelf-lives larger than two years at 10-15°C [31].
3.1.1.A. Ester Prodrugs from OH Functionalities of Drugs
Case Study 1
Ganciclovir (GCV) is a promising antiviral compound which exhibits significant activity against human cytomcgalovirus. However the low partition coefficient of GCV results in poor ocular bioavailability. In order to improve corneal permeation of GCV following topical administration, Mitra et al. have reported short chain lipophilic mono-ester prodrugs of GCV with varying side chain from one carbon to four carbon atoms (Fig. 2). GCV mono-ester prodrugs have shown increased corneal permeability compared to parent GCV. Corneal permeability has been found to increase disproportionately with increasing side chain length or lipophilicity of the prodrugs. GCV monovalerate containing four carbon side chains have shown highest permeability with a six fold increase compared to parent GCV. These prodrugs undergo facile enzymatic hydrolysis to active GCV in cornea, leading to lower prodrug concentration in this tissue and therefore generating high driving force for prodrug diffusion. Thus prodrug permeability exhibits a linear relationship with their hydrolysis rate. The hydrolysis rate in corneal homogenate and iris ciliary body has been also found to increase with as carbon chain length ascends from acetate to valerate ester [27, 32].
Fig. (2).
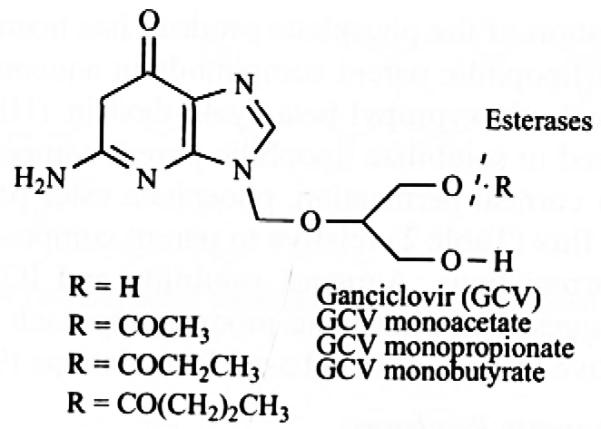
Monoester prodrugs of Ganciclovir.
3.1.1B. Ester Prodrugs from COON Functionalities of Drugs
Case Study 2
Prostaglandin analogs, PGF2α have been widely used as ocular hypotensive agents. In carboxylic acid forms of these compounds exhibit poor permeability and cause irritation to the eye. The carboxylic acid functionality of prostaglandins (PGF2α and its analogs) has been utilized to develop various alkyl/aryl ester prodrugs which resulted in 2-3 fold higher lipophilicity and 25-40 fold enhanced in vitro corneal permeability. These agents were found to be 10-30 times more potent ocular hypotensive agent than their parent molecules. However, due to ocular side effects such as conjunctival hyperemia and superficial irritation these prodrugs have limited clinical application [33-35]. Later on, two isopropyl ester derivatives of modified PGF2α analogs have been developed (latanoprost, travoprost) where the modification was made on one of the side chain (omega chain) of PGF2α backbone by attaching phenyl ring at the 17-position. These prostaglandin analogs exhibit high selectivity to the prostaglandin F receptor (FP receptor) with low affinity toward non-specific receptors and thereby lower some of the side effects. Latanoprost, travoprost and one other PGF2α analog, unoprostone isopropyl are currently being used clinically (Fig. 3) [26, 36, 37]. Latanoprost and travoprost undergo facile hydrolysis by esterases present in cornea to biologically active latanoprost acid and travoprost acid respectively. Cornea slowly releases latanoprost acid into anterior parts of the eye [36]. The maximum concentration of latanoprost acid has been detected in aqueous humour 1-2 hrs after topical application [38]. Travoprost and other ester prodrugs of PGF2α analog hydrolyze during passage through the cornea by butyrylcholine esterase and carboxyl esterases [39].
Fig. (3).
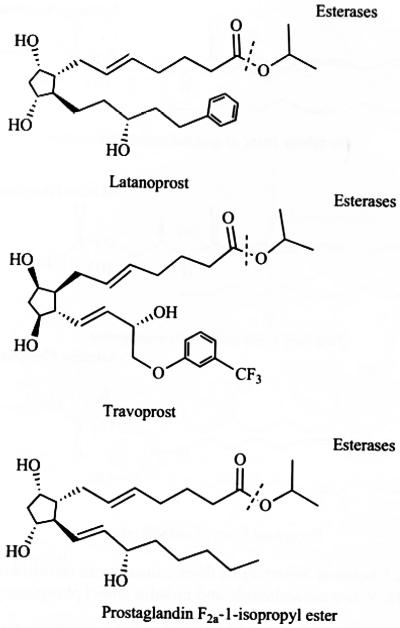
Chemical structure of prostaglandin prodrugs.
3.1.2. Phosphate Ester Prodrugs
Phosphate ester prodrugs are typically designed for hydroxyl functionalities of poorly water-soluble drugs. Presence of dianionic phosphate promoiety in phosphate prodrugs enhances aqueous solubility of the parent drugs [8-11]. These compounds exhibit adequate to excellent chemical stability which opens up the possibility of developing topical eye drop formulations [40]. These prodrugs can be rapidly hydrolyzed to parent drugs by alkaline phosphatases present in the eye tissues [41].
Case Study 3
Most cannabinoids have poor aqueous solubility which limits their application by topical administration. In order to improve aqueous solubility, phosphate ester prodrugs of three cannabinoids (arachidonylethanolamide, R-methanandamide and noladin ether) have been synthesized (Fig. 4) and their physicochemical properties have been studied. These prodrugs have shown significantly enhanced aqueous solubility (Table 2) compared to their parent drugs with adequate chemical stability in buffer solutions [42]. These compounds undergo facile enzymatic hydrolysis on the surface of the cornea by alkaline phosphatase to their lipophilic parent compounds, which subsequently permeate cornea Corneal permeation of the phosphate prodrug has been compared with that of lipophilic parent compounds in aqueous formulations where hydroxypropyl-beta-cyclodextrin (HP-beta-CD) has been used to solubilize lipophilic parent compounds. During in vitro corneal permeation, phosphate ester prodrugs exhibit lower flux (Table 2) relative to parent compounds in HP-beta-CD formulations. Aqueous solubility and IOP reducing efficacy suggest that phosphate prodrug approach is a potential alternative to cyclodextrin based formulations [9].
Fig. (4).
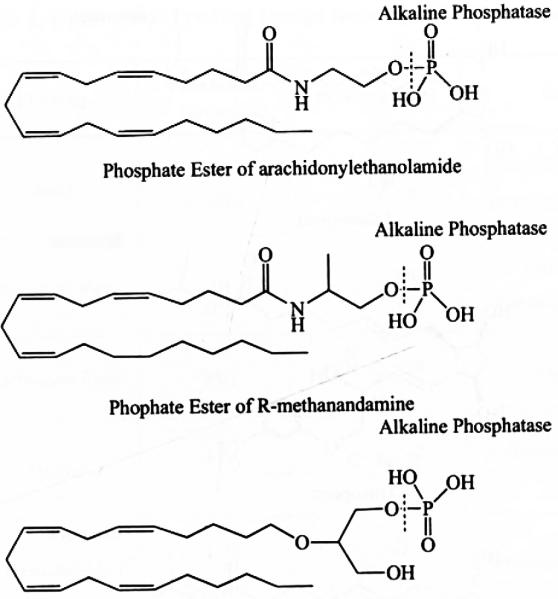
Chemical Structure of three cannabinoid (arachidonylethanolamide, R-methanandamide and noladin ether) phosphate esters.
Table 2.
Aqueous Solubility and Steady State Fluxes of Cannabinoids and their Phosphate Ester Prodrugs [42]
| Compounds | Solubility at pH 7.4 (μg mL−1) |
Steady State Fluxes (nmol (cm2.h)−1 |
|---|---|---|
| Arachidonylethanolamide | 0.4 | 39.30 (5 % CD, Tris buffer) |
| Arachidonylethanolamide phosphate ester | >5000 | 26.66 (Tris buffer) |
| R-methanandamide | - | 54.89 (5 % CD, Tris buffer) |
| R-methanandamide phosphate ester | >5000 | 23.95 (Tris buffer) |
| Noladin ether | <0.1 | 27.61 (5 % CD, Tris buffer) |
| Noladin ether phosphate ester | >5000 | 14.07 (5 % CD, Tris buffer) |
CD:Cyclodextrin
3.1.3. Carbamate Prodrugs
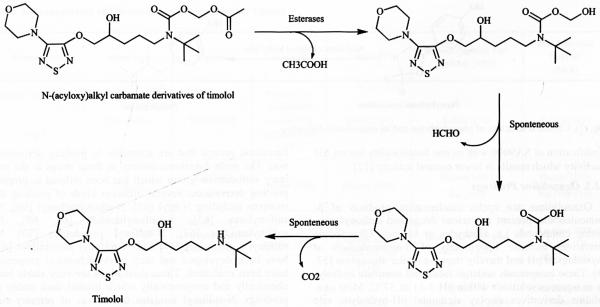
Carbamate prodrugs can be prepared from amine and carboxyl functionalities. Although amines can be easily acylated, carbamate prodrugs are rarely used in ophthalmic delivery because of the relatively high in vivo enzymatic stability [43]. However, the problem can be overcome by introducing an enzymatically labile ester group in the carbamate structure resulting in N-(acyloxy)alkyl carbamates, which are highly stable in aqueous solutions [7]. These compounds also exhibit improved in vitro corneal penetration [44, 45]. N-(acyloxy)alkyl carbamates exhibit high susceptibility to enzymatic bioreversion to the active parent. Esterase-catalzed hydrolysis of terminal ester linkage in these derivatives lead to an unstable (acyloxy)alkyl carbonyl intermediate which undergoes spontaneous decomposition to parent amine via a labile carbamic acid (Fig. 5) [46,47]. However such a prodrug approach has limited applicability to primary amines since N-(acyloxy)alkyl carbonyl derivatives of primary amines undergo intramolecular acyl transfer reaction leading to formation of stable N-acylated derivatives [48].
Fig. (5).
Hydrolysis of N-(acyloxy)alkyl carbamate derivatives of timolol to parent timolol.
Case Study 4
This strategy can be used to prepare N-(acyloxy) alkyl carbamate derivatives oftimolol where secondary amine group of timolol is utilized to make prodrug derivatives. This derivative exhibits 100-500 times faster hydrolysis in plasma than in buffer solution (pH 7.4) at 37°C with high aqueous stability (3-5 years at 4°C, iii 4). Esterase-catalyzed hydrolysis of this prodrug results in a hemiacetal, which spontaneously convert to parent timolol via formation of carbamic acid intermediate (Fig. 5). This prodrug results in five fold enhanced in vitro corneal penetration [7].
3.1.4. Oxime Prodrugs
Oximes are derivatives of ketones, which provide an opportunity to modify drug molecules, lacking hydroxyl, amine or carboxyl functionalities. Several oxime or methoxime derivatives of known β-adrenergic blockers i.e. alprenolol [49], betaxolol [13], propranolol [50] and timolol [51]. have been synthesized and studied as potential antiglaucoma agents [13, 52-55]. Synthesis of these derivatives involves oxidation of secondary hydroxyl functional groups present in the original β-blocker alcohols, using activated dimethyl sulfoxide (Pfitzner-Moffat oxidation) followed by coupling of resulting ketone by additing either hydroxylamine or methoxyamine in the same reaction mixture. Oxime or methoxime derivatives exist in alternative Z (syn) or E (anti) configuration. These compounds exhibit significant and long lasting intraocular pressure (IOP) lowering activity with improved therapeutic index. Oxime prodrugs undergo sequential hydrolysis to parent β-blockers. The activation process involves initial hydrolysis (by oxime hydrolase) of oxime derivatives to ketone which further undergoes enzymatic hydrolysis by ketone reductase to parent β-blockers (S−(−) stereoisomer). Ocular distribution of oxime hydrolase and ketone reductases is primarily limited to iris-ciliary body [56].
