Abstract
IgG-Fab fragment, a model antibody protein was hydrophobically modified by a novel approach of ion-pairing complexation. Three different sulphated ion-pairing agents were utilised including sodium dodecyl sulphate, taurocholic acid and dextran sulphate (DS). The formations of hydrophobic ion-pairing (HIP) complexes were dependant on pH and molar ratio of ion-pairing agent to Fab. Aqueous solubilities of HIP complexes were very low compared to Fab alone. In particular, when dextran sulphate was added as ion-pairing agent, formed Fab:DS HIP complexes were least soluble in water. Further, nanoparticles (NPs) loaded with drug and Fab:DS HIP complex were prepared and characterised with respect to encapsulation efficiency and size. We observed significant improvement in encapsulation efficiency for Fab:DS HIP complex-loaded nanoparticles. This study demonstrates a novel approach of formulating antibody-loaded nanoparticles which can also be employed for delivery of large antibodies.
Keywords: Antibody fragment, dextran sulphate, hydrophobic ion-pairing complex, IgG-Fab fragment, nanoparticles, protein
Introduction
Monoclonal antibodies represent one of the most effective classes of protein therapeutics being developed. Currently, 28 monoclonal antibodies are approved for clinical applications by US-FDA and many are in clinical trials. Amongst antibody therapeutics, several recombinant antibody fragments are emerging because of low molecular weight and minimal immunogenicity (Li and Zhu, 2010). So far, 54 antibody fragments have been entered in clinical studies and amongst them 30 are Fabs, 19 are scFvs and 5 are third-generation versions such as miniaturised antibodies (Nelson and Reichert, 2009). These small antibody fragments are less immunogenic and maintain similar target specificity of full length antibodies. In addition, some have greater efficacy and more applications than full length monoclonal antibodies. However, these highly potent therapeutics require frequent administration due to their short biological half-lives (Nelson and Reichert, 2009; El Sanharawi et al., 2010).
Several formulation strategies have been employed to enable sustained delivery of antibody therapeutics by nanoparticulate based dosage forms. However, such development represents a real challenge to scientists. The most commonly employed technique to encapsulate proteins into biodegradable nanoparticles is water in oil in water (W/O/W) double emulsion solvent evaporation method. In this process, protein in aqueous solution is emulsified with organic phase containing polymer to form w/o primary emulsion. Subsequently, this emulsion is added to large quantity of external aqueous phase containing surfactant (polyvinyl alcohol, PVA). The mixture is then stirred to evaporate organic solvent and the nanoparticles are separated by centrifugation. However, one of the limiting factors in developing nanoparticulate formulation of protein therapeutics is their hydrophilic nature. Because of hydrophilic nature, these molecules partition poorly into polymer matrix and rapidly penetrate to the external aqueous phase during encapsulation process leading to poor encapsulation efficiency (Cui et al., 2006; Gaudana et al., 2011a).
The hydrophobic ion-pairing (HIP) complexation has emerged as an alternative approach which represents a paradigm shift in the delivery of therapeutic proteins and peptides. HIP complex is formed by electrostatic interactions between ionizable groups of such drug molecules with oppositely charged groups of surfactant or polymer. The complex is reversible in nature and can easily dissociate in the presence of excess of oppositely charged ions (Gaudana et al., 2011a). Figure 1 depicts a schematic of HIP complexation process. The formed HIP complex is highly lipophilic in nature and is able to partition largely in to polymer matrix during encapsulation process (Meyer and Manning, 1998; Lengsfeld et al., 2002). As a result, HIP complexation significantly enhances encapsulation efficiency. However, lipophilicity of HIP complex depends on the type of ion-pairing agent employed for complexation. Hence, to prepare complexes with enough hydrophobicity in order to improve encapsulation efficiency, it may be necessary to screen ion-pairing agents. Moreover, the selection of ion-pairing agents also depends on properties of therapeutic proteins being complexed such as isoelectric point, molecular weight and number of charges in both protein and ion-pairing agent. Till now, this approach has been employed for the delivery of various peptides and proteins such as insulin, melittin, leuprolide, bovine serum albumin (BSA) and lysozyme (Choi and Park, 2000; Yoo et al., 2001; Shi et al., 2008; Yang et al., 2009; Sun et al., 2010; Gaudana et al., 2011a; Sun et al., 2011). Sodium dodecyl sulphate (SDS) is the most commonly employed ion-pairing agent in HIP complexation (Shi et al., 2008; Yang et al., 2009). In addition, there are also few reports regarding use of dextran sulphate and bile acids such as cholic acid and deoxycholic acid (Dalwadi and Sunderland, 2009; Yang et al., 2009; Sun et al., 2011; Gaudana et al., 2011a). To the best of our knowledge, application of HIP complexation in delivery of monoclonal antibody-based protein therapeutics has never been reported. Antibodies are large molecules with very complex three-dimensional structure with high density of both cationic (lysine and arginine) and anionic (aspartic acid and glutamic acid) amino acids which renders complexation of these molecules more challenging. In this study, we have investigated the effects of various sulphated ion-pairing agents (dextran sulphate, taurocholic acid and SDS; Figure 1) on HIP complex formation with IgG-Fab fragment and subsequent hydrophobicity enhancement. In addition, we have also developed IgG-Fab fragment and HIP complex-loaded nanoparticulate dosage forms utilising two different fabrication methodologies.
Figure 1.
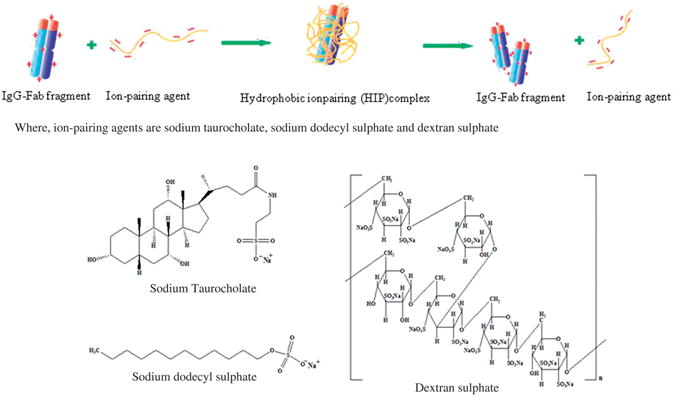
A schematic representation of HIP complexation process.
