Summary
Background
Lymphangioleiomyomatosis (LAM) is a rare, progressive and frequently lethal cystic lung disease that almost exclusively affects women and has no proven therapies. An improved understanding of the pathogenesis has identified promising molecular targets for clinical trials. Although barriers, modifiers, and benefits for clinical trial participation in common diseases such as cancer have been studied, we are unaware of such evaluations concerning rare diseases.
Methods
We performed a survey of a population-based registry of 780 LAM subjects in North America to identify predictors of trial participation. Logistic regression analysis evaluated the association of demographic and clinical features with trial participation.
Results
41 of 263 (16%) LAM patient respondents in North America had participated in a clinical trial. Age, disease duration, lack of any college education, use of oxygen therapy, and presentation without chest pain were associated with trial participation in unadjusted analyses. Multivariate analyses indicate that patient age was the strongest independent predictor for trial participation (OR = 2.07, p = 0.004 per decade greater of patient age). Common reasons reported against trial participation included not meeting enrollment criteria (44%), drug toxicity (25%), and stable disease (20%). The most frequent reason reported for trial participation was to help future patients (85%).
Conclusions
Study entry criteria, drug toxicity, and stability of disease are barriers to trial enrollment among subjects with LAM. Older LAM patients and those with more advanced disease are more likely to have participated in clinical trials. Altruism is commonly a motivating factor.
Keywords: Lymphangioleiomyomatosis, Clinical trial, Survey, Rare diseases, Trial participation
Introduction
For rare, chronic, and terminal illnesses that have no known cure, clinical trials offer the best hope of discovering an effective treatment. However, trials may not yield helpful results if an insufficient number of patients participate. Difficulty in recruitment to a trial may limit the statistical power of the study to detect a possible treatment effect. Furthermore, the external validity of the trial is limited if the study sample is not representative of the population at large in which the treatment might be used. Previous literature has established the barriers to participation in clinical trials in specific disease categories (e.g. coronary artery disease patients) and underrepresented demographic groups (e.g. women and African Americans). For women in general, the major perceived barriers to participation in clinical trials include increased burden on health and time, inconvenience of appointments, and increased risk of harm.1–3 Barriers and benefits involved in participating in randomized controlled trials of patients with cancer (representative of chronic, terminal diseases of large population size) have also been well studied and the major barriers include fear of trials, competing clinical trials, and information overload.4 In women with gynecologic malignancies, distrust of trials and time burdens have been cited as causes for unwillingness to participate.1,3,5 To our knowledge, such barriers have not been evaluated in the setting of rare diseases.
Lymphangioleiomyomatosis (LAM) is a rare, progressive, frequently lethal cystic lung disease that almost exclusively affects women with an estimated prevalence of 2 patients per million people.6,7 It occurs in up to 40% of women with the tuberous sclerosis complex, and can also present in a sporadic form that involves the lung, lymphatics, and kidney (in the form of angiomyolipomas (AMLs)6,7), but without systemic genetic disease. Among all LAM patients, mortality at 10 years has been estimated to be approximately 10–20% from the onset of symptoms8,9 and 30% at 10 years from the time of lung biopsy.10 The current treatments for LAM are primarily based on the antagonism of estrogen action and remain empiric and unproven.11 Improved understanding of the molecular pathogenesis of the disease has identified several promising molecular targets for clinical trials. In particular, dysregulation in mTOR signaling pathway due to loss of the growth regulatory protein, tuberin, has been shown to result in abnormal cell proliferation in LAM. An FDA approved mTOR inhibitor called sirolimus has been reported to restore normal cell growth in the laboratory and to cause tumor regression in animal models of tuberin deficiency. The Cincinnati Angiomyolipoma Sirolimus Trial (registration identifier, NCT00457808) was a proof-of-principle trial involving 20 patients with angiomyolipomas, including 11 with LAM,12 which showed an improvement in lung function after 1 year of treatment. A large, 3-year, randomized controlled trial called the Multicenter International LAM Efficacy of Sirolimus (MILES) trial (NCT00414648) opened in 2006. Octreotide, which slows the production of chyle through reduction in splanchnic blood flow, has been tested in LAM patients in a clinical trial at the National Heart, Lung, and Blood Institute (NCT00005906). There are also three open AML trials that are enrolling tuberous sclerosis and LAM patients at the current time, all based on tumor volume endpoints (NCT NCT00790400, NCT00457964, NCT00792766).