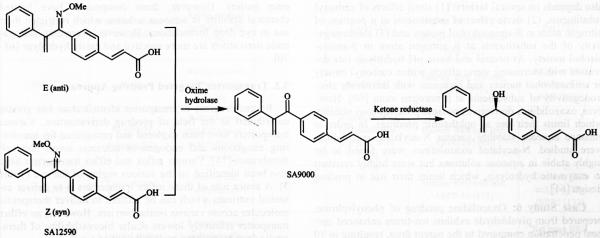
Case Study 5
Ethacrynic acids (ECA) which is a sulfhydryl (SH)-reactive diuretic is a known ocular hypotensive agent. However ECA and ils derivatives (such as SA9000) exhibit corneal toxicity with poor corneal permeability. In an attempt to overcome these barriers, an oxime derivative of SA9000 (Fig. 6) has been developed and IOP reducing efficacy, corneal toxicity, and in vitro SH reactivity of this compound have been studied. This prodrug showed improved IOP reduction and enhanced corneal penetration relative to SA9000 following topical administration. The carbonyl functionality in SA9000 accelerates SH reactivity which leads to protein binding, thereby resulting in corneal toxicity. Modification of SA9000 with oxime functionality lowers SH reactivity which results in lower corneal toxicity [12].
Fig. (6).
Hydrolysis of oxime derivatives (ethacrynic acid a an example) .
3.1.5. Oxazolidine Prodrugs
Oxazolidines are cyclic condensation products of β-aminoalcohols, present in various drugs and carbonyl containing compounds i.e. aldehydes or ketones. Oxazolidine derivatives increase lipophilicity of β-aminoalcohols at physiological pH and thereby improve ocular absorption [57, 58]. These compounds undergo facile and complete hydrolysis in aqueous solutions within pH 1-11 at 37°C. Most oxazolidine derivatives display sigmoidal pH -hydrolytic rate profile where maximum hydrolysis rate is observed at pH 7-7.5 [59]. The rate of hydrolysis of oxaz.olidine derivatives also depends on several factors: (1) steric effects of carbonyl substituents, (2) steric effect of substituents at a position of nitrogen atom in β-aminoalcohol moiety and (3) electronegativity of the substituents at β nitrogen atom in β-aminoalcohol moiety. At neutral and basic pH hydrolysis rate decreases with increasing steric effects within carbonyl moiety or aminoalcohol moiety and increases with increasing electronegativity of substituents at β nitrogen atom [60]. However, oxazolidines derivatives possess poor aqueous stability which limits their use as ophthalmic prodrugs. In order to enhance aqueous stability various N-acylated oxazolidines were studied. N-acylated oxazolidines were found to be highly stable in aqueous solutions but were highly resistant to enzymatic hydrolysis, which limits their use in prodrug design [61].
Case Study 6
Oxazolidine prodrug of phenylephrine, prepared from pivaldehyde exhibits ten-times enhanced corneal penetration compared to the parent drug, resulting in 10 to 15 fold reduction in the phenylephrine dose and thereby reducing the side effects caused by systemic absorption of phenylephrine. In rabbits the prodrug shows 10 fold increase in mydriatic response compared to topically instilled phenylephrine hydrochloride. However it converts to phenylephrine in aqueous solution at pH (1-7.4) with short halflifes (6-13 min) [20, 62, 63]. Hydrolysis rate of phenylephrine oxazolidine has been found to decrease with increasing steric crowding of substituents (R and R′ in Fig. 7) derived from the carbonyl component [57, 64]. Thus the prodrug has to be formulated in a non-aqueous vehicle such as sesame oil for the preparation of phenylephrine oxazolidine eye drops.
Fig. (7).
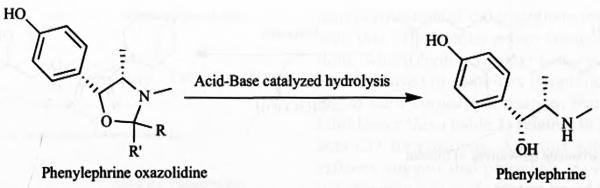
Chemical structure of phenylephrine and its oxazolidine derivative.
3.1.6. Prodrugs Derived from Sulfonamide Functional Groups
Drugs like carbonic acid inhibitors (such as acetazolamide, methazolamide and ethoxyzolamide) do not contain functional groups that are amenable to prodrug derivatization. The main functional moiety in these drugs is the primary sulfonamide group which has been utilized to prepare prodrug derivatives. Several different kinds of prodrug derivatives including N-acyl [65], N-alkoxycarbonyl [66], N-sulfonylurea [67], N-sulfonylimidate [68, 69], N-sulfonylamidine [67], N-sulfonyl pseudourea [70], N-sulfonyl sufoximines [67] and N-sulfonyl sulfilimines [67] have been developed and their physicochemical properties have been evaluated. These derivatives are very stable both chemically and enzymatically which limited their utility as prodrugs. N-sulfonyl imidates derivatives of primary sulfonamide moiety have been shown to readily hydrolyze in aqueous solution to yield sulfonamide and a carboxylic acid ester moiety. However, these compounds have limited chemical stability in aqueous solution which restricted their use in eye drop formulations. However, secondary sulfonamide derivatives are more reactive and easily hydrolyze [68-70].
3.2. Transporter Targeted Prodrug Approach
Recent progress in transporter identification has greatly contributed to the field of prodrug derivatization. Various transporters have been explored and recognized for transferring exogenous and endogenous nutrients across the cell membranes [74]. Various influx and effiux transporters have also been identified on the various region of the eye (Table 3). A major role of these influx transporters is to deliver essential nutrients which can be utilized to deliver therapeutic molecules across various ocular barriers. However, an effiux transporter relatively lowers ocular bioavailability of therapeutic drug by pushing molecules out of a cell.
Table 3.
Transporters and Receptors in Various Ocular Tissues
| Transporters | Substrates | Cornea | Conjunctiva | Retina | Retinal Pigment Epit- helium (RPE) |
Blood-Retinal Barrier (BRB) |
|---|---|---|---|---|---|---|
| Influx Transporters | Dipeptides | Rabbit [90] | Primary cul- tured rabbit conjunctival epithelial cells [100] |
Rabbit [101] | Rabbit [102] | Rabbit [103] |
| PepT1 | ||||||
| GLUT1 | Glucose | Rat [104] Human [105] |
Human [106] | Human [107] | Human [107] Rat [108] |
Human [107] Rat [108-110] |
| ENT1 | Nucleoside | Rabbit [111] Rabbit corneal epithelial cells [112] |
Rabbit [113] | Rabbit [114] Cultured human retinal cell line [115] |
Human RPE cell line, ARPE-19 [114] |
Rat inner BRB cell line, TR- iBRB2 [116] |
| MCTs | Monocarboxylate | Rabbit [117] | - | Rat [118] | Rat [118] Human RPE and A RPE- 19 cells [119] |
Rat inner BRB cell line, TR- iBRB2 [92] |
| SVCT2 | Vitamin C(Ascor- bic acid) |
Rabbit corneal epithelial cells [120] |
- | Rat [121] | Rat [121] Human RPE cells [96] Primary cultures of cat RPE [122] |
Rat [123] |
| SMVT | Biotin | Rabbit corneal epithelial cells [124] |
- | Rabbit [94] | Human RPE cell line, ARPE-19 [94] |
Rat inner BRB cell line, TR- iBRB2[125] |
| Riboflavin | Riboflavin (vitamin B2) |
Rabbit cornea and Rabbit cor- neal epithelial cells [126] |
- | Human-derived retinoblastoma cell line, Y-79 [127] |
Human RPE cell line, ARPE-19[128] |
- |
| LAT1, LAT2 | Large neutral amino acids |
Human and rabbit cornea [129] |
- | Human [130] | Human RPE cell line, ARPE-19 [131] |
Rat inner BRB cell line, TR- iBRB2[132] |
| ASCT1 | Neutral amino acids |
Rabbit corneal epithelial cells and rabbit cornea [133] |
- | - | Rat inner BRB cell line, TR- iBRB2[134] |
|
| B(0,+) | Neutral and cati- onic amino acids |
Human, Rabbit [135] |
Rabbit [136] Rabbit [137] Human [138] |
- | Rat [139] | |
| Reduced-folate transporter (RFT) |
Reduced folate | - | - | Rabbit [140] | Human RPE cells [141, 142] Rabbit [140] Mouse [141] |
Mouse [141] Rat inner BRB cell line, TR- iBRB2[143] |
| Proton-coupled folate transporter (PCFT) |
Folate | - | - | Mouse [144, 145] | Mouse [144] | Rat inner BRB cell line, TR- iBRB2[143] |
| Folate receptor alpha |
Folic acid | - | - | Human [146] Mouse [144] |
Mouse [141, 146] Human RPE cells [141] |
Mouse [141] |
| Transfeirin | Transferrin | Human [147] Bovine [148] |
Human [147] Bovine [148] |
Rat [149] | Human [147, 150] Rat [149, 150] |
Human and Rat [150] |
| Efflux Transporters | Various drugs | Corneal epithe- lial cells, human and rabbit cornea [151] |
Rabbit con- junctival epithelial cells [152] |
Bovine retinal endothelial cells [153] Rabbit retina [83] |
Human RPE cell line, ARPE-19[154] Human RPE [155] |
Mouse [156] |
| P-glycoprotein, MDR 1 | ||||||
| Multidrug resis- tance associated proteins (MRPs) |
Rabbit cornea and human cor- neal cells [157] Freshly excised human corneal epithelial [158] |
Human [157] | - | Human RPE cell line, ARPE-I9[154] |
Mouse [156] | |
| Breast cancer resis- tance protein (BCRP) |
Human corneal epithelial cells [159] Freshly excised human corneal epithelial [158] |
- | - | Human RPE cell line, ARPE-19[149] |
Mouse [151] |
Anticancer [75], antifungal [76], antiviral [77], steroids [78] and fluroquinolones [79, 80] are known substrates of efflux transporters, which lowers ocular bioavailability. Several strategies have been applied to evade drug effiux, among which prodrug derivatization is one of the most successfully utilized approach for improving ocular bioavailability of therapeutic agents.
Prodrugs have been synthesized in such a way that (a) chemically modified drug will have lower affinity towards effiux transporter such as quinidine prodrugs [81-83], or (b) chemically modified drug will have higher affinity towards influx transporter which otherwise are not recognized as such by a transporter such as peptide and amino acid prodrugs (acyclovir [84-86] and ganciclovir [87-89]). Hence higher ocular bioavailability of therapeutic agents can be achieved. In addition to peptide (PepT1) [87, 90], amino acid (LAT1, LAT2, B(0,+)) [84, 91] and monocarboxylic acid (MCT) [92, 93] transporters, recently various vitamin transporters such biotin [94] and ascorbic acid (SVCT2) [95-98] have been utilized for the delivery of various ocular prodrugs.