Materials and methods
Materials
Human IgG-Fab fragment was purchased from Athens Research & Technology (Athens, GA) and used after purification by dialysis. Dextran sulphate sodium salt (molecular weight 5000Da), Poly (dl-lactide-co-glycolide) (PLGA 85:15, molecular weight of 50000-75000 Da), taurocholic acid and SDS were procured from Sigma Aldrich (St. Louis, MO). NuPAGE® gels were purchased from Invitrogen™ Life Technologies (Carlsbad, CA). Precision Plus Protein™ Kaleidoscope™ Standards was purchased from BIO-RAD (Hercules, CA). Bicinchoninic acid (BCA) protein assay and micro-BCA protein assay kits were purchased from Fisher Scientific (Pittsburgh, PA). All the solvents and other reagents of analytical grade were purchased from Fisher Scientific and used as received without any further purification. Double distilled water (DDW) was used throughout the study.
Methods
Preparation of HIP complexes of Fab
Prior to complexation, Fab was dialysed against histidine buffer pH 5.5 to remove excessive salt and concentration was adjusted to 5 mg/ml. Stocks of ion-pairing agents were prepared in DDW. Briefly, pH of the Fab solution was adjusted with 0.1 N HCl to achieve more ionisation of basic amino acids providing more positive charges. Following pH adjustment, the solution of ion-pairing agent was added slowly to Fab solution. HIP complex was formed spontaneously as both aqueous solutions were mixed in an optimum ratio. Once formed, the HIP complex was vigorously vortexed for 5 min followed by centrifugation at 12000RPM for 15 min to separate supernatant. The resulting complex was redispersed and lyophilised into powder. Uncomplexed Fab was measured in the supernatant using BCA assay.
Effect of Fab solution pH on HIP complex formation
Effect of four different pH (5.5, 5.0, 4.5 and 4) on HIP complexation was studied. pH of Fab solution was adjusted with 0.1 N HCl followed by addition of ion-pairing agent to Fab fragment solution. Once formed, HIP complex was vigorously vortexed for 5 min followed by centrifugation at 12000 RPM for 15 min to separate supernatant. Uncomplexed Fab was measured in the supernatant using BCA assay.
Effect of ion-pairing agent to Fab molar ratio on HIP complex formation
HIP complexes were prepared with different molar ratios of ion-pairing agents to Fab. The four different molar ratios were studied. These ratios represent the addition of different amounts of ion-pairing agents into Fab solution. Once formed, HIP complex was vigorously vortexed for 5min followed by centrifugation at 12000 RPM for 15 min to separate supernatant. Uncomplexed Fab was measured in the supernatant using BCA assay.
Characterisation of the HIP complexes
Determination of Fab binding efficiency
The amount of Fab within the complexes was determined by indirect method. Briefly, free (uncomplexed) Fab remaining in the supernatant was measured by BCA assay kit. The percentage binding efficiency was expressed as the percentage Fab difference between the initial amount of Fab and the free amount in the supernatant relative to the initial amount of Fab added for the complex preparation. Percentage binding efficiency of Fab was calculated according to Equation (1) (Sun et al., 2011).
| (1) |
where Mi denotes initial amount of Fab added and Mf represents amount of free Fab measured in supernatant.
Aqueous solubility of HIP complexes
For determining aqueous solubility, HIP complex with 0.1 mg of Fab was added to 1 ml of water in Eppendorf tube and kept on shaker bath for 24 h at room temperature. Subsequently, the solution was centrifuged at 12000 rpm over 15 min. The supernatant was filtered through 0.45 μm syringe filter. The amount of complex in solution was determined by measuring protein concentration in supernatant with micro BCA assay. Solubility studies were carried out in triplicate (Patel et al., 2005).
Dissociation of Fab fragment from HIP complexes
(1) In phosphate buffered saline and water
Dissociation of Fab from HIP complex was studied to characterise the nature of interaction between Fab and ion-pairing agent. Briefly, HIP complexes containing 0.1 mg of Fab was incubated in the presence of 100 mM PBS or water. The solution was vortexed and kept for equilibrium for 6 h at room temperature. Then, the solution was subjected to centrifugation and supernatant was collected. Concentration of dissociated protein in the supernatant was then measured with micro BCA assay.
(2) Simulated body fluid
Dissociation of Fab from Fab:DS HIP complex was also studied in simulated body fluid (SBF) in order to mimic body environment. SBF was prepared as per previously published protocol (Marques Margareth et al., 2011). Briefly, HIP complexes containing 0.1 mg of Fab was incubated in the presence of SBF. The solution was vortexed and kept for equilibrium for 6 h at room temperature. Then, the solution was subjected to centrifugation and supernatant was collected. Concentration of dissociated protein in the supernatant was then measured with micro BCA assay.
Fourier transform infrared spectroscopy (FTIR)
FTIR analysis of Fab:DS HIP complexes was carried out with an infrared spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The samples were brought into intimate contact with the diamond crystal by applying a loading pressure. The samples were placed on diamond crystal top-plate of Attenuated Total Reflectance (ATR) accessory and scanned between 750–4000 cm−1. Spectra obtained using this device represents an average of 32 individual scan possessing a spectral resolution of 4 cm−1.