The particular challenges of trial recruitment in rare diseases may differ from those barriers studied previously in other groups. We are unaware of any published studies evaluating predictors of participation, barriers, and perceived benefits of clinical trials in rare disease populations. Therefore, we studied persons affected with LAM in order to determine the demographic and clinical factors associated with clinical trial participation and to investigate the reasons for and against the decision to enroll in a clinical trial.
Methods
We developed a questionnaire to document respondents’ demographic information, clinical data, and reasons for choosing to enroll or not to enroll in clinical trials. We asked subjects whether they had participated in at least one clinical trial and allowed the subjects to specify the particular trial/s in free text. Themes relating to barriers, motivating factors and modifiers to participation obtained from published literature were used to generate questions. A focus group using semi-structured interviewing techniques (including open-ended and free-response formats) of LAM patients was conducted. The responses from this session and ensuing discussion were used to generate a semi-structured survey consisting of both multiple choice and free-response questions that required about 15–30 min to complete. Face and content validity were ensured by using different questions to elicit the same information.
With the help of the LAM Foundation, a nonprofit organization founded to further patient and physician support and education and to enhance research efforts in LAM, we contacted LAM patients and/or family members in North America who had previously given consent to allow disclosure of their names to researchers. The questionnaire was distributed to 780 self-identified LAM patients (all patients and caregivers of current (n = 621) and deceased (n = 159) patients for which addresses were available listed in the Foundation’s population-based registry) requesting that they complete either the online questionnaire or the mailed questionnaire between July 2008 and December 2008. The questionnaire was sent both in paper form and via email with a link to a web-based survey site (www.surveymonkey.com). Respondents could return the questionnaire by mail, fax, or email, and they received no incentive for participation. Each questionnaire was assigned a random LAM identification number. Data from completed questionnaires were entered into the web-based survey site and were exported as a Microsoft Excel database. This study was approved by the institutional review board of the University of Cincinnati College of Medicine.
The predictor variables examined included age, educational level, income level, region of residence, disease duration, forced expiratory volume in 1 s (FEV1) (at diagnosis and most recent), presentation type (i.e. pneumo-thorax or dyspnea), employment status, insurance status, length of diagnosis, location of care, and main provider type (pulmonologists or other). Descriptive characteristics of the respondents, identified barriers and motivating factors regarding clinical trial participation were calculated as percentages of the total responses in each category. In the unadjusted analysis, the main outcome of presence or absence of trial participation utilized Fisher’s exact test/Chi square, as appropriate, with two tailed p-values < 0.05 considered statistically significant. Variables found to be associated at a p value of <0.05 were considered for inclusion in the multivariate logistic regression model. The final model was selected using backwards deletion. Analyses were performed using Stata statistical software, version 9 (College Station, Texas).
Results
Of 780 questionnaires distributed, 263 were returned and included in the analysis. There was a very low response rate from family members of deceased patients (1/159). Amongst surviving members of the database, the response rate was 262/621(42%). 41 of 263 (16%) respondents in North America had participated in a clinical trial for LAM. The responding population was predominantly Caucasian and similar in age to the LAM Foundation registry overall. Clinical trial participants were older (mean age = 53) than non-participants (mean age = 49, p value = 0.001) and tended to have longer disease duration (Table 1). Those subjects citing altruism as a motivating factor for clinical trial enrollment were older and had longer disease duration than those that did not (p value = 0.01 and 0.002, respectively; data not shown). The presenting symptoms leading to diagnosis were similar between participants and non-participants with the exception of chest pain, which was more common among those not enrolled in a clinical trial (24% vs 10%, p = 0.04). Approximately 51% had undergone either surgical lung biopsy or biopsy of abdominal tissues or lymph nodes, but there was no difference in tissue confirmation rates between trial participants and non-participants. In both groups, the majority of respondents were non-smokers and under the care of pulmonologists. Clinical trial participants had a slightly lower most recent FEV1% predicted, but it did not reach statistical significance (59% vs 65%, p = 0.39). More clinical trial participants were on supplemental home oxygen than nonparticipants (63% vs 40%, p = 0.009). There was no observed association between region of residence, as classified by the US Census Bureau, and trial participation (data not shown). Although the majority of subjects in both groups were college educated, there were fewer with advanced education among the clinical trial participants (73% vs 90%, p = 0.009) (Table 2). In addition, clinical trial participants were more likely to have experienced disease related job loss (47% vs 24%, p = 0.008). Income and current job status did not differ between the two groups, and almost all subjects had some health insurance.