In transporter targeted prodrug approach a promoiety such as amino acid, peptide or vitamin, which is a substrate of respective transporter, is conjugated to parent drug molecule with ultimate aim of improving its bioavailability at the target site. Various transporters have been targeted in the eye for improving drug bioavailability following topical route. For successful prodrug delivery an ideal transporter must be highly expressed at desired ocular region in order to facilitate optimal and rapid drug uptake. In addition, it must have high capacity to avoid inhibition of an excess prodrug or nutrients recognized by the same transporter. This approach has distinct feature relative to other approaches as the prodrug is specifically recognized by a particular transporter expressed on the cell surface.
3.3. Receptor Targeted Prodrug Approach
In addition to transporter targeted delivery, drug targeting to specific receptor using carrier mediated absorption is emerging as a clinically significant approach. Receptors useful for prodrug targeting have been identified in various region of the eye (Table 3). Receptors are responsible for the internalization of nutrients, such as folate, vitamin B12 and transferrin. Due to the importance of these receptors, numbers of investigator have examined the use of drug-receptor conjugation for drug delivery and drug targeting. Internalization of such conjugates has been achieved successfully by receptor-mediated endocytosis. So far folate receptor has been utilized as an ideal candidate for tumor targeted drug delivery but less attention has been given to receptor theory for ocular drug delivery [160]. Recently our laboratory has started working on the extension of targeted prodrug approach by synthesizing receptor targeted prodrugs for ocular drug delivery. However, much attention is needed to explore and extend receptor based prodrug approach.
3.4. Stereoisomeric Dipeptide Prodrug Approach
An idea of modulating the enzymatic hydrolysis rate of prodrugs and its implications in drug delivery is a growing concept which has high clinical significance. Extended availability of intact prodrug at the target site is a crucial requirement for effective drug absorption and higher bioavailability. Systemic drug delivery (intravenous or oral) is a potential route for the treatment of various ophthalmic disorders [161]. Transporter targeted prodrug strategy has been utilized to increase the ocular bioavailability of various drug molecules following systemic administration [99, 103, 162-164]. Among all, peptide transporter (PepT) was utilized most significantly for dipeptide prodrug delivery due to availability of this transporter at various ocular tissues (Table 3).
A major problem associated with orally administered dipeptide prodrugs is their rapid metabolism into parent compound resulting in limited availability of intact prodrug at transporter site of target ocular tissue [162, 165, 166]. Our laboratory has addressed this problem by designing stereoisomeric dipeptide prodrug for enhancing residence time of intact prodrug in the systemic circulation so that its translocation by ocular influx transporter can be maximized. Basic theory behind this concept was to synthesize enzymatically stable prodrugs by averting its early hydrolysis and elevating oral as well as ocular bioavailability after oral administration [167]. Hydrolytic enzymes (peptidases and esterases) responsible for the bioreversion of dipeptide prodrugs are stereospecific and have high affinity for L-isomers. All dipeptide prodrugs studied so far were based on L-amino acid isomers, which are natural substrates for these enzymes. Talluri et al. have designed series of stereoisomeric prodrug by incorporating D-isomers into the dipeptide moieties at a particular position to modulate its rate of metabolism. Results from this work were comparable with other studies and provides evidence that incorporation of one D-amino acid into a dipeptide does not eradicate its affinity towards PepT transporter [168-170]. Moreover, it also possesses higher stability against metabolizing enzymes which could result in higher cellular permeability (Table 4). Furthermore it was observed that inclusion of two D-amino acid into a dipeptide moiety can not only increase the enzymatic stability but simultaneously abolishing its affinity towards PepT transporter [167, 171]. This novel concept has shown that the metabolic stability as well as the cellular permeability can be modulated with the incorporation of a D-isomer of an aminoacid at a definite position into a dipeptide conjugate. This idea can be further extended to a range of therapeutic molecules particularly for enhancing their tissue bioavailability .
Table 4.
Half lives of Stereolsomeric Acyclovir (ACV) Dipeptide Prodrugs In Tissue and Cell Homogenates (Table Regenerated from Reference [167])
| Prodrug | t½ (hrs) | ||
|---|---|---|---|
| Rat Liver Homogenate |
Rat Intestinal Homogenate | Caco-2 cell Homogenate |
|
| LLACV | <0.08 | <0.08 | 7.52 ± 0.40 |
| LDACV | 0.49 ± 0.02 | 1.01 ± 0.07 | 52.80 ± 8.42 |
| DLACV | 2.82 ± 0.18 | 6.27 ± 0.25 | no degradation |
| DDACV | no degradation | no degradation | no degradation |
3.5. Lipid Prodrug
Molecules can cross cell membranes through passive diffusion. In the eye, drug absorption takes place either through corneal route (cornea-aqueous humor-intraocular tissues) or non-corneal route (conjunctiva-sclera-choroid/RPE) [172]. Due to lipophilic nature of cornea and other intraocular tissues, both hydrophilic and hydrophobic drugs take transcellular pathway to cross ocular membrane. In addition to these, sustained drug delivery for prolonged periods of time at the target site is required especiaDy for the treatment of eye diseases at posterior segment of eye like vitreous, retina and choroid. In order to improve lipophilicity of hydrophilic drug molecules and hence to improve corneal permeation, the lipid prodrug approach has been developed Lipid prodrugs are chemical entities where a drug molecule is covalently bound to a lipid moiety, such as fatty acid, diglyceride or phosphoglyceride. Lipid prodrugs diffuse readily across cell membrane by facilitated diffusion and thereby result in improved cellular absorption (Table 5). It also shows sustained delivery of parent drug molecule at the site of action [173, 174].
Table 5.
Recent Trends in Lipid Prodrug Derivatization
| Drug | Chemical Modification | Disease | Inferences | References |
|---|---|---|---|---|
| Cidofovir (CDV) | Hexadecyloxypropyl (HDP- CDV) and Octadecyloxyethyl (ODE-CDV) ester derivatives |
Poxvirus, Herpesvirus, Adenovirus, Polyomavi- rus |
Immensely improved antiviral efficacy (EC50) from μMrange (for parent compound) to nM range (for lipid conjugates) |
[165, 178-183] |
| Ganciclovir (GCV) |
1-O-hexadecylpropanediole- 3-phospho-ganciclovir (HDP- p-GCV) |
Herpes simplex virus (HSV), Cytomegalovirus (CMV) in retinitis |
Prolonged in vivo antiviral activity (for 4-6 weeks) with no significant toxicity relative to parent com- pound (less than 1 week) upon intravitreal injec- tion |
[184] |
| Elicidic acid conjugates of GCV (E-GCV) |
Human cytomegalovirus (HCMV), Herpes sim- plex virus (HSV) and varicella zoster virus (VZV) |
Improved in vitro efficacy (5-30 fold reduction in antiviral dose) against HCMV and HSV. E-GCV compared to GCV at equimolar doses proved more efficacious with reduction of mortality rate. |
[185] | |
| Foscamet or Phosphonoformic acid (PFA) |
1-O-octadecyl-sn-glycerol-3- phosphonofonnate (ODG- PFA) |
Human cytoinegalovirus (HCMV) |
ODG-PFA had longer vitreous half life and sus- tained drug level in retina at the end ofte nth week after intravitreal injection (concentration of 32 μM at the 10th week was 10imes higher than IC90 value against HCMV for foscamet) in rabbits. |
[186] |
| Peptide nucleic acid (PNA) |
Cationic peptide-decanoic acid-Nuclear Antisense |
- | Nuclear antise nse activity of peptide nucle ic acid was higher up to 2 ordecs of magnitude for cationic peptide-decanoic acid-nuclear antise nse compare to peptide nucleic acid alone. |
[187] |
However high lipophilicity of molecules can result in limited permeability as it will stick inside the lipid membrane of cornea. Schoenwald and Ward reported a parabolic relationship between the lipophilicity and permeability of drug molecules across the rabbit cornea. Maximal permeability is observed for prodrugs with log P value of about 2-4 where P is defined as octanol/pH 7.4 buffer partition coefficients [22]. So depending on the hydrophilicity of each drug molecule, lipid chain length needs to be adjusted in order to get maximum permeability across the cornea. Intraocular permeation can be further enhanced by conjugating a targeting moiety (receptor/transporter) at one end of lipid prodrug, which is being currently explored (Patent: WO 2009/158633 A1) in our laboratory.
Case Study 7
5-Fluorouracil (5-FU) is an antimetabolite which has failed to exhibit significant benefit to intraocular cell proliferation, resulting from many vision threating vitre-oretinal diseases as it has short vitreous half-life after perioperative infusion [175]. Therefore 5-FU implant is required in proliferative vitreoretinopathy [176]. Recently Cheng et al. has reported two lipid derivatives of 5-Fluorouracil nucleoside analog, 2′-deoxy-5-fluorouridine (5-F-2dUrd) in order to achieve sustained intravitreal drug release, and thereby achieving drug delivery by simple intravitreal injection (Fig. 8). An alkoxyalkyl phospholipid residue is covalently anchored to 5-F-2dUrd to obtain hexa-decyloxypropyl 5-fluoro-2′-deoxyuridine 5′-monophosphate (HDP-P-5F-2dUrd), and hexadecyloxypropyl 5-fluoro-2′-deoxyuridine 3′,5′-cyclic monophosphate (HDP-cP-5-F-2dUrd).Compared to 5-FU both lipid prodrugs exhibit longer vitreous half life with higher non-toxic dose. In addition, the potency of these prodrugs against cell proliferation jumped 11.6 times and 3.5 times for HDP-P-5F-2dUrd and HDP-cP-5-F-2dUrd respectively [177].
Fig. (8).
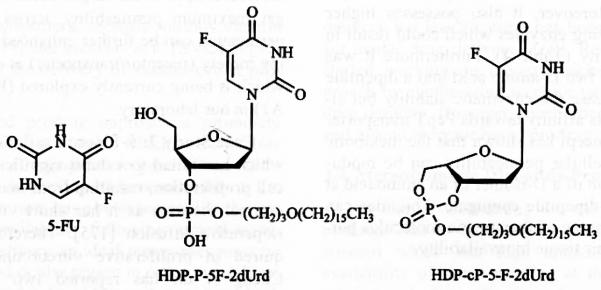
Chemical Structure of lipid prodrugs of 5-Fluorouracil.
Conjugated lipid chain with 5-F-2dUrd enhances cellular uptake of lipid prodrugs by inner limiting membrane. Inside the cells these lipophilic nucleotide converts back to the corresponding nucleoside triphosphate which exhibit antiproliferative activity.
4. RECENT FORMULATION STRATEGIES FOR OCULAR PRODRUG DELIVERY
Sustain drug concentration at desired site is an important feature of ocular delivery. In addition to prolong drug release, sustain delivery formulations can also utilize to prevent drug loss from pre-corneal site. Formulations such as microparticles [194], nanoparticles [28, 195], and liposomes [196] have been utilized with an aim to delivery prodrug for achieving sustained drug delivery at ocular sites. These colloidal formulations can be delivered alone or by suspending into a gel in order to modify drug release at various ocular sites. Solid lipid microparticles have been designed with an aim to improve stability of encapsulated dopamine prodrug in physiological environment Solid matrix of lipid microparticles has also provided the sustained delivery of dopamine prodrug for longer period of time [194]. Iwala et a1. have demonstrated the sustained release of dipeptide prodrugs of acyclovir (ACV) encapsulated into nanoparticle formulation. In this study, stereoisomenc dipeptide (L-valine-L-valine and L-valine-D-valine) prod rugs of acyclovir encapsulated into PLGA nanoparticles have shown ideal biphasic drug release. Moreover, nanoparticle formulations suspended in thermosensitive gels were able to prolong the release of ACV pro drugs by eliminating initial burst release [195]. In another study, Gaudana et al. have demonstrated the role of hydrophobic ion pairing (HIP) complexation for improving prodrug entrapment in nanoparticles. Dipeptide prodrug of dexamethasone was complexed using dextran SUlphate as complexing polymer. This novel principle of IDP complexation has significantly enhanced entrapment of dexamethasone prodrug in nanoparticles by overcoming partitioning limitation of hydrophilic prodrug [28]. Prodrug retention at pre-corneal and vitreous site bas been improved by delivering intravitreal injection of liposome containing tilisolol prodrug [196]. These approaches can have particular importance to treat posterior segment eye disease such as diabetic retinopathy and age-related macular degeneration [197].