Evaluation of Fab stability using sodium dodecyl sulphatepolyacrylamide gel (SDS-PAGE) electrophoresis
To investigate the structural integrity of IgG-Fab fragment, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as per previously published protocol (Panyam et al., 2003). Briefly, protein samples were mixed with Laemelli's buffer and boiled for 5 min. Then a 20 μL aliquot of the mixture was loaded onto a well of a pre-cast NuPAGE 4–12% w/v Bis-Tris gel (Invitrogen, Carlsbad, CA). The samples were then subjected to electrophoresis at 60V. A blue silver staining was employed for protein visualisation (Candiano et al., 2004).
Preparation of nanoparticles (NPs)
Lyophilised Fab:DS HIP complex (most hydrophobic) was employed for nanoparticles preparation. PLGA (85:15) was employed for the nanoparticles fabrication. Nanoparticles were prepared by two different methods; (i) Modified nanoprecipitation method and (ii) Solid in oil in water (S/O/W) emulsion solvent evaporation method.
Modified nanoprecipitation method
Modified nanoprecipitation method published earlier was utilised for NPs fabrication (Gaudana et al., 2013). Effect of different drug to polymer ratios (1:10 and 1:15) on encapsulation efficiency was studied. Briefly, 1mg of Fab in HIP complex was used for preparation of NPs. A protein compatible solvent, i.e. DMSO was employed to dissolve PLGA and HIP complex. A mixture containing HIP complex and polymer was added slowly to 1% poloxamer solution. Nanoparticles formed instantaneously as both the solutions were mixed. Prepared NPs were washed two times with DDW to remove surface bound Fab, poloxamer and DMSO. This study was carried out in triplicate. Blank NPs were also prepared by employing only polymer in similar amounts.
Solid in oil in water (S/O/W) emulsion solvent evaporation method
To prepare NPs, PLGA(85:15) was selected as a polymer. Nanoparticles were prepared by solid in oil in water (S/O/W) emulsion solvent evaporation method published earlier with minor modifications (Gaudana et al., 2011a). Briefly, HIP complex with 1mg of Fab was used for preparation of NPs. PLGA(85:15) was dissolved in methylene chloride. PLGA solution was gradually added to the earlier prepared HIP complex. Votexing time and volumes of methylene chloride were optimised to obtain S/O dispersion. About 1–2 ml of methylene chloride was required to completely disperse the HIP complex. Sonication was performed for about ≈2 min using tip sonicator (Fisher 100 Sonic dismembrator, Fisher Scientific) at power output of 9–10W to obtain the fine S/O dispersion. This S/O dispersion was added to an external aqueous phase (10 ml, 1% PVA) followed by further sonication for ≈2 min. This procedure resulted in S/O/W nanoemulsion which was kept on a stirring at room temperature for 15min followed by complete evaporation of methylene chloride using a Rotavap. After evaporation, the nanodispersion was centrifuged for 20 min at 20 000 rpm. Prepared NPs were washed two times with DDW to remove surface bound Fab and PVA. This study was carried out in triplicate. Blank nanoparticles were also prepared by employing only polymer in similar amounts.
Characterisation of nanoparticles
Encapsulation efficiency
The encapsulation efficiency (EE%) was measured using indirect method. The amount of encapsulated drug was calculated using mass balance by subtracting the amount of the free drug present in the supernatant from the initial drug amount. The amount of Fab remaining in the supernatant was measured by BCA assay kit. Percent encapsulation efficiency was calculated using Equation (2) (Niculae et al., 2012).
| (2) |
Particle size and zeta potential measurement
The mean particle size and polydispersity of the various NPs suspensions were measured at 25 °C by dynamic light scattering method (Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK). A dilute sample of the nanosuspension was examined for particle size analysis. The particle size of different samples was evaluated and represented as Z-average diameter. The zeta potential of the NPs was measured using the zeta potential analysis mode of the instrument. To study the effect of pH, suspensions of nanoparticles were prepared in 10 mM HEPES buffer of different pH (pH adjusted either with 0.1 N hydrochloric acid or 0.1 N sodium hydroxide) and particle size and zeta potential were measured immediately (Sahoo et al., 2002).
Morphology
Morphology of nanoparticles was analysed by scanning electron microscopy (SEM). For SEM analysis, freeze dried specimen was applied on a sticky carbon film positioned on an aluminum stub. Specimens were sputter coated with gold-palladium and imagined with the field-emission SEM XL30 (FEI, Hillsboro, OR) at 2–5 kV.
Results and discussion
In these studies, application of hydrophobic ion-pairing complexation in developing antibody nanocarriers was demonstrated using human IgG-Fab fragment (48kDa) as a model antibody protein.
Preparation and characterisation of HIP complexes
HIP complexes of human IgG-Fab fragment were prepared and characterised with respect to % Fab binding efficiency. We have selected three common sulphated ion-pairing agents (dextran sulphate, SDS and taurocholic acid) to prepare HIP complexes with Fab. The pKa of sulphate group in all three agents is <2 and hence all these molecules carry negative charge above pH 2 (Sacco and Dellacherie, 1986; Elkins and Mullis, 2004; Lovaglio et al., 2011). In addition, we have also investigated some other ion-pairing agents such as sodium cholate (pKa 5.2), sodium deoxycholate (pKa 6.3) and oleic acid (pKa 8) however, their high pKa (>5) with less positive charges on Fab above these pH restricted the formation of HIP complexes (Silen and Forte, 1975; Mathias et al., 1981).