Table 1.
General characteristics and clinical features associated with clinical trial participation.
| Variables | Overall number measured |
Clinical trial participants (n = 41) |
Non-participants (N = 222) |
p-value |
|---|---|---|---|---|
| Age, mean (SD) | 263 | 53 (8) | 49 (10) | 0.001 |
| Caucasian, number (%) | 263 | 36 (88) | 191 (86) | 1.00 |
| Disease duration, years (SD) | 239 | 11 (7) | 9 (6) | 0.060 |
| Established disease,a number (%) | 208 | 38 (18) | 170 (82) | 0.020 |
| No established disease,a number (%) | 55 | 3 (8) | 48 (92) | |
| Presentation withb | ||||
| Dyspnea, number (%) | 263 | 28 (68) | 142 (64) | 0.594 |
| Kidney tumor, number (%) | 263 | 7 (17) | 30 (14) | 0.547 |
| Pneumothorax, number (%) | 263 | 14 (34) | 91 (41) | 0.411 |
| Chest pain, number (%) | 263 | 4 (10) | 54 (24) | 0.039 |
| Asymptomatic, number (%) | 263 | 2 (5) | 22 (10) | 0.304 |
| Ever smoker, number (%) | 256 | 16 (39) | 68 (32) | 0.355 |
| Diagnosis by | ||||
| Surgical lung biopsy, number (%) | 111/263 (42%) | 17 (41) | 94 (42) | 0.917 |
| Abdominal/lymph node biopsy, number (%) | 23/263 (9%) | 4 (10) | 19 (9) | 0.803 |
| Under the care of a pulmonologist, number (%) | 211/263 (80%) | 36 (88) | 175 (79) | 0.185 |
| Main care at an academic medical center, number (%) | 126/263 (48%) | 19 (46) | 107 (48) | 0.827 |
| Recent FEV1, mean % predicted (SD) | 84 | 59 (26) | 65 (27) | 0.394 |
| At diagnosis FEV1, mean % predicted (SD) | 79 | 72 (18) | 72 (23) | 0.990 |
| Oxygen therapy, number (%) | 221 | 24 (63) | 73 (40) | 0.009 |
Established disease = disease duration of greater than 3 years.
Symptoms not mutually exclusive.
Table 2.
Socioeconomic factors associated with clinical trial participation.
| Variables | Overall number measured |
Clinical trial participants |
Non-participants | p-value |
|---|---|---|---|---|
| Employed, number (%) | 263 | 17 (41) | 98 (44) | 0.751 |
| On Disability, number (%) | 178 | 16 (50) | 47 (32) | 0.056 |
| College educated, number (%) | 263 | 30 (73) | 199 (90) | 0.009 |
| Income, number (%) | 227 | |||
| Less than $30,000/year | 3 (9) | 25 (13) | 0.775 | |
| Greater than $100,000/year | 9 (28) | 54 (28) | 1.00 | |
| Insurance coverage, number (%) | 256 | 41 (100) | 212 (99) | 0.447 |
| Disease related job loss (%) | 179 | 15 (47) | 35 (24) | 0.008 |
Results of multivariate analyses indicate that patient age was the strongest independent predictor for trial participation (OR = 2.07, CI 1.26–3.41, p = 0.004 for each decade greater in patient age) (Table 3). Oxygen use and absence of chest pain presentation approached but did not meet statistical significance criteria.
Table 3.