5. OCULAR PRODRUG PATENTS
Pharmaceutical companies and academic organizations have implemented prodrug strategies to overcome the recent ophthalmic delivery challenges which are confirmed by the trends seen in the published and filed U.S. patents. It is beyond the scope of this review to cover all advancements in the field of ocular prodrug design and hence, we have summarized recent patents published in the field of ocular prodrug delivery in Table 6.
Table 6.
Recent Ocular Prodrug Patents
| Inventor | Drug | Promoiety | Disease | United States Patent |
|---|---|---|---|---|
| Allergan | Dexamethasone, bimatoprost |
amino acid, peptide, monocar- boxylic acid, organic anion, cation nucleoside |
posterior segment eye disease |
7714024 [188] |
| Allergan | anti-glaucoma, ocular hy- potensive compounds |
acetylcholinester, psuedoacetylcholine |
glaucoma | 6350780[189] |
| University of Georgia Research Foundation |
etoposide, vincristine, fluo- cinolone and other steroids |
carotenoid (zeaxanthine) | macular and retinal disease |
20070259843[190] |
| Novagali Pharnia SA | steroids | lipophilic ester | posterior segment eye disease |
20070280995[191] |
| University of Florida and University of North Texas Health Science Center |
steroidal quinol compounds | phosphate or tertiary amide ester |
cataract or glaucoma | 7572781 [192] |
| University of Missouri Kansas City |
acyclovir and ganciclovir | dipeptide, tripeptide ester | herpes virus infection | WO 2003/03048190 |
| University of Missouri Kansas City |
nucleoside, nucleotide, oligonucleotide, peptide |
lipophilic linker | ocular diseases | WO 2009/158633 A1 |
| Cellgate, Inc. | anti-bacterial, anti-viral, anti-fungal |
guanidine, amidino, arginine | ocular infections | 7229961[193] |
6.CONCLUSION
Prodrug derivatization is an adaptable method that can be applicable for series of parent drug molecule. For successful prodrug utilization, recognition of drug properties and participation of barriers at target site are critical factors. Most of the prodrugs are used for improving drug penetration by enhancing lipophilicity and more recently by modulating aqueous solubility. Prodrug strategy has revealed promising outcome for the delivery of ophthalmic drugs. The recent progress in the field of prodrug design holds a promising future for ophthalmic drug delivery. Prodrugs have become an integral part of the drug design and delivery process, as exemplified by the growing number of approved prodrugs and patents. Growing utilization of coherent prodrug approach at the initial phase of drug discovery will lead to the development of composite with improved physicochemical properties.
ACKNOWLEDGEMENTS
This work was supported by NIH grants ROI EY 09171-16 and ROI EY 10659-14.
REFERENCES
- [1].Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci. 2006;95(6):1177–95. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- [2].Testa B, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism. Wiley-VCH Verlag GmbH; Weinheim: 2003. [Google Scholar]
- [3].Kwatra D, Vaishya R, Gaudana R, Jwala J. In: Prodrugs and Targeted Delivery Towards Beller ADME Properties. Rautio Jarkko., editor. Vol. 47. Wiley-VCH; Germany: 2011. pp. 181–201. [Google Scholar]
- [4].Faulkner R, Sharif NA, Orr S, Sall K, Dubiner H, Whitson JT, Moster M, Craven ER, Curtis M, Pailliotet C, Martens K, Dahlin D. Aqueous humor concentrations of bimatoprost free acid, bimatoprost and travoprost free acid in cataract surgical patients administered multiple topcal ocular doses of LUMIGAN or TRAVATAN. J. Ocul. Pharmacal. Ther. 2010;26(2):147–56. doi: 10.1089/jop.2009.0098. [DOI] [PubMed] [Google Scholar]
- [5].Fukano Y, Kawazu K. Disposition and metabolism of a novel prostanoid antiglaucoma medication, tafluprost, following ocular administration to rats. Drug. Metab. Dispos. 2009;37(8):1622–34. doi: 10.1124/dmd.108.024885. [DOI] [PubMed] [Google Scholar]
- [6].Majumdar S, Hingorani T, Srirangam R, Gadepalli RS, Rimoldi JM, Repka MA. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: delineation of passive diffusion versus tranporter involvement. Pharm. Res. 2009;26(5):1261–9. doi: 10.1007/s11095-008-9730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alexander J, Cargill R, Michelson SR, Schwam H. (Acyloxy)alkyl carbamates as novel bioreversible prodrugs for amines: increased permeation through biological membranes. J. Med. Chem. 1988;31(2):318–22. doi: 10.1021/jm00397a008. [DOI] [PubMed] [Google Scholar]
- [8].Lallemand F, Varesio E, Felt-Baeyens O, Bossy L, Hopfgartner G, Gurny R. Biological conversion of a water-soluble prodrug of cyclosporille A. Eur. J. Pharm. Biopharm. 2007;67(2):555–61. doi: 10.1016/j.ejpb.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [9].Juntunen J, Jarvinen T, Niemi R. In-vitro corneal permeation of cannabinoids and their water-soluble phosphate ester prodrugs. J. Pharm. Pharmacol. 2005;57(9):1153–7. doi: 10.1211/jpp.57.9.0009. [DOI] [PubMed] [Google Scholar]
- [10].Mäntylä A, Gamier T, Rautio J, Nevalainen T, Yepsälainen J, Koskinen A, Croft SL, Järvinen T. Synthesis, in vitro evaluation, and antileishmanial activity of water-soluble prodrugs of buparvaquone. J. Med. Chem. 2004;47(1):188–95. doi: 10.1021/jm030868a. [DOI] [PubMed] [Google Scholar]
- [11].Juntunen J, Huuskonen J, Laine K, Niemi R, Taipale H, Nevalainen T, Pate DW, Jarvinen T. Anandamide prodrugs. 1. Water-soluble phosphate esters of arachidonylethanolamide and R-methanandamide. Eur. J. Pharm. Sci. 2003;19(1):37–43. doi: 10.1016/s0928-0987(03)00044-7. [DOI] [PubMed] [Google Scholar]
- [12].Shimazaki A, Kirihara T, Rao PV, Tajima H, Matsugi T, Epstein DL. Effects of the new ethacrynic acid oxime derivative SA12590 on intraocular pressure in cats and monkeys. Biol. Pharm. Bull. 2007;30(8):1445–9. doi: 10.1248/bpb.30.1445. [DOI] [PubMed] [Google Scholar]
- [13].Farag HH, Wu WM, Barros MD, Somogyi G, Prokai L, Bodor N. Ocular-specific chemical delivery systems of betaxolol for safe local treatment of glaucoma. Drug Des. Discov. 1997;15(2):117–30. [PubMed] [Google Scholar]
- [14].Järvinen T, Niemi R. In: Prodrug Approaches to Ophthalmic Drug Delivery. Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J, editors. V. Springer; New York: 2007. pp. 125–155. [Google Scholar]
- [15].Neises B, Angew WS. Simple method for the esterification of carboxylic acids bernhard neises and wolfgang steglich. Chem. Int. Ed. 1978;17:522. [Google Scholar]
- [16].Hussain A, Truelove JE. Prodrug approaches to enhancement of physicochemical properties of drugs IV: novel epinephrine prodrug. J. Pharm. Sci. 1976;65(10):1510–2. doi: 10.1002/jps.2600651023. [DOI] [PubMed] [Google Scholar]
- [17].Wei CP, Anderson JA, Leopold I. Ocular absorption and metabolism of topically applied epinephrine and a dipivalyl ester of epinephrine. Invest. Ophthalmol. Vis. Sci. 1978;17(4):315–21. [PubMed] [Google Scholar]
- [18].Mandell AI, Stentz F, Kitabchi AE. Dipivalyl epinephrine: a new pro-drug in the treatment of glaucoma. Ophthalmology. 1978;85(3):268–75. doi: 10.1016/s0161-6420(78)35668-2. [DOI] [PubMed] [Google Scholar]
- [19].Niemi R, Huuskonen J, Laine K, Jarvinen T. Synthesis, hydrolysis, and intraocular pressure lowering effects of fadolmidine prodrugs. Int. J. Pharm. 2005;295(1-2):121–7. doi: 10.1016/j.ijpharm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [20].Chien DS, Schoenwald RD. Improving the ocular absorption of phenylephrine. Biopharm. Drug Dispos. 1986;7(5):453–62. doi: 10.1002/bdd.2510070506. [DOI] [PubMed] [Google Scholar]
- [21].Kawakami S, Ohshima N, Hirayama R, Al MH, Kitahara T, Sakaeda T, Mukai T, Nishida K, Nakamura J, Nakashima M, Sasaki H. Biodistribution and pharmacokinetics of O-palmitoyl tilisolol, a lipophilic prodrug of tilisolol, after intravenous administration in rats. Bioi. Pharm. Bull. 2002;25(8):1072–6. doi: 10.1248/bpb.25.1072. [DOI] [PubMed] [Google Scholar]
- [22].Chang SC, Bundgaard H, Buur A, Lee VH. Improved corneal penetration of timolol by prodrugs a a means to reduce systemic drug load. Invest. Ophthalmol. Vis. Sci. 1987;28(3):487–91. [PubMed] [Google Scholar]
- [23].Subonen P, Jarvinen T, Koivisto S, Urtti A. Different effects of pH on the permeation of pilocarpine and pilocarpine prodrugs across the isolated rabbit cornea. Eur. J. Pharm. Sci. 1998;6(3):169–76. doi: 10.1016/s0928-0987(97)10002-1. [DOI] [PubMed] [Google Scholar]
- [24].Jarvinen T, Auriola S, Peura P, Suboneo P, Urtti A, Vepsalainen J. Synthesis and identification of pilocarpic acid diesters, prodrugs of pilocarpine. J. Pharm. Biomed. Anal. 1991;9(6):457–64. doi: 10.1016/0731-7085(91)80247-7. [DOI] [PubMed] [Google Scholar]
- [25].Woltersdorf OW, Jr., Schwam H, Bicking JB, Brown SL, deSolms SJ, Fishman DR, Graham SL, Gautheron PD, Hoffman JM, Larson RD. Topically active carbonic anhydrase inhibitors. I. O-acyl derivatives of 6-hydroxybenzothiazole-2-sulfonamide. J. Med. Chem. 1989;32(11):2486–92. doi: 10.1021/jm00131a011. [DOI] [PubMed] [Google Scholar]
- [26].Russo A, Riva I, Pizzolante T, Noto F, Quaranta L. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin. Ophthalmol. 2008;2(4):897–905. [PMC free article] [PubMed] [Google Scholar]
- [27].Macha S, Duvvuri S, Mitra AK. Ocular disposition of novel lipophilic diester prodrugs of ganciclovir following intravitreal administration using microdialysis. Curr. Eye Res. 2004;28(2):77–84. doi: 10.1076/ceyr.28.2.77.26233. [DOI] [PubMed] [Google Scholar]
- [28].Gaudana R, Pareoky A, Vaishya R, Samanta SK, Mitra AK. Developmeor and characterization of nanoparticulate formulation of a water soluble prodrug of dexamethasone by HIP complexation. J. Microencapsul. 2011;28(1):10–20. doi: 10.3109/02652048.2010.520093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Civiale C, Bucaria F, Piazza S, Peri O, Miano F, Enea V. Ocular permeability screening of dexamethasone esters through combined cellular and tissue systems. J. Ocul. Pharmacol. Ther. 2004;20(1):75–84. doi: 10.1089/108076804772745482. [DOI] [PubMed] [Google Scholar]