Hydrophobic ion pairing complexation was optimised with respect to pH of Fab solution and molar ratio of ion-pairing agents to Fab. Effects of four different pH conditions on binding efficiency of Fab have been evaluated. Briefly, a pH of Fab solution was adjusted using 0.1 N HCl to 5.5, 5.0, 4.5 and 4 (Figure 2). A higher binding efficiency (more than 90%) has been observed when pH was less than 5 in case of both the dextran sulphate and SDS which might be attributed to ionisation of basic amino acids in greater extent under these conditions. Protonation of these amino acids are dependent on pH of surrounding medium which promote complexation by ionic interactions amongst oppositely charged species. In case of taurocholic acid (TA), binding efficiency also showed ascending trend with reduction in pH. However, extent of binding was very low under all studied pH conditions. These results may be attributed to relatively less hydrophobic nature of TA (in comparison to SDS) and lower charge density relative to Fab. In some cases, it may require longer incubation for complexation while in this case we have incubated it only for 5 min. Similar results were observed with bovine serum albumin (BSA). Fifty percent binding efficiency was obtained following 10min incubation of BSA with TA. However, binding efficiency was significantly enhanced up to 90% following 3h incubation (data not shown). Subsequently, effect of different molar ratios of ion-pairing agents to Fab on binding efficiency was also evaluated (Figure 3). A rise in binding efficiency of Fab was noted with increase in molar ratios. At molar ratio which corresponds to approximate charge ratio of 1:1, maximum % binding efficiency of Fab was observed with dextran sulphate or SDS. However, further increment in molar ratio did not show any significant increase in binding efficiency. On the other hand, when taurocholic acid (TA) was used as ion-pairing agent, maximum percentage binding efficiency was achieved with molar ratio of 125 which corresponds to an approximate charge ratio of 4:1 (TA:Fab). Several reports discuss complexation at higher molar ratio especially when there is a large difference in molar masses of both the electrolytes, as in our case (Chen et al., 2003). The molecular weight of Fab is 48kDa which is much higher compared to that of TA (molecular weight 515.7).
Figure 2.
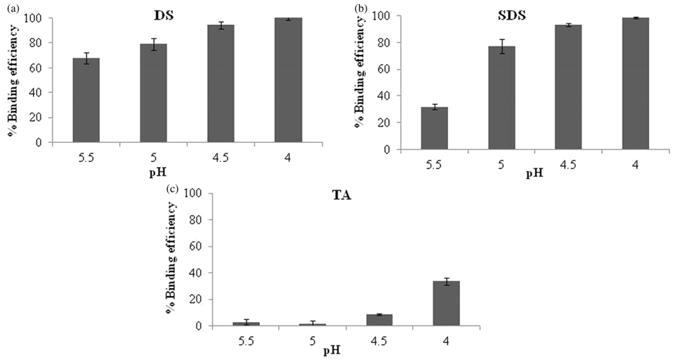
Effect of pH on % binding efficiency of Fab with different ion-pairing agents: (a) Dextran sulphate, (b) SDS, and (c) TA. Note: Values are represented as mean ± SD (n = 3).
Figure 3.
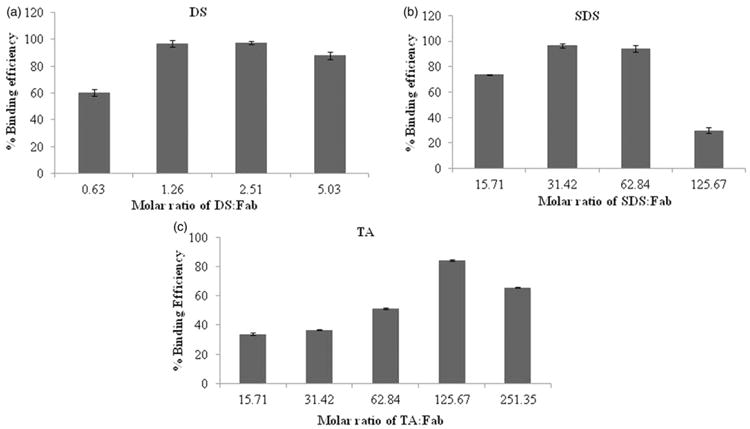
Effect of molar ratio on % binding efficiency of Fab with different ion-pairing agents: (a) Dextran sulphate, (b) SDS, and (c) TA. Note: Values are represented as mean ± SD (n = 3).
In this study, hydrophobicity of Fab was elevated by complexing with ion-pairing agents. The octanol:water partition coefficient is more common approach to determine hydrophobicity/hydrophilicity of molecule. However, complexes formed with Fab were insoluble in octanol and hence not amenable to this method. Therefore, to assess the augmentation in hydrophobicity, aqueous solubility of resultant HIP complexes was measured. All the complexes have shown very low aqueous solubility compared with Fab. The Fab:DS complex was least soluble in water. As shown in Figure 4, the ability of HIP complexes to reduce the aqueous solubility of Fab has been confirmed.
Figure 4.
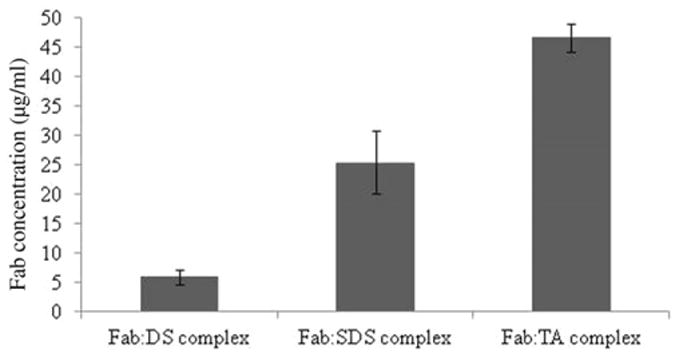
Aqueous solubility of HIP complexes. Note: Values are represented as mean ± SD (n = 3).
Ionic interactions are the driving forces for the HIP complexation which are delicate and can be dismantle in the presence of higher ionic strength. The dissociation studies indicate how ionic forces may be essential for complexation and can be disrupted in the presence of strong ionic medium such as PBS. To test this hypothesis, dissociation study of complexes was carried out in phosphate buffered saline (PBS) and DDI water as well. Results in Figure 5(a) demonstrate that more than 90% of Fab dissociated from Fab:DS HIP complex in PBS. However, negligible dissociation was observed in water which confirms existence of ionic interactions between Fab and DS.
Figure 5.