Multivariate logistic regression model for factors associated with clinical trial participation.
| Predictor variables | Odds ratio |
95%CI | P value |
|---|---|---|---|
| Age, per additional decade | 2.07 | 1.26, 3.41 | 0.004 |
| Disease duration, per year | 1.01 | 0.95, 1.07 | 0.823 |
| College educated | 0.47 | 0.18, 1.26 | 0.134 |
| Oxygen therapy | 2.08 | 0.93, 4.62 | 0.074 |
| Presentation with chest pain | 0.29 | 0.08, 1.05 | 0.059 |
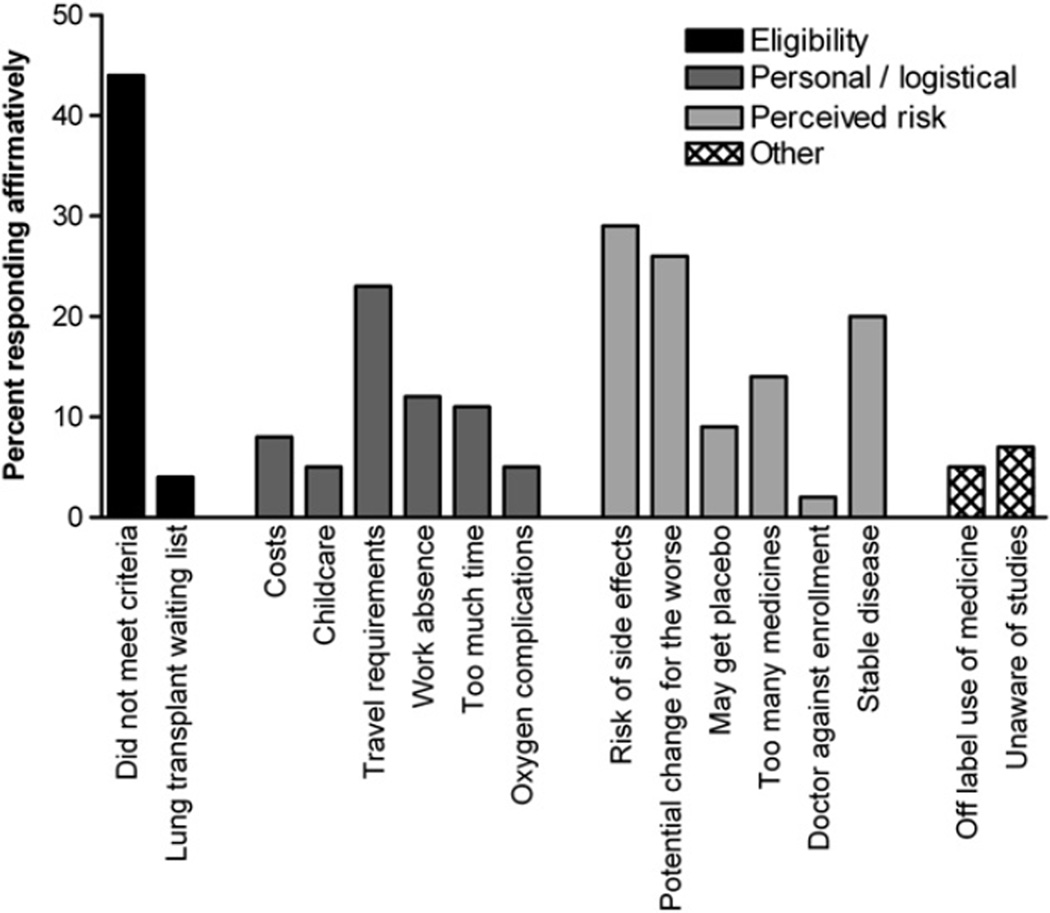
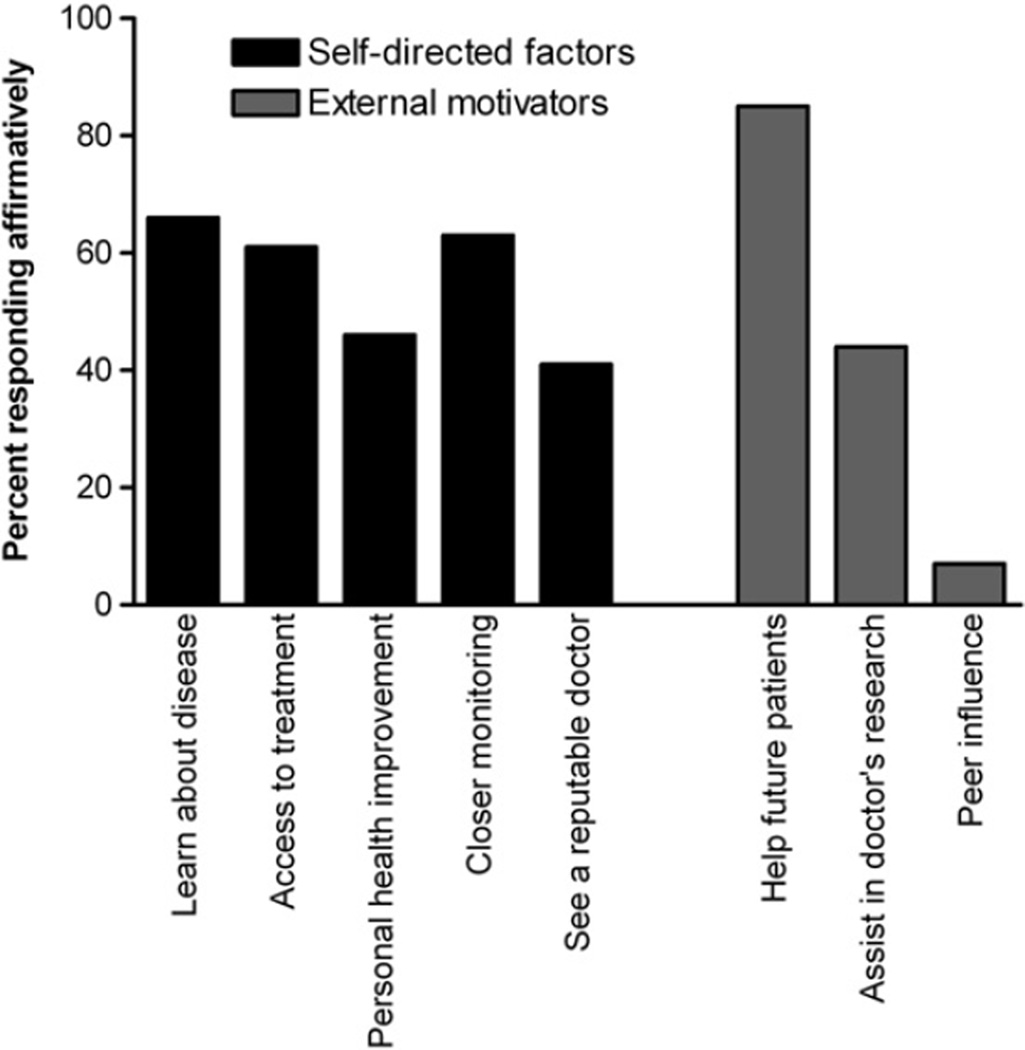
The most common reasons against trial participation were not meeting enrollment criteria (44%), potential drug side effects (29%), and stable disease (26%) (Fig. 1). When grouped into common themes for non-participation, 46% reported eligibility issues, 36% personal/logistical concerns, and 45% cited perceived risks (total not equal to 100% as responses were not mutually exclusive). Notably, awareness of studies, study costs, risk of placebo assignment, and off-label medication use were not widely cited as obstacles to trial participation. The most frequent reasons given for trial participation were to help future patients (85%), learn about the disease (66%), closer monitoring (63%), and access to therapies (61%) (Fig. 2).
Figure 1.
Reasons cited for the decision against participation in a clinical trial among patients with LAM (N = 222).
Figure 2.
Reasons cited for the decision to participate in a clinical trial among patients with LAM (N = 41).
Discussion
Over 7000 rare diseases, each involving fewer than 200,000 US residents, affect nearly 30 million people in the United States.13 Clinical investigators in rare disease research regularly face challenges specific to the study of uncommon disorders, such as the recruitment of sufficient numbers of study subjects for adequately powered clinical trials. Strategies that have been used to attempt to surmount these challenges include the creation of multi-institutional and international collaborations, adoption of specialized study designs, biostatistical techniques developed to maximize data from small numbers of subjects, and partnerships with patient advocacy organizations.13 However, we are unaware of any published study that systematically evaluates the predictors, barriers and motivating factors for clinical trial enrollment among patients with rare lung diseases such as LAM. We found that the predictors of trial participation in this rare disease differ from prior studies of cancer patients or underrepresented minority groups in general such as women.