- [30].Duvvuri S, Majumdar S, Mitra AK. Role of metabolism in ocular drug delivery. Curr. Drug Metab. 2004;5(6):507–15. doi: 10.2174/1389200043335342. [DOI] [PubMed] [Google Scholar]
- [31].Bundgaard H, Buur A, Chang SC, Lee VHL. Timolol prodrugs: synthesis, stability and lipophilicity of various alkyl, cycloalkyl and aromatic esters of timolol. Int. J. Pharm. 1988;46:77–88. [Google Scholar]
- [32].Tirucherai GS, Dias C, Mitra AK. Corneal permeation of ganciclovir: mechanism of ganciclovir permeation enhancement by acyl ester prodrug design. J. Ocul. Pharmacol. Ther. 2002;18(6):535–48. doi: 10.1089/108076802321021081. [DOI] [PubMed] [Google Scholar]
- [33].Camras CB, Siebold BC, Lustgarten JS, Serle JB, Frisch SC, Podos SM, Bito LZ. Maintained reduction of intraocular pressure by prostaglandin F2 alpha-1-isopropyl ester applied in multiple doses in ocular hypertensive and glaucoma patients. Ophthalmology. 1989;96(9):1329–1337. doi: 10.1016/s0161-6420(89)32717-5. [DOI] [PubMed] [Google Scholar]
- [34].Camber O, Edman P, Olsson LI. Permeability of prostaglandin F2α and prostaglandin F2α esters across cornea in vitro. Int. J. Pharm. 1986;29:259–266. [Google Scholar]
- [35].Villumsen J, Aim A, Soderstrom M. Prostaglandin F2 alpha-isopropylester eye drops: effect on intraocular pressure in open-angle glaucoma. Br. J. Ophthalmol. 1989;73(12):975–9. doi: 10.1136/bjo.73.12.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Suzuki ER, Suzuki CL. Efficacy and safety of travoprost alone or in combination with other agents for glaucoma and ocular hypertension: patient considerations. Clin. Ophthalmol. 2010;4:1165–71. doi: 10.2147/OPTH.S6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Melamed S. Neuroprotective properties of a synthetic docosanoid, unoprostone isopropyl: clinical benefits in the treatment of glaucoma. Drugs Exp. Clin. Res. 2002;28(2-3):63–73. [PubMed] [Google Scholar]
- [38].Sjoquist B, Stjemschantz J. Ocular and systemic pharmacokinetics of latanoprost in humans. Surv. Ophthalmol. 2002;47(Suppl 1):S6–12. doi: 10.1016/s0039-6257(02)00302-8. [DOI] [PubMed] [Google Scholar]
- [39].Camber O, Edman P. Factors influencing the corneal permeability of prostaglandin F2α and its isopropyl ester in vitro. Int. J. Pharm. 1987;37:27–32. [Google Scholar]
- [40].Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug. Discov. 2008;7(3):255–70. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- [41].McComb RB, Bowers GN, Jr, Posen S. Alkaline Phosphatase. Plenum Press; New York and London: 1979. [Google Scholar]
- [42].Juntunen J, Vepsalainen J, Niemi R, Laine K, Jarvinen T. Synthesis, in vitro evaluation, and intraocular pressure effects of water-soluble prodrugs of endocannabinoid noladin ether. J. Med. Chem. 2003;46(23):5083–6. doi: 10.1021/jm030877j. [DOI] [PubMed] [Google Scholar]
- [43].Reddy IK. Ocular theraputics and drug delivery: a multidisciplinary approach. 1st CRC Press; 1995. [Google Scholar]
- [44].Li Z, Bitha P, Lang SA, Lin YI. Synthesis of (alkoxycarbonyloxy)methyl, (acyloxy)methyl and (oxodioxolenyl)methyl carhamates a bioreversible prodrug moieties for amines. Bioorg. Med. Chem. Lett. 1997;7(22):2909–2912. [Google Scholar]
- [45].Alexander J, Cargill R, Michelson SR, Schwam H. (Acyloxy)alkyl carbamates as novel bioreversible prodrugs for amines: increased permeation through biological membranes. J. Med. Chem. 1988;31(2):318–22. doi: 10.1021/jm00397a008. [DOI] [PubMed] [Google Scholar]
- [46].Gogate US, Repta AJ, Alexander J. N-(Acyloxyalkoxycarbonyl) derivatives as potential prodrugs of amines. I. kinetics and mechanism of degradation in aqueous solutions. Int. J. Pharm. 1987;40:235–248. [Google Scholar]
- [47].Gogate US, Repta AJ. N-(Acyloxyalkoxycarbonyl) derivative as potential prodrugs of amines. II. esterase-catalysed release of parent amines from model prodrug. Int. J. Pharm. 1987;40:249–255. [Google Scholar]
- [48].Simplicio AL, Clancy JM, Gilmer JF. Prodrugs for amines. Molecules. 2008;13(3):519–47. doi: 10.3390/molecules13030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bodor N, Elkoussi A. Improved delivery through biological membranes. LVI. Pharmacological evaluation of alprenoxime-a new potential antiglaucoma agent. Pharm. Res. 1991;8(11):1389–95. doi: 10.1023/a:1015849123020. [DOI] [PubMed] [Google Scholar]
- [50].Manna F, Chimenti F, Bolasco A, Lena R, Filippelli A, Falciani M, Fontana M. Beta-adrenoreceptor blocking heterocyclic oximes and ethers. Farmaco. 1996;51(11):699–706. [PubMed] [Google Scholar]
- [51].Bodor N, Farag HH, Somogyi G, Wu WM, Barros MD, Prokai L. Ocular-specific delivery of timolol by sequential bioactivation of its oxime and methoxime analogs. J. OCul. Pharmacol. Ther. 1997;13(5):389–403. doi: 10.1089/jop.1997.13.389. [DOI] [PubMed] [Google Scholar]
- [52].Bodor N. Retrometabolic approaches for drug design and targeting. Pharmazie. 1997;52(7):491–4. [PubMed] [Google Scholar]
- [53].Polgar P, Bodor N. Minimal cardiac electrophysiological activity of alprenoxime, a site-activated ocular beta-blocker, in dogs. Life Sci. 1995;56(14):1207–13. doi: 10.1016/0024-3205(95)00060-j. [DOI] [PubMed] [Google Scholar]
- [54].Bodor N, Prokai L. Site- and stereospecific ocular drug delivery by sequential enzymatic bioactivation. Pharm. Res. 1990;7(7):723–5. doi: 10.1023/a:1015863521513. [DOI] [PubMed] [Google Scholar]
- [55].Bodor N, ElKoussi A, Kano M, Nakamura T. Improved delivery through biological membranes. 26. Design, synthesis, and pharmacological activity of a novel chemical delivery system for beta-adrenergic blocking agents. J. Med. Chem. 1988;31(1):100–6. doi: 10.1021/jm00396a015. [DOI] [PubMed] [Google Scholar]
- [56].Bodor N, Buchwald P. Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. AAPS J. 2005;7(4):E820–33. doi: 10.1208/aapsj070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bundgaard H, Johansen M. Pro-drugs as drug delivery systems XX. Oxazolidines as potential pro-drug types for β-aminoalcohols, aldehyde or ketones. Int. J. Pharm. 1982;10(2):165–175. [Google Scholar]
- [58].Walker RB, Dholakia VN, Brasfield KL, Bakhtiar R. Effect of hydroxypropyl-beta-cyclodextrin on the central stimulant activity of (−)-ephedrine and an oxazolidine prodrug in rats. General Pharmacal. 1998;30(5):725–731. doi: 10.1016/s0306-3623(97)00341-8. [DOI] [PubMed] [Google Scholar]
- [59].Hu L. In: Prodrug approaches to drug delivery. Wang B, Siahaan TJ, Soltero R, editors. John Wiley & Sons Inc.; 2005. pp. 125–165. [Google Scholar]
- [60].Miller-Meeks MJ, Farrell TA, Munden PM, Folk JC, Rao C, Schoenwald RD. Phenylephrine prodrug, report of clinical lrials. Ophthalmology. 1991;98(2):222–6. [PubMed] [Google Scholar]
- [61].Buur A, Bundgaard H. Prodrugs as drug delivery systems. XXVIII. Structural factors influencing the rate of hydrolysis of oxazolidines—a potential prodrug type. Int. J. Pharm. 1984;18(3):325–334. [Google Scholar]
- [62].Schoenwald RD, Folk JC, Kumar V, Piper JG. In Vivo comparison of phenylephrine and phenylephrine oxazolidine instilled in the monkey eye. J. Ocul. Pharmacal. Ther. 1987;3(4):333–340. doi: 10.1089/jop.1987.3.333. [DOI] [PubMed] [Google Scholar]
- [63].Chien DS, Schoenwald RD. Ocular pharmacokinetics and pharmacodynamics of phenylephrine and phenylephrine oxazolidine in rabbit eyes. Pharm. Res. 1990;7(5):476–83. doi: 10.1023/a:1015808514727. [DOI] [PubMed] [Google Scholar]
- [64].Johansen M, Bundgaard H. Prodrug as drug delivery systems XXV: hydrolysis of oxazolidines–a potential new prodrug type. J. Pharm. Sci. 1983;72(11):1294–8. doi: 10.1002/jps.2600721115. [DOI] [PubMed] [Google Scholar]
- [65].Larsen JD, Bundgaard H, Lee VH. Prodrug forms for the sulfonamide group. II. Water-soluble amino acid derivatives of N-methylsulfonamides as possible prodrugs. Int. J. Pharm. 1988;47:103–110. [Google Scholar]
- [66].Lopes F, Moreira R, Iley J. Acyloxymethyl as a drug protecting group. Part 6: N-acyloxymethyl- and N-[(aminocarbonyloxy)methyl]sulfonamides as prodrugs of agents containing a secondary sulfonamide group. Bioorg. Med. Chem. 2000;8(4):707–16. doi: 10.1016/s0968-0896(00)00015-8. [DOI] [PubMed] [Google Scholar]
- [67].Larsen JD, Bundgaard H. Prodrug form for the sulfonamide group. I. Evaluation of N-acyl derivatives, N-sulfonylamidines, N-sulfonylsulfilimines and sulfonylureas as possible prodrug derivatives. Int. J. Pharm. 1987;37(1-2):87–95. [Google Scholar]
- [68].Larsen JD, Bundgaard H. Prodrug fonns for the sulfonamide group. III. Chemical and enzymatic hydrolysi of various N-sulfonyl imidates–a novel prodrug form for a sulfonamide group or an ester function. Int. J. Pharm. 1989;51:27–38. [Google Scholar]
- [69].Bundgaard H, Larsen JD. N-sulfonyl imidates as a novel prodrug form for an ester function or a sulfonamide group. J. Med. Chem. 1988;31(11):2066–9. doi: 10.1021/jm00119a002. [DOI] [PubMed] [Google Scholar]
- [70].Larsen JD, Bundgaard H. Prodrug forms for the sulfonamide group. IV. Kinetics of hydrolysis of N-sulfonyl pseudourea derivatives. Acta Pharm. Nord. 1989;1(1):31–40. [PubMed] [Google Scholar]
- [71].Pavan-Langston D, North RD, Jr., Geary PA, Kinkel A. Intraocular penetration of the soluble antiviral, Ara AMP. Arch. Ophthalmol. 1976;94(9):1585–8. doi: 10.1001/archopht.1976.03910040415018. [DOI] [PubMed] [Google Scholar]
- [72].Qiu Y, Schoenwald RD, Guillory JK. Physicochemical characterization of high- and low-melting phenylephrine oxazolidines. Pharm. Res. 1993;10(10):1507–15. doi: 10.1023/a:1018939728993. [DOI] [PubMed] [Google Scholar]
- [73].Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agents. Curr. Top. Med. Chem. 2007;7(9):849–54. doi: 10.2174/156802607780636735. [DOI] [PubMed] [Google Scholar]
- [74].Lee VH. Membrane transporters. Eur. J. Pharm. Sci. 2000;11(Suppl 2):S41–50. doi: 10.1016/s0928-0987(00)00163-9. [DOI] [PubMed] [Google Scholar]