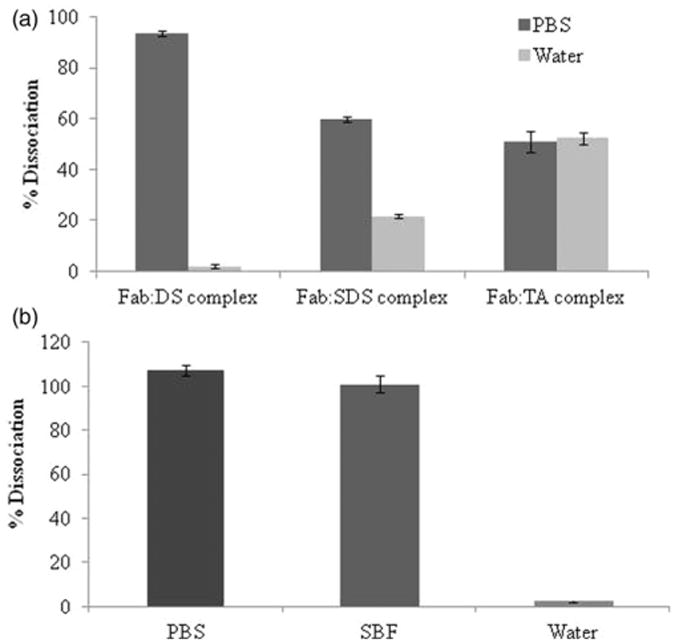
Dissociation of HIP complexes. (a) Comparative dissociation of Fab HIP complexes in PBS and water. (b) Dissociation of Fab:DS HIP complexes in different medium (PBS, SBF and water). Note: PBS: phosphate buffered saline; SBF: simulated body fluid. Values are represented as mean ± SD (n = 3).
In contrast, HIP complex between SDS and Fab have shown around 60% dissociation in PBS. This observation may suggest the presence of hydrophobic interactions between hydrophobic amino acids of Fab and long hydrophobic chain of SDS in addition to ionic interactions. The presence of both hydrophobic and ionic interactions hindered dissociation of such HIP complexes in presence of PBS. However, when TA was employed as ion-pairing agent; complexes are formed solely due to hydrophobic interactions and therefore dissociated about equally in the presence of both PBS and water. Alternatively, it can also be explained by higher aqueous solubility of Fab:TA HIP complexes. Moreover, there are also possibility of strong hydrogen bonding between TA and Fab as it possesses both hydrogen acceptor and donor groups. Other researchers have reported presence of ionic interaction between protein with SDS and bile acids (Yang et al., 2009; Sun et al., 2011). However, in this case, with SDS and TA as ion-pairing agent the complexation may preferentially involve hydrophobic interactions rather than ionic interactions. The reason might be the complex structure of antibody over other proteins studied in earlier reports. Based on solubility and dissociation results, we have selected the most hydrophobic Fab:DS HIP complex for further studies.
Further, in order to mimic in vivo situation, we have also carried out dissociation of Fab:DS HIP complexes into simulated body fluid. Results are presented in Figure 5(b). Our results showed that Fab:DS HIP complexes were completely dissociated in the presence of SBF demonstrating in vivo applicability.
FTIR spectroscopy was also performed to understand the nature of interactions between amino groups of basic amino acids in Fab and sulphate group of DS. Previously, FTIR analysis was performed by other investigators to characterise interactions between oppositely charged ionic groups (Dai and Dong, 2007). The observed characteristics peaks for sulphate group of DS in the IR region are: (A) 804.31 cm−1: S–O–S vibration, (B) 983.6 cm−1: symmetric SOO- stretching vibration and (C) 1226.7 cm−1: asymmetric SOO- stretching vibration. The observed peaks are consistent with previously published results. The ionic interactions can be interpreted in terms of attenuation in observed IR peaks for sulphate group or shift in the peaks (Sun et al., 2011). Due to such interactions between amino and sulphate groups, a peak for sulphate group in the IR region has been diminished. The IR spectra of Fab alone, dextran sulphate and Fab:DS HIP complex are shown in Figure 6. In addition to nature of interaction, the native structure of the protein was also examined using IR spectroscopy. In the IR spectrum of Fab, three main bands were observed at 1633.7.9 cm−1 (amide I), 1537.2 cm−1 (amide II) and 3284.7 cm−1 (amide A band) (Yang et al., 2009). From the spectra, it can be confirmed that the amide I band (1630-1700 cm−1) of the complex and pure Fab was similar. It clearly indicates that the native secondary structure conformation of Fab in the complex was retained.
Figure 6.
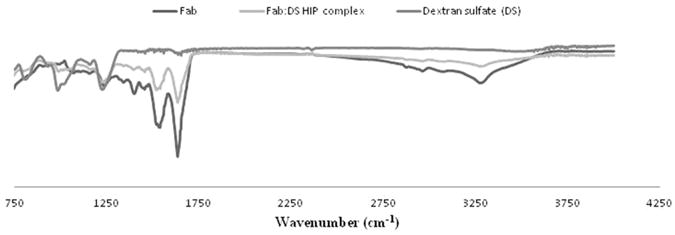
Infrared spectra of Fab, Fab:DS HIP complex and Dextran sulphate.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis analysis has been used extensively to demonstrate the stability of proteins. To study the effect of HIP complexation on structural integrity of Fab, reducing SDS-PAGE was performed. A representative image of the stained gel containing visible bands of proteins is shown in Figure 7. Lane 1 represents molecular weight marker, lane 2 is the Fab dissociated from Fab:DS HIP complex and lane 3 is the Fab standard. The presence of bands in lane 2 and 3 at approximately 24 kDa indicates the structural similarity between components represented by the bands. This study confirms that Fab dissociated from HIP complex did not suffer covalent aggregation or degradation by fragmentation. There are no deleterious effects of HIP complexation on Fab structure observed.
Figure 7.