Specific characteristics of the LAM patient population may explain these differences. While some of the same factors and reasons found in previous studies apply to the LAM patient population, other barriers are unique and may arise due to the small patient population that consists mostly of women. The small size of the group allows for a greater connection between individuals, and as a result, a strong “group persona” is evident that is not usually present with larger patient populations. This combined with the tight friendships that LAM patients have with each other may influence decisions made by individuals. Therefore, certain motivating factors and barriers to clinical trial participation may exist because of the group persona and thus may be unique to rare diseases. We found that subjects who were older and had their diagnosis for at least 3 years were more likely to participate in a clinical trial. Furthermore, the association observed between age and disease duration with altruistic motivation suggests that as people with a rare disease become older and have more shared experiences they become more altruistic toward the community with the disease. This is in contrast to evaluations of patients with gynecological diseases, in which older patients are far less likely to participate in trials.5,14 Moreover, LAM patients are far more likely to cite altruistic intent, as opposed to greater access to treatment for themselves. These distinctions perhaps may be secondary to the importance of group identity. Interestingly, peer influence was not a reason frequently cited for participation in our patients, suggesting that the impact of the group identity is not overt, but rather reflective of a general feeling of community and shared cause in this small, geographically disperse yet tightly knit group.
The most common reason cited for trial non-participation was that the subject did not meet enrollment criteria. Although stringent inclusion and exclusion criteria can lead to a homogenous study population that is best suited to answer the scientific question, they may create a barrier to successful trial enrollment in studies of rare diseases, where the pool for potential study subjects is already very small. The study impact of an individual being excluded from trial participation in more common diseases (e.g. cancers) is minimal as there typically are hundreds of alternative potential participants available. In the MILES trial, for example, study recruitment has been limited by potential participants failing to meet predefined FEV1 criterion (<70% predicted) and the requirement for definitive diagnosis, which in more than half of cases requires tissue biopsy. Relaxing such requirements might allow for greater enrollment, but could have potential negative consequences. For example, without the preset FEV1 criterion, a study might have a reduced capacity to detect an improvement in lung function, and without requiring definitive diagnosis of LAM, patients with a disease mimicking LAM could be exposed to undue risk. Creative solutions to reconcile these different vantage points are necessary for successful trials to be effectively conducted in rare diseases.
Perhaps as interesting as the identified predictors and reasons for clinical trial participation were those factors that were not cited by our population. Studies in patients with cancer and cardiovascular disease identify mistrust of experimental methods and randomization as major barriers to trial participation.1,15 Risk of taking placebo or off-label medication use are often identified as threats to trial recruitment when there is a placebo control arm or commercially available therapeutic with a different FDA indication. Our data suggests that these are not major barriers to trial enrollment in this rare disease; although it is conceivable that people who are taking drugs off label may be less likely to respond to questionnaires. Furthermore, it appears that economic factors, geographic location and time burdens play a limited, if any, role in the decision to participate in a clinical trial in subjects with this disease. Similarly, inadequate knowledge about available trials or lack of advanced education does not appear to be an important determinant of trial participation. These findings should inform future targeted interventions to improve enrollment in rare diseases.
There are several potential limitations of this study that warrant discussion. First, the person who responds to surveys is most likely someone who approves of medical research and is invested in the pursuit of scientific knowledge. Thus, persons who do not enroll in clinical trials because they dislike or distrust the process (or purpose) of the clinical trials may have been selectively excluded. We attempted to address this limitation by including patients’ perspectives from those who had and had not participated in clinical research in the focus group based generation of the survey instrument. Second, survey sampling methodology can greatly impact the representativeness of a reference population. We attempted to mitigate this potential weakness by partnering with a patient advocacy organization with a mature population-based registry16,17 and offering subjects multiple survey mediums, including paper and electronic forms. In addition, although we tried to be as inclusive as possible of both living and deceased patient experiences, we had a very poor response rate amongst family members of deceased patients. While the survey design was carefully designed and pretested, researcher bias still may exist. For example, in the question “Why did you choose not to participate in a clinical trial,” the choices offered represent the researchers’ hypotheses for the reasons. Even though an “other” choice with a fill-in the-blank was available, the subject might feel more compelled to simply check a pre-existing box. During the process of survey design, we attempted to model the survey in a patient relevant manner and to minimize the influence of any pre-existing biases of the research team.