- [75].Tsuji A. P-glycoprotein-mediated efflux transport of anticancer drugs at the blood-brain barrier. Ther. Drug Monit. 1998;20(5):588–90. doi: 10.1097/00007691-199810000-00024. [DOI] [PubMed] [Google Scholar]
- [76].Cannon RD, Lamping E, Holme AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. Efflux-mediated antifungal drug resistance. Clin. Microbial. Rev. 2009;22(2):291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kim RB. Drug emux transporters and antiviral drug therapy. Expert. Opin. Ther. Targets. 2000;4:439–446. [Google Scholar]
- [78].Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm. Bowel Dis. 2004;10(5):578–83. doi: 10.1097/00054725-200409000-00012. [DOI] [PubMed] [Google Scholar]
- [79].Barot M, Golculgandhi MR, Haghnegahdar M, Dalvi P, Mitra AK. Effect of emergence of fluoroquinolone resistance on intrinsic expression of P-glycoprotein phenotype in corneal epithelial cells. J. Ocul. Pharmacal. Ther. 2011;27(6):553–9. doi: 10.1089/jop.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Marquez B, Caceres NE, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Identification of the efflux transporter of the Ouoroquinolone antibiotic ciprofloxacin in murine macrophages: studies with ciprofloxacin-resistant cells. Antimicrob. Agents Chemother. 2009;53(6):2410–6. doi: 10.1128/AAC.01428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Katragadda S, Talluri RS, Mitra AK. Modulation of P-glycopmtein-mediated efflux by prodrug derivatization: an approach involving peptide transporter-mediated influx across rabbit cornea. J. Ocul. Pharmacal. Ther. 2006;22(2):110–20. doi: 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- [82].Jain R, Majumdar S, Nashed Y, Pal D, Mitra AK. Circumventing P-glycoprotein-mediated cellular emux of quinidine by prodrug derivatization. Mol. Pharm. 2004;1(4):290–9. doi: 10.1021/mp049952s. [DOI] [PubMed] [Google Scholar]
- [83].Duvvuri S, Gandhi MD, Mitra AK. Effect of P-glycoprotein on the ocular disposition of a model substrate, quinidine. Curr. Eye. Res. 2003;27(6):345–53. doi: 10.1076/ceyr.27.6.345.18187. [DOI] [PubMed] [Google Scholar]
- [84].Katragadda S, Jain R, Kwatra D, Hariharan S, Mitra AK. Pharmacokinetics of amino acid ester prodrugs of acyclovir after oral administration: interaction with the transporters on Caco-2 cells. Int. J. Phram. 2008;362(1-2):93–101. doi: 10.1016/j.ijpharm.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Anand BS, Katragadda S, Nashed YE, Mitra AK. Amino acid prodrug of acyclovir as possible antiviral agents against ocular HSV-1 infections: interactions with the neutral and cationic amino acid transporter on tbe corneal epithelium. Curr. Eye Res. 2004;29(2-3):153–66. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- [86].Nasbed YE, Mitra AK. Synthesis and characterization of novel dipeptide ester prodrugs of acyclovir. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2003;59(9):2033–9. doi: 10.1016/s1386-1425(03)00007-6. [DOI] [PubMed] [Google Scholar]
- [87].Janoria KG, Boddu SH, Natesan S, Mitra AK. Vitreal pharmacokinetics of peptide-transporter-targeted prodrugs of ganciclovir in conscious animals. J. Ocul. Pharmacal. Ther. 2010;26(3):265–71. doi: 10.1089/jop.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kansara V, Hao Y, Mitra AK. Dipeptide monoester ganciclovir prodrug for transscleral drug delivery: targeting the oligopeptide transporter on rabbit retina. J. Ocul. Pharmacal. Ther. 2007;23(4):321–34. doi: 10.1089/jop.2006.0150. [DOI] [PubMed] [Google Scholar]
- [89].Patel K, Trivedi S, Luo S, Zhu X, Pal D, Kern ER, Mitra AK. Synthesis, physicochemical properties and antiviral activitie of ester prodrugs of ganciclovir. Int. J. Pharm. 2005;305(1-2):75–89. doi: 10.1016/j.ijpharm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- [90].Anand B, Nashed Y, Mitra A. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. Curr. Eye Res. 2003;26(3-4):151–63. doi: 10.1076/ceyr.26.3.151.14893. [DOI] [PubMed] [Google Scholar]
- [91].Balakrishnan A, Jain-Vakkalagadda B, Yang C, Pal D, Mitra AK. Carrier mediated uptake of L-tyrosine and its competitive inhibition by model tyrosine linked compounds in a rabbit corneal cell line (SIRC)--strategy for the design of transporter/receptor targeted prodrugs. Int. J. Pharm. 2002;247(1-2):115–25. doi: 10.1016/s0378-5173(02)00405-2. [DOI] [PubMed] [Google Scholar]
- [92].Hosoya K, Kondo T, Tomi M, Takanaga H, Obtsuki S, Terasaki T. MCT1-mediated transport of L-lactic acid at the inner blood-retinal barrier: a possible route for delivery of monocarboxylic acid drug to the retina. Pharm. Res. 2001;18(12):1669–76. doi: 10.1023/a:1013310210710. [DOI] [PubMed] [Google Scholar]
- [93].Tsuji A, Tarnai I, Nakanishi M, Terasaki T, Hamano S. Intestinal brush-border transport of the oral cephalosporin antibiotic, cefdinir, mediated by dipeptide and monocarboxylic acid transport systems in rabbits. J. Pharm. Pharmacal. 1993;45(11):996–8. doi: 10.1111/j.2042-7158.1993.tb05645.x. [DOI] [PubMed] [Google Scholar]
- [94].Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetic of biotinylatcd ganciclovir: role of sodium-dependent multivitamin transporter expressed on retina. J. Ocul. Pharmacal. Ther. 2009;25(1):39–49. doi: 10.1089/jop.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dalpiaz A, Pavan B, Scaglianti M, Vitali F, Bortolotti F, Biondi C, Scalturin A, Manfredini S. Vitamin C and 6-amino-vitamin C conjugates of diclofenac: synthesis and evaluation. Int. J. Pharm. 2005;291(1-2):171–81. doi: 10.1016/j.ijpharm.2004.07.054. [DOI] [PubMed] [Google Scholar]
- [96].Manfredini S, Venuani S, Pavan B, Vitali F, Scaglianti M, Bortolotti F, Biondi C, Scatturin A, Prasad P, Dalpiaz A. Design, synthesis and in vitro evaluation on HRPE cells of ascorbic and 6-bromoa corbic acid conjugates with neuroactive molecules. Bioorg. Med. Chem. 2004;12(20):5453–63. doi: 10.1016/j.bmc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- [97].Dalpiaz A, Pavan B, Scaglianti M, Vitali F, Bortolotti F, Biondi C, Scanurin A, Tanganelli S, Ferraro L, Prasad P, Manfredini S. Transporter-mediated effects of diclofcnamic acid and its a corbyl pro-drug in the in vivo neurotropic activity of ascorbyl nipecotic acid conjugate. J. Pharm. Sci. 2004;93(1):78–85. doi: 10.1002/jps.10532. [DOI] [PubMed] [Google Scholar]
- [98].Manfredini S, Pavan B, Venuani S, Scaglianti M, Compagnone D, Biondi C, Scatturin A, Tanganelli S, Ferraro L, Prasad P, Dalpiaz A. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acid, liable to increase neurotropic activity. J. Med. Chem. 2002;45(3):559–62. doi: 10.1021/jm015556r. [DOI] [PubMed] [Google Scholar]
- [99].Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting thc oligopcptide transporter on the rabbit cornea. Pharm. Res. 2002;19(8):1194–202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- [100].Basu SK, Haworth LS, Bolger MB, Lee VH. Proton-driven dipeptide uptake in primary cultured rabbit conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 1998;39(12):2365–73. [PubMed] [Google Scholar]
- [101].Majumdar S, Macha S, Nashed Y, Mitra AK. Expression of peptide transporters on the rabbit retina: A strategy to improve retinal delivery of ganciclovir. Lett. Drug Des. Discov. 2004;1:73–77. [Google Scholar]
- [102].Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE. Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE); lack of functional activity in RPE plasma membranes. Pharm. Res. 2003;20(9):1364–72. doi: 10.1023/a:1025741723724. [DOI] [PubMed] [Google Scholar]
- [103].Atluri H, Anand BS, Patel J, Mitra AK. Mechanism of a model dipeptide transport across blood-ocular barriers following systemic administration. Exp. Eye. Res. 2004;78(4):815–22. doi: 10.1016/j.exer.2003.10.020. [DOI] [PubMed] [Google Scholar]
- [104].Takahashi H, Kaminski AE, Zieske JD. Glucose transporter I expression is enhanced during corneal epithelial wound repair. Exp. Eye Res. 1996;63(6):649–59. doi: 10.1006/exer.1996.0159. [DOI] [PubMed] [Google Scholar]
- [105].Gurses L, Doganay S, Mizrak B. Expression of glucose transporter protein-I (Glut-I) in ocular urface squamous neoplasia. Cornea. 2007;26(7):826–30. doi: 10.1097/ICO.0b013e3180645814. [DOI] [PubMed] [Google Scholar]
- [106].Gherzi R, Melioli G, De Luca M, D'Agostino A, Guastella M, Traverso CE, D'Anna F, Franzi AT, Cancedda R. High expression levels of the "erythroid/brain" type glucose transporter (GLUT1) in the basal cells of human eye conjunctiva and oral mucosa reconstituted in culture. Exp. Cell Res. 1991;195(1):230–6. doi: 10.1016/0014-4827(91)90522-v. [DOI] [PubMed] [Google Scholar]
- [107].Mantych GJ, Hageman GS, Devaskar SU. Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology. 1993;133(2):600–7. doi: 10.1210/endo.133.2.8344201. [DOI] [PubMed] [Google Scholar]
- [108].Ban Y, Rizzolo LJ. Regulation of gLucose transporters during development of the retinal pigment epithelium. Brain. Res. Dev. Brain Res. 2000;121(1):89–95. doi: 10.1016/s0165-3806(00)00028-6. [DOI] [PubMed] [Google Scholar]
- [109].Fernandes R, Suzuki K, Kumagai AK. Inner blood-retinal barrier GLUTI in long-term diabetic rats: an immunogold electron microscopic study. Invest. Ophthalmol. Vis. Sci. 2003;44(7):3150–4. doi: 10.1167/iovs.02-1284. [DOI] [PubMed] [Google Scholar]
- [110].Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat invest. Ophthalmol. Vis. Sci. 1992;33(2):377–83. [PubMed] [Google Scholar]
- [111].Majumdar S, Gunda S, Mitra A. Functional expression of a sodium dependent nucleoside transporter on rabbit cornea: Role in corneal permeation of acyclovir and idoxuridine. Curr. Eye Res. 2003;26(3-4):175–83. doi: 10.1076/ceyr.26.3.175.14895. [DOI] [PubMed] [Google Scholar]
- [112].Majumdar S, Tirucherai GS, Pal D, Mitra AK. Functional differences in nucleoside and nucleobase transporters expressed on the rabbit corneal epithelial cell line (SIRC) and isolated rabbit cornea. AAPS PharmSci. 2003;5(2):E15. doi: 10.1208/ps050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hosoya K, Horibe Y, Kim KJ, Lee VH. Nucleoside transport mechanisms in the pigmented rabbit conjunctiva. Invest. Ophthalmol. Vis. Sci. 1998;39(2):372–7. [PubMed] [Google Scholar]