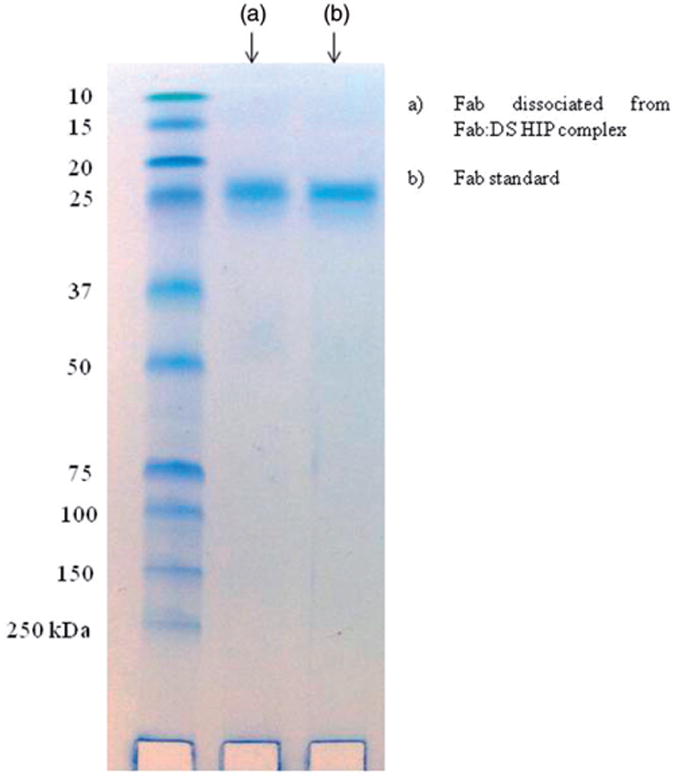
SDS-PAGE gel electrophoresis of Fab. (a) Fab dissociated from Fab:DS HIP complex and (b) Fab standard.
Preparation and characterisation of nanoparticles
The primary goal in this study was to achieve higher encapsulation of Fab in NPs by employing minimal amounts of polymer. To achieve this goal, we have prepared the most hydrophobic Fab:DS HIP complex loaded NPs utilising PLGA (85:15) as a polymer. The most hydrophobic PLGA (85:15) was selected so that hydrophobic interactions with HIP complex would increase which will subsequently lead to higher encapsulation.
Nanoprecipitation and solid in oil in water emulsion solvent evaporation methods reported earlier were employed for nano-particles preparation with slight modifications. These methods of preparation offer significant advantages over conventional water in oil in water (W/O/W) double emulsion method. In the conventional method, protein is initially dissolved in an aqueous phase and then subjected to emulsification in the presence of an organic phase using sonication. Due to hydrophilic nature, these molecules show very poor partitioning into polymer matrix and rapidly penetrate to the external aqueous phase during encapsulation process leading to poor encapsulation efficiency (Cui et al., 2006; Gaudana et al., 2011a).
In this study, nanoparticles have been prepared by employing two different fabrication methods: (a) nanoprecipitation method and (b) S/O/W method. Nanoparticles loaded with both Fab and Fab:DS HIP complex were prepared with two different ratios of protein to polymer (1:10 and 1:15) via nanoprecipitation method. The highest encapsulation of Fab in nanoparticles was achieved with protein:PLGA ratio of 1:15 when HIP complex was employed. In contrast, with Fab alone, poor encapsulation efficiency was achieved. NPs characterisations data are given in Table 1. The increased encapsulation might be attributed to more partitioning of HIP complex into polymer matrix and lower solubility into aqueous external phase. The postulated rationale is that very hydrophobic Fab:DS complex because of its low water solubility partitions more into hydrophobic PLGA(85:15) matrix and precipitate with polymer during nanoprecipitation. On the other side, higher water solubility of Fab favours its diffusion to aqueous external phase leading to poor encapsulation efficiency.
Table 1.
Zeta potential, particle size, polydispersity and encapsulation efficiency of various batches of nanoparticles prepared by nanoprecipitation method.
| Formulation | Polymer | Drug | D:P ratio | % Encapsulation efficiency | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|---|---|
| A | PLGA(85:15) | Fab:DS HIP | 1:10 | 37.61 ± 17.0 | 178.05 ± 24.0 | 0.147 ± 0.07 | −22.7 ±1.9 |
| B | PLGA(85:15) | Fab:DS HIP | 1:15 | 70.76 ± 14.09 | 192.45 ±2.05 | 0.092 ±0.041 | −22.5 ±3.3 |
| C | PLGA(85:15) | Fab | 1:10 | 11.17 ± 6.62 | 154.55 ±0.77 | 0.112 ±0.005 | −18.7 ±5.4 |
| D | PLGA(85:15) | Fab | 1:15 | 18.73 ± 1.58 | 199.1 ± 1.97 | 0.190 ±0.004 | −15.1 ±4.5 |
Note: Values are given as mean±SD (n = 3).
Both Fab and Fab:DS HIP complex-loaded nanoparticles were also prepared by solid in oil in water emulsion solvent evaporation method. Similar to nanoprecipitation method, higher encapsulation efficiency was achieved with Fab:DS complex with average % encapsulation efficiency of 85% (Table 2). However, when Fab alone was used % EE was less than 15% (Table 2).
Table 2.
Zeta potential, particle size, polydispersity and encapsulation efficiency of various batches of nanoparticles prepared by solid in oil in water (S/O/W) method.
| Formulation | Polymer | Drug | D:P ratio | % Encapsulation efficiency | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|---|---|
| E | PLGA(85:15) | Fab:DS HIP | 1:15 | 85.25 ±3.11 | 171.15 ± 11.10 | 0.106 ±0.024 | −19.2 ± 1.2 |
| F | PLGA(85:15) | Fab | 1:15 | 14.97 ±2.12 | 188.3 ±4.80 | 0.048 ±0.021 | −19.6 ± 3.15 |
Note: Values are given as mean ± SD (n = 3).