We conducted a study of barriers, motivating factors and predictors of clinical trial participation among individuals with a rare lung disease. Our findings indicate that the motivation for and against clinical trial participation among those with rare diseases may be different from those with more common diseases like heart disease or cancer. Specifically, altruism is a dominant motivating factor, while older LAM patients and those with more advanced disease and greater impact on lifestyle are more likely to have participated in clinical trials. The impact of stringent study entry criteria, drug toxicity and stability of disease are important obstacles to successful trial enrollment, and may play a more significant role in clinical trials of rare diseases than of common ones. Future research should investigate barriers, motivating factors, and predictors of clinical trial participation in other rare diseases and determine effective recruitment methods for achieving successful clinical trial enrollment.
Acknowledgments
We would like to acknowledge the assistance of the LAM Foundation and all patients who participated.
Abbreviations
- LAM
lymphangioleiomyomatosis
- AML
angiomyolipomas
Footnotes
Sources of support: NIH Clinical Research Loan Repayment Grant (BWK) and a Dean’s Scholars Clinical Research grant from the University of Cincinnati (BWK).
Conflict of interest
None of the authors have actual or potential conflict of interest in the subject matter in this manuscript.
The following describes each author’s contributions to this manuscript: Study Design (BWK, ACS, LRY, NO, JTH, SB, FXM), subject enrollment and data acquisition (BWK, ACS, LRY, NO, JTH, SB, FXM), statistical analysis (BWK, JTH, LRY), manuscript preparation (BWK, ACS, LRY, NO, JTH, SB, FXM).
References
- 1.Cheung AM, Lee Y, Kapral M, Scher J, Ho I, Lui-Yee D, et al. Barriers and motivations for women to participate in cardiovascular trials. J Obstet Gynaecol Can. 2008 Apr;30(4):332–337. doi: 10.1016/S1701-2163(16)32802-X. [DOI] [PubMed] [Google Scholar]
- 2.Sharp L, Cotton SC, Alexander L, Williams E, Gray NM, Reid JM. Reasons for participation and non-participation in a randomized controlled trial: postal questionnaire surveys of women eligible for TOMBOLA(Trial Of Management of Borderline and Other Low-Grade Abnormal smears) Clin Trials. 2006;3(5):431–442. doi: 10.1177/1740774506070812. [DOI] [PubMed] [Google Scholar]
- 3.Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007 May 14;167(9):905–912. doi: 10.1001/archinte.167.9.905. [DOI] [PubMed] [Google Scholar]
- 4.Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007 Oct;60(10):990–1001. doi: 10.1016/j.jclinepi.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Mathews C, Restivo A, Raker C, Weitzen S, Disilvestro P. Willingness of gynecologic cancer patients to participate in clinical trials. Gynecol Oncol. 2009 Jan;112(1):161–165. doi: 10.1016/j.ygyno.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol. 2007 Apr;36(4):398–408. doi: 10.1165/rcmb.2006-0372TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006 Jan 1;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SR, Whale CI, Hubbard RB, Lewis SA, Tattersfield AE. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004 Sep;59(9):800–803. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) 1999 Sep;78(5):321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Matsui K, Beasley MB, Nelson WK, Barnes PM, Bechtle J, Falk R, et al. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol. 2001 Apr;25(4):479–484. doi: 10.1097/00000478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 11.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008 Feb;133(2):507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 12.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, Kaye E, Krischer J, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2008 Nov 12; doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006 Jan 15;106(2):258–270. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 15.Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: a metaanalysis and systematic review of patient-reported factors. Lancet Oncol. 2006 Feb;7(2):141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 16.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006 May;129(5):1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 17.Young LR, Almoosa KF, Pollock-Barziv S, Coutinho M, McCormack FX, Sahn SA. Patient perspectives on management of pneumothorax in lymphangioleiomyomatosis. Chest. 2006 May;129(5):1267–1273. doi: 10.1378/chest.129.5.1267. [DOI] [PubMed] [Google Scholar]