- [114].Majumdar S, Macha S, Pal D, Mitra AK. Mechanism of ganciclovir uptake by rabbit retina and human retinal pigmented epithelium cell line ARPE-19. Curr. Eye Res. 2004;29(2-3):127–36. doi: 10.1080/02713680490504678. [DOI] [PubMed] [Google Scholar]
- [115].Williams EF, Ezeonu I, Dutt K. Nucleoside transport sites in a cultured human retinal cell line established by SV-40 T antigen gene. Curr.Eye Res. 1994;13(2):109–18. doi: 10.3109/02713689409042405. [DOI] [PubMed] [Google Scholar]
- [116].Nagase K, Tomi M, Tachikawa M, Hosoya K. Functional and molecular characterization of adenosine transport at the rat inner blood-retinal barrier. Biochim. Biophys. Acta. 2006;1758(1):13–9. doi: 10.1016/j.bbamem.2006.01.011. [DOI] [PubMed] [Google Scholar]
- [117].Giasson C, Bonanno JA. Facilitated transport of lactate by rabbit corneal endothelium. Exp. Eye Res. 1994;59(1):73–81. doi: 10.1006/exer.1994.1082. [DOI] [PubMed] [Google Scholar]
- [118].Gerhart DZ, Leino RL, Drewes LR. Distribution of monocarboxylate transporters MCTI and Men in rat retina. Neuroscience. 1999;92(1):367–75. doi: 10.1016/s0306-4522(98)00699-x. [DOI] [PubMed] [Google Scholar]
- [119].Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 2003;44(4):1716–21. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- [120].Talluri RS, Katragadda S, Pal D, Mitra AK. Mechanism of L-ascorhic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr. Eye Res. 2006;31(6):481–9. doi: 10.1080/02713680600693629. [DOI] [PubMed] [Google Scholar]
- [121].Salceda R, Contreras-Cubas C. Ascorbate uptake in normal and diabetic rat retina and retinal pigment epithelium. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2007;146(1-2):175–9. doi: 10.1016/j.cbpc.2007.02.015. [DOI] [PubMed] [Google Scholar]
- [122].Khatami M, Stramm LE, Rockey JH. Ascorbate transport in cultured cat retinal pigment epithelial cells. Exp. Eye. Res. 1986;43(4):607–15. doi: 10.1016/s0014-4835(86)80027-6. [DOI] [PubMed] [Google Scholar]
- [123].Hosoya K, Minamizono A, Katayama K, Terasaki T, Tomi M. Vitamin C transport in oxidized form across the rat blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 2004;45(4):1232–9. doi: 10.1167/iovs.03-0505. [DOI] [PubMed] [Google Scholar]
- [124].Janoria KG, Hariharan S, Paturi D, Pal D, Mitra AK. Biotin uptake by rabbit corneal epithelial cells: role of sodium-dependent multivitamin transporter (SMVT) Curr. Eye Res. 2006;31(10):797–809. doi: 10.1080/02713680600900206. [DOI] [PubMed] [Google Scholar]
- [125].Ohkura Y, Akanuma S, Tachikawa M, Hosoya K. Blood-to-retina transport of biotin via Na+-dependent multivitamin transporter (SMVT) at the inner blood-retinal barrier. Exp. Eye Res. 2010;91(3):387–92. doi: 10.1016/j.exer.2010.06.010. [DOI] [PubMed] [Google Scholar]
- [126].Hariharan S, Janoria KG, Gunda S, Zhu X, Pal D, Mitra AK. Identification and functional expression of a carrier-mediated riboflavin transport system on rabbit corneal epithelium. Curr.Eye.Res. 2006;31(10):811–24. doi: 10.1080/02713680600899655. [DOI] [PubMed] [Google Scholar]
- [127].Kansara V, Pal D, Jain R, Mitra AK. Identification and functional characterization of riboflavin transporter in human-derived retinoblastoma cell line (Y-79): mechanisms of cellular uptake and translocation. J. Ocul. Pharmacal. Ther. 2005;21(4):275–87. doi: 10.1089/jop.2005.21.275. [DOI] [PubMed] [Google Scholar]
- [128].Said HM, Wang S, Ma TY. Mechanism of riboflavin uptake by cultured human retinal pigment epithelial ARPE-19 cells: possible regulation by an intracellular Ca2+-caLmodulin-mediated pathway. J. Physiol. 2005;(566):369–77. doi: 10.1113/jphysiol.2005.085811. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jain-Vakkalagadda B, Dey S, Pal D, Mitra AK. Identification and functional characterization of a Na+-indepcndent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest. Ophthalmol. Vis. Sci. 2003;44(7):2919–27. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- [130].Pow DV. Amino acids and their transporters in the retina. Neurochem. Int. 2001;38(6):463–84. doi: 10.1016/s0197-0186(00)00114-5. [DOI] [PubMed] [Google Scholar]
- [131].Gandhi MD, Pal D, Mitra AK. Identification and functional characterization of a Na(+)-indepcndent large neutral amino acid transporter (LATI) on ARPE-19 cells. Int. J. Pharm. 2004;275(1-2):189–200. doi: 10.1016/j.ijpharm.2004.01.035. [DOI] [PubMed] [Google Scholar]
- [132].Tomi M, Mori M, Tachikawa M, Katayama K, Terasaki T, Hosoya K. L-type amino acid transporter 1-mediated L-Ieucine transport at the inner blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 2005;46(7):2522–30. doi: 10.1167/iovs.04-1175. [DOI] [PubMed] [Google Scholar]
- [133].Katragadda S, Talluri RS, Pal D, Mitra AK. Identification and characterization of a Na+-dependent neutral amino acid transporter, ASCT1, in rabbit corneal epithelial cell culture and rabbit cornea. Curr. Eye Res. 2005;30(11):989–1002. doi: 10.1080/02713680500306439. [DOI] [PubMed] [Google Scholar]
- [134].Yoneyama D, Shinozaki Y, Lu WL, Tomi M, Tachikawa M, Hosoya K. Involvement of system A in the retina-to-blood transport of 1-proline acro s the inner blood-retinal barrier. Exp. Eye Res. 2010;90(4):507–13. doi: 10.1016/j.exer.2010.01.003. [DOI] [PubMed] [Google Scholar]
- [135].Jain-Vakkalagadda B, Pal D, Gunda S, Nashed Y, Ganapathy V, Mitra AK. Identification of a Na+-dependent cationic and neutral amino acid tran porter, B(0,+), in human and rabbit cornea. Mol. Pharm. 2004;1(5):338–46. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- [136].Hosoya K, Horibe Y, Kim KJ, Lee VH. Na(+)-dependent Larginine transport in the pigmented rabbir conjunctiva. Exp. Eye Res. 1997;65(4):547–53. doi: 10.1006/exer.1997.0354. [DOI] [PubMed] [Google Scholar]
- [137].Kompella UB, Kim KJ, Shiue MH, Lee VH. Possible exi tence of Na(+)-coupled amino acid transport in the pigmented rabbit conjunctiva. Life Sci. 1995;57(15):1427–31. doi: 10.1016/0024-3205(95)02105-r. [DOI] [PubMed] [Google Scholar]
- [138].Jager K, Bonisch U, Risch M, Worlitzsch D, Paulsen F. Detection and regulation of cationic amino acid transporters in healthy and diseased ocular surface. Invest. Ophthalmol. Vis. Sci. 2009;50(3):1112–21. doi: 10.1167/iovs.08-2368. [DOI] [PubMed] [Google Scholar]
- [139].Tornquist P, Alm A. Carrier-mediated transport of amino acids through the blood-retinal and the blood-brain barriers. Graefes. Arch. Clin. Exp. Ophthalmol. 1986;224(1):21–5. doi: 10.1007/BF02144127. [DOI] [PubMed] [Google Scholar]
- [140].Kansara V, Mitra AK. Evaluation of an ex vivo model implication for carrier-mediated retinal drug delivery. Curr.Eye Res. 2006;31(5):415–26. doi: 10.1080/02713680600646890. [DOI] [PubMed] [Google Scholar]
- [141].Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. Expression and differential polarization of the reduced-folate transporter-I and the folate receptor alpha in mammalian retinal pigment epithelium. J. Biol. Chem. 2000;275(27):20676–84. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- [142].Huang W, Prasad PD, Kekuda R, Leibach FH, Ganapathy V. Characterization of N5-methyltetrahydrofolate uptake in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1997;38(8):1578–87. [PubMed] [Google Scholar]
- [143].Hosoya K, Fujita K, Tachikawa M. Involvement of reduced folate carrier 1 in the inner blood-retinal barrier transport of methyltetrabydrofolate. Drug Metab. Pharmacokinet. 2008;23(4):285–92. doi: 10.2133/dmpk.23.285. [DOI] [PubMed] [Google Scholar]
- [144].Bozard BR, Ganapathy PS, Duplantier J, Mysona B, Ha Y, Roon P, Smith R, Goldman ID, Prasad P, Martin PM, Ganapathy V, Smith SB. Molecular and biochemical characterization of folate transport proteins in retinal Muller cells. Invest. Ophthalmol. Vis. Sci. 2010;51(6):3226–35. doi: 10.1167/iovs.09-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, Ganapathy V, Prasad PD. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest. Ophthalmol. Vis. Sci. 2007;48(11):5299–305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest. Ophthalmol. Vis. Sci. 1999;40(5):840–8. [PubMed] [Google Scholar]
- [147].Baudouin C, Brignole F, Fredj-Reygrobellet D, Negre F, Bayle J, Gastaud P. Transferrin receptor expression by retinal pigment epithelial cells in proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 1992;33(10):2822–9. [PubMed] [Google Scholar]
- [148].Kompella UB, Sundaram S, Ragbava S, Escobar ER. Luteinizing hormone-releasing hormone agonist and transferrin functionalizations enhance nanoparticle delivery in a novel bovine ex vivo eye model. Mol. Vis. 2006;12:1185–98. [PubMed] [Google Scholar]
- [149].Yefimova MG, Jeanny JC, GuiIJonneau X, Keller N, Nguyen-Legros J, Sergeant C, Guillou F, Courtois Y. Iron, ferritin, transferrin and transferrin receptor in the adult rat retina. Invest. Ophlhalmol. Vis. Sci. 2000;41(8):2343–51. [PubMed] [Google Scholar]
- [150].Gnana-Prakasam JP, Martin PM, Smith SB, Ganapathy V. Expression and function of iron-regulatory proteins in retina. IUBMB Life. 2010;62(5):363–70. doi: 10.1002/iub.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Dey S, Patel J, Anand BS, Jain-Vakkalagadda S, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expres ion of P-glycoprotein (MDRI) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44(7):2909–18. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- [152].Saha P, Yang JJ, Lee VH. Existence of a p-glyeoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 1998;39(7):1221–6. [PubMed] [Google Scholar]
- [153].Maines LW, Antonetti DA, Wolpert EB, Smith CD. Evaluation of the role of P-glycoprotein in the uptake of paroxetine, clozapine, phenytoin and carbamazapine by bovine retinal endothelial cell. Neuropharmacology. 2005;49(5):610–7. doi: 10.1016/j.neuropharm.2005.04.028. [DOI] [PubMed] [Google Scholar]