The resultant nanoparticles were also characterised with respect to particle size and zeta potential (Tables 1 and 2). In all cases, size of nanoparticles varied from 150 to 200 nm with narrow size distribution. Prepared NPs carried negative zeta potential which is in accordance with previously published studies with PLGA nanoparticles (Chen et al., 2008; Zhang et al., 2008; Ghotbi et al., 2011).
Effect of pH on nanoparticle characteristics was also evaluated. Table 3 represents influence of pH on nanoparticle size and zeta potential. From the results, it can be depicted that there was no effect of pH on size of nanoparticles prepared using S/O/W method. It clearly signifies that there is no aggregation in nanoparticle at different pH. Similarly, in case of nanoparticles prepared with nanoprecipitation method, there was no effect on particle size until pH 5 however; we have observed some increase in size at pH 4 which is in accordance with the zeta potential results. Results of Table 3 demonstrate that all the nanoparticles carried negative zeta potential at studied pH and zeta potential was reduced with lowering in pH. However, the change in zeta potential for nanoparticles prepared with S/O/W method was not significant. On the other hand, the zeta potential for nanoparticles prepared with nanoprecipitation method was drastically reduced at lower pH which might contributed to increment in size due to aggregation. The difference in behaviours between both the batches can be explained by different preparation methods, use of different surfactant and different solvent during preparation. However, it is beyond the scope of the article to elucidate detail mechanism for different behaviours.
Table 3.
Effect of pH on Fab:DS HIP NPs size and zeta potential. (a) Particle size of Fab:DS HIP NPs with respect to pH and (b) Zeta potential of Fab:DS HIP NPs with respect to pH.
| No | Method of preparation | Drug | pH | Particle size (nm) |
|---|---|---|---|---|
| (a) Particle size of Fab:DS HIP NPs with respect to pH | ||||
| 1 | Nanoprecipitation method | Fab:DS HIP | 7.45 | 222.10 ±5.54 |
| 6 | 217.55 ±0.97 | |||
| 5 | 216.05 ± 1.77 | |||
| 4 | 284.70 ±9.41 | |||
| 2 | S/O/W method | Fab:DS HIP | 7.45 | 171.90 ±3.87 |
| 6 | 174.40 ±2.77 | |||
| 5 | 175.47 ±3.23 | |||
| 4 | 173.90 ±2.17 | |||
| (b) Zeta potential of Fab:DS HIP NPs with respect to pH. | Zeta potential (mV) | |||
|
|
||||
| 1 | Nanoprecipitation method | Fab:DS HIP | 6 | −10.25 ± 0.07 |
| 4 | −3.63 ±0.09 | |||
| 2 | S/O/W method | Fab:DS HIP | 6 | −5.47 ± 1.68 |
| 4 | −4.80 ±0.66 | |||
Note: Values are represented as mean ± SD (n = 3).
Scanning electron microscopy was performed to study the nanoparticle surface morphology. We have analysed morphology of Fab:DS HIP complex-loaded nanoparticles prepared by S/O/W method (with highest encapsulation efficiency). Results of this study are shown in Figure 8. Result confirmed that Fab:DS HIP complex-loaded particles prepared with S/O/W method have smooth surface and spherical shape. In previous studies also, researcher observed this kind of morphology for nanoparticle prepared by S/O/W method. The results are in accordance with previously published results (Gaudana et al., 2011b; Kashi et al., 2012).
Figure 8.

Scanning electron microscopy image of Fab:DS HIP-loaded NPs prepared by solid in oil in water (S/O/W) method.
Conclusions
This study for the first time shows the feasibility of forming HIP complex of an antibody such as IgG-Fab with different kinds of ion-pairing agents. Study confirms the involvement of basic amino acids in a protein in the formation of HIP complexation. Dissociation studies of HIP complex in the presence of higher ionic strength medium as well as FTIR studies have revealed the presence of strong ionic interactions between basic amino acids of Fab and sulphate groups present in DS. Moreover, there was no any deleterious effect of HIP complexation on Fab structural integrity. We have successfully prepared and characterised nanoparticles of Fab in HIP complex form using nanoprecipitation and S/O/W methods. Significant encapsulation of Fab in nanoparticles has been obtained with low PLGA amounts using the HIP technique. Taken altogether, HIP complexation can be a promising approach to enhance encapsulation of large proteins such as full length antibody and other antibody fragments based therapeutic molecules in nanocarriers.
Acknowledgments
Declaration of interest: This study was supported by NIH grants R01 EY-09171 and R01 EY-10659.