- [154].Mannermaa E, Vellonen KS, Ryhanen T, Kokkonen K, Ranta VP, Kaamiranta K, Urtti A. Efflux protein expression in human retinal pigment epithelium cell line. Pharm. Res. 2009;26(7):1785–91. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- [155].Kennedy BG, Mangini NJ. P-glycoprotein expre sion in human retinal pigment epithelium. Mol. Vis. 2002;8:422–30. [PubMed] [Google Scholar]
- [156].Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Expression of ATP-binding cassette transporters at the inner blood-retinal barrier in a neonatal mouse model of oxygen-induced retinopathy. Brain Res. 2009;1283:186–93. doi: 10.1016/j.brainres.2009.05.095. [DOI] [PubMed] [Google Scholar]
- [157].Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 2007;336(1):12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Vellonen KS, Mannerrnaa E, Tumer H, Hakli M, Wolosin JM, Tervo T, Honkakoski P, Urtti A. EfIluxing ABC transporters in human corneal epithelium. J. Pharm. Sci. 2010;99(2):1087–98. doi: 10.1002/jps.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (SCRP) in human corneal epithelial cells. Curr. Eye Res. 2009;34(1):1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Dal Pozzo F, Andrei G, Lebeau I, Beadle JR, Hostetler KY, De Clercq E, Snoeck R. In vitro evaluation of the anti-orf virus activity of alkoxyalkyl esters of CDV, cCDV and (S)-HPMPA. Antiviral. Res. 2007;75(1):52–7. doi: 10.1016/j.antiviral.2006.11.010. [DOI] [PubMed] [Google Scholar]
- [161].anoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007;4(4):371–88. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- [162].Anand BS, Katragadda S, Mitra AK. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: intestinal absorption and liver metabolism. J. Pharmacal. Exp. Ther. 2004;311(2):659–67. doi: 10.1124/jpet.104.069997. [DOI] [PubMed] [Google Scholar]
- [163].Anand BS, Hill JM, Dey S, Maruyama K, Bbattacharjee PS, Myle ME, Nashed YE, Mitra AK. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-I epithelial and stromal keratitis in the rabbit eye model. Invest. Ophthalmol. Vis. Sci. 2003;44(6):2529–34. doi: 10.1167/iovs.02-1251. [DOI] [PubMed] [Google Scholar]
- [164].Anand BS, Dey S, Mitra AK. Current prodrug strategies via membrane transporters/receptors. Expert Opin. Biol. Ther. 2002;2(6):607–20. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- [165].Granero GE, Amidon GL. Stability of valacyclovir: implications for its oral bioavailability. Int. J. Pharm. 2006;317(1):14–8. doi: 10.1016/j.ijpharm.2006.01.050. [DOI] [PubMed] [Google Scholar]
- [166].Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob. Agents Chemother. 1995;39(12):2759–64. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Talluri RS, Samanta SK, Gaudana R, Mitra AK. Synthesis, metabolism and cellular permeability of enzymatically stable dipeptide prodrug of acyclovir. Int. J. Pharm. 2008;361(1-2):118–24. doi: 10.1016/j.ijpharm.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Friedrichsen GM, Jakobsen P, Taub M, Begtrup M. Application of enzymatically stable dipeptides for enhancement of intestinal permeability. Synthesis and in vitro evaluation of dipeptide-coupled compounds. Bioorg. Med. Chem. 2001;9(10):2625–32. doi: 10.1016/s0968-0896(01)00066-9. [DOI] [PubMed] [Google Scholar]
- [169].Steffansen B, Lepist EI, Taub ME, Larsen BD, Frokjaer S, Lennernas H. Stability, metabolism and transport of D-Asp(OBzl)-AJa-a model prodrug with afftnity for the oligopeptide transporter. Eur. J. Phann. Sci. 1999;8(1):67–73. doi: 10.1016/s0928-0987(98)00062-1. [DOI] [PubMed] [Google Scholar]
- [170].Tamura K, Lee CP, Smith PL, Borchardt RT. Metabolism, uptake, and transepithelial tran port of the stereoisomers of Val-Val-Val in the human intestinal cell line, Caco-2. Pharm. Res. 1996;13(11):1663–7. doi: 10.1023/a:1016436606183. [DOI] [PubMed] [Google Scholar]
- [171].Talluri RS, Gaudana R, Hariharan S, Mitra AK. Pharmacokinetics of stereoisomeric dipeptide prodrugs of acyclovir following intravenous and oral administrations in rats: a study involving in vivo corneal uptake of acyclovir following oral dosing. Ophthalmol. Eye Dis. 2009:121–31. doi: 10.4137/oed.s2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm. Res. 2009;26(5):1197–216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Cheng L, Hostetler KY, Lee J, Koh HJ, Beadle JR, Besho K, Toyoguchi M, Aldem K, Bovet JM, Freeman WR. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrug of ganciclovir and cyclic cidofovir. Invest. Ophthalmol. Vis. Sci. 2004;45(11):4138–44. doi: 10.1167/iovs.04-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J. Infect. Dis. 2004;190(3):499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- [175].Cbarteris DG, Aylward GW, Wong D, Groenewald C, Asaria RH, Bunce C. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of e tabli hed proliferative vitreoretinopathy. Ophthalmology. 2004;111(12):2240–5. doi: 10.1016/j.ophtha.2004.05.036. [DOI] [PubMed] [Google Scholar]
- [176].Cardillo JA, Farah ME, Mitre J, Morales PH, Costa RA, Melo LA, Kuppermann B, Jorge R, Ashton P. An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy. Br. J. Ophthalmol. 2004;88(9):1201–5. doi: 10.1136/bjo.2003.039917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cheng L, Hostetler K, Valiaeva N, Tammewar A, Freeman WR, Beadle J, Bartsch DU, Aldern K, Falkenstein I. Intravitreal crystalline drug delivery for intraocular proliferation disease. Invest. Ophthalmol. Vis. Sci. 2010;51(1):474–81. doi: 10.1167/iovs.09-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Randhawa P, Farasati NA, Shapiro R, Hostetler KY. Ether lipid ester derivatives of cidofovir inhibit polyoma virus BK replication in vitro. Antimicrob. Agents Chemother. 2006;50(4):1564–6. doi: 10.1128/AAC.50.4.1564-1566.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Williams-Ariz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, Beadle JR, Wan WB, Hosteder KY, Kern ER. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 2005;49(9):3724–33. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hartline CB, Gustin KM, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 2005;191(3):396–9. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
- [181].Buller RM, Owen G, Schriewer J, Melman L, Beadle JR, Hosteder KY. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318(2):474–81. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- [182].Beadle JR, Hartline C, Aldern KA, Rodriguez N, Harden E, Kern ER, Hostetler KY. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exbibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents. Chemother. 2002;46(8):2381–6. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kern ER, Hartline C, Harden E, Keith K, Rodriguez N, Beadle JR, Hostetler KY. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 2002;46(4):991–5. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Cheng L, Hostetler KY, Chaidhawangul S, Gardner MF, Beadle JR, Keefe KS, Bergeron-Lynn G, Severson GM, Soules KA, Mueller AJ, Freeman WR. Intravitreal toxicology and duration of efficacy of a novel antiviral lipid prodrug of ganciclovir in liposome formulation. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1523–32. [PubMed] [Google Scholar]
- [185].Andrei G, Snoeck R, Neyts J, Sandvold ML, Myhren F, De Clercq E. Antiviral activity of ganciclovir elaidic acid ester against herpesviruse. Antiviral. Res. 2000;45(3):157–67. doi: 10.1016/s0166-3542(00)00070-x. [DOI] [PubMed] [Google Scholar]
- [186].Cheng L, Hostetler KY, Gardner MF, Avila CP, Jr., Bergeron-Lynn G, Severson GM, Freeman WR. Intravitreal pharmacokinetics in rabbits of the foscamet lipid prodrug: 1-O-octadecyl-sn-glycerol-3-phosphonoformate (ODG-PFA) Curr. Eye Res. 1999;18(3):161–7. doi: 10.1076/ceyr.18.3.161.5366. [DOI] [PubMed] [Google Scholar]
- [187].Koppelhu U, Shiraishi T, Zachar V, Pankratova S, Nielsen PE. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjug. Chem. 2008;19(8):1526–34. doi: 10.1021/bc800068h. [DOI] [PubMed] [Google Scholar]
- [188].Hugbes PM, Olejnik O, Chang-lin J. Compositions and methods for the intraocular transport of therapeutic agents. 2007. U.S. Patent 7714024.
- [189].Garst ME, Adorante JS. Methods and compositions for drug delivery. 2002. U.S. Patent 6350780.
- [190].Marcus DM, Chu CK. Methods and compositions for treatment of macular and retinal disease. 2007. U.S. Patent 20070259843.
- [191].Rabinovich-guilatt L, Lambert G. Use of asteroid prodrug for the treatment of disease of the posterior segment of the eye. 2007. U.S. Patent 20070280995.
- [192].Prokai L, Prokai K, Simpkins J, Agarwal N. Prodrugs for use a ophthalmic agents. 2007. U.S. Patent 7572781.
- [193].Rothbard JB, Wender PA, Mcgrane LP, Sista VLS, Kirschberg TA. Compositions and methods for enhancing drug delivery across and into ocular tissues. 2007 U.S. Patent 7229961. [Google Scholar]
- [194].Dalpiaz A, Cacciari B, Mezzena M, Strada M, Scalia S. Solid lipid microparticle for the stability enhancement of a dopamine prodrug. J. Pharm. Sci. 2010;99(11):4730–7. doi: 10.1002/jps.22178. [DOI] [PubMed] [Google Scholar]
- [195].wala J, Boddu SH, Shah S, Sirimulla S, Pal D, Mitra AK. Ocular sustained release nanoparticles containing stereoisomeric dipeptide prodrugs of acyclovir. J. Ocul. Pharmacol. Ther. 2011;27(2):163–72. doi: 10.1089/jop.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kawakami S, Yamamura K, Mukai T, Nishida K, Nakamura J, Sakaeda T, Nakashima M, Sasaki H. Sustained ocular delivery of tilisolol to rabbits after topical administration or intravitreal injection of lipophilic prodrug incorporated in liposome. J. Pharm. Pharmacol. 2001;53(8):1157–61. doi: 10.1211/0022357011776423. [DOI] [PubMed] [Google Scholar]
- [197].Barot M, Gokulgandhi MR, Mitra AK. Mitocbondrial dysfunction in retinal diseases. Curr. Eye Res. 2011;36(12):1069–77. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]