References
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–33. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Chen J, Heitmann JA, Hubbe MA. Dependency of polyelectrolyte complex stoichiometry on the order of addition. 1. Effect of salt concentration during streaming current titrations with strong poly-acid and poly-base. Colloids Surf A: Physicochem Eng Aspects. 2003;223:215–30. [Google Scholar]
- Chen Y, Wang F, Benson HA. Effect of formulation factors on incorporation of the hydrophilic peptide dalargin into PLGA and mPEG-PLGA nanoparticles. Biopolymers. 2008;90:644–50. doi: 10.1002/bip.21013. [DOI] [PubMed] [Google Scholar]
- Choi SH, Park TG. Hydrophobic ion pair formation between leuprolide and sodium oleate for sustained release from biodegradable polymeric microspheres. Int J Pharm. 2000;203:193–202. doi: 10.1016/s0378-5173(00)00457-9. [DOI] [PubMed] [Google Scholar]
- Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114:242–50. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Dai WG, Dong LC. Characterization of physiochemical and biological properties of an insulin/lauryl sulfate complex formed by hydrophobic ion pairing. Int J Pharm. 2007;336:58–66. doi: 10.1016/j.ijpharm.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Dalwadi G, Sunderland B. An ion pairing approach to increase the loading of hydrophilic and lipophilic drugs into PEGylated PLGA nanoparticles. Eur J Pharm Biopharm. 2009;71:231–42. doi: 10.1016/j.ejpb.2008.08.004. [DOI] [PubMed] [Google Scholar]
- El Sanharawi M, Kowalczuk L, Touchard E, Omri S, De Kozak Y, Behar-Cohen F. Protein delivery for retinal diseases: From basic considerations to clinical applications. Prog Retin Eye Res. 2010;29:443–65. doi: 10.1016/j.preteyeres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Elkins CA, Mullis LB. Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl Environ Microbiol. 2004;70:7200–9. doi: 10.1128/AEM.70.12.7200-7209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudana R, Gokulgandhi M, Khurana V, Kwatra D, Mitra AK. Design and evaluation of a novel nanoparticulate-based formulation encapsulating a HIP complex of lysozyme. Pharm Dev Technol. 2013;18:752–9. doi: 10.3109/10837450.2012.737806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudana R, Khurana V, Parenky A, Mitra AK. Encapsulation of protein-polysaccharide HIP complex in polymeric nanoparticles. J Drug Deliv. 2011a;2011 doi: 10.1155/2011/458128. Article ID 458128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudana R, Parenky A, Vaishya R, Samanta SK, Mitra AK. Development and characterization of nanoparticulate formulation of a water soluble prodrug of dexamethasone by HIP complexation. J Microencapsul. 2011b;28:10–20. doi: 10.3109/02652048.2010.520093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghotbi Z, Haddadi A, Hamdy S, Hung RW, Samuel J, Lavasanifar A. Active targeting of dendritic cells with mannan-decorated PLGA nanoparticles. J Drug Target. 2011;19:281–92. doi: 10.3109/1061186X.2010.499463. [DOI] [PubMed] [Google Scholar]
- Kashi TS, Eskandarion S, Esfandyari-Manesh M, Marashi SM, Samadi N, Fatemi SM, Atyabi F, Eshraghi S, Dinarvand R. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine. 2012;7:221–34. doi: 10.2147/IJN.S27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengsfeld CS, Pitera D, Manning M, Randolph TW. Dissolution and partitioning behavior of hydrophobic ion-paired compounds. Pharm Res. 2002;19:1572–6. doi: 10.1023/a:1020429321350. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin. 2010;31:1198–207. doi: 10.1038/aps.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovaglio RB, Dos Santos FJ, Jafelicci M, Jr, Contiero J. Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids Surf B Biointerfaces. 2011;85:301–5. doi: 10.1016/j.colsurfb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Marques Margareth RC, Raimar L, May A. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18:15–28. [Google Scholar]
- Mathias PM, Harries JT, Muller DP. Optimization and validation of assays to estimate pancreatic esterase activity using well-characterized micellar solutions of cholesteryl oleate and tocopheryl acetate. J Lipid Res. 1981;22:177–84. [PubMed] [Google Scholar]
- Meyer JD, Manning MC. Hydrophobic ion pairing: Altering the solubility properties of biomolecules. Pharm Res. 1998;15:188–93. doi: 10.1023/a:1011998014474. [DOI] [PubMed] [Google Scholar]
- Nelson AL, Reichert JM. Development trends for therapeutic antibody fragments. Nat Biotechnol. 2009;27:331–7. doi: 10.1038/nbt0409-331. [DOI] [PubMed] [Google Scholar]
- Niculae G, Lacatusu I, Badea N, Meghea A. Lipid nanoparticles based on butyl-methoxydibenzoylmethane: In vitro UVA blocking effect. Nanotechnology. 2012;23:315704. doi: 10.1088/0957-4484/23/31/315704. [DOI] [PubMed] [Google Scholar]
- Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(D,L-lactide-co-glycolide) nano- and micro-particles. J Control Release. 2003;92:173–87. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- Patel K, Trivedi S, Luo S, Zhu X, Pal D, Kern ER, Mitra AK. Synthesis, physicochemical properties and antiviral activities of ester prodrugs of ganciclovir. Int J Pharm. 2005;305:75–89. doi: 10.1016/j.ijpharm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Sacco D, Dellacherie E. Interaction of a macromolecular polyanion, dextran sulfate, with human hemoglobin. FEBS Lett. 1986;199:254–8. doi: 10.1016/0014-5793(86)80490-2. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–14. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- Shi K, Cui F, Yamamoto H, Kawashima Y. Investigation of drug loading and in vitro release mechanisms of insulin-lauryl sulfate complex loaded PLGA nanoparticles. Pharmazie. 2008;63:866–71. [PubMed] [Google Scholar]
- Silen W, Forte JG. Effects of bile salts on amphibian gastric mucosa. Am J Physiol. 1975;228:637–44. doi: 10.1152/ajplegacy.1975.228.2.637. [DOI] [PubMed] [Google Scholar]
- Sun S, Liang N, Kawashima Y, Xia D, Cui F. Hydrophobic ion pairing of an insulin-sodium deoxycholate complex for oral delivery of insulin. Int J Nanomedicine. 2011;6:3049–56. doi: 10.2147/IJN.S26450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Liang N, Piao H, Yamamoto H, Kawashima Y, Cui F. Insulin-S.O (sodium oleate) complex-loaded PLGA nanoparticles: Formulation, characterization and in vivo evaluation. J Microencapsul. 2010;27:471–8. doi: 10.3109/02652040903515490. [DOI] [PubMed] [Google Scholar]
- Yang L, Cui F, Shi K, Cun D, Wang R. Design of high payload PLGA nanoparticles containing melittin/sodium dodecyl sulfate complex by the hydrophobic ion-pairing technique. Drug Dev Ind Pharm. 2009;35:959–68. doi: 10.1080/03639040902718039. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Choi HK, Park TG. Protein-fatty acid complex for enhanced loading and stability within biodegradable nanoparticles. J Pharm Sci. 2001;90:194–201. doi: 10.1002/1520-6017(200102)90:2<194::aid-jps10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2008;2:1696–702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]


