Abstract
Propionibacterium acnes is a key therapeutic target in acne, yet this bacterium has become resistant to standard antibiotic agents. We investigated whether the human antimicrobial protein granulysin is a potential candidate for the treatment of acne. Granulysin and synthetic granulysin-derived peptides possessing a helix–loop–helix motif killed P. acnes in vitro. Modification of a helix–loop–helix peptide, 31–50, by substitution of a tryptophan for the valine at amino acid 44 (peptide 31–50v44w) to increase its interaction with bacterial surfaces also increased its antimicrobial activity. Moreover, when synthesized with d- rather than l-type amino acids, this peptide (d-31– 50v44w) became less susceptible to degradation by proteases and more effective in killing P. acnes. Granulysin peptides were bactericidal, demonstrating an advantage over standard bacteriostatic antibiotics in their control of P. acnes. Moreover, peptide d-31–50v44w killed P. acnes in isolated human microcomedone preparations. Importantly, peptides 31–50, 31–50v44w, and d-31–50v44w also have potential anti-inflammatory effects, as demonstrated by suppression of P. acnes-stimulated cytokine release. Taken together, these data suggest that granulysin peptides may be useful as topical therapeutic agents, providing alternatives to current acne therapies.
Keywords: acne, antimicrobial peptide, inflammation, innate immunity, skin
Since their introduction approximately 60 y ago, antibiotics have been our most powerful weapons against microbial invaders. The effectiveness of traditional antibiotics has, however, been severely limited by the development of multidrug-resistant bacterial strains. In particular, analysis of clinical isolates of Propionibacterium acnes, a major etiologic agent of acne vulgaris, has indicated increasing resistance to standard antibiotic therapies, making the treatment of acne more challenging (Cooper, 1998; Coates et al, 2002; Ross et al, 2003). Thus, there is a need for the development of new antimicrobial agents for the treatment of acne and other diseases with an infectious component.
Throughout evolution, single and multicellular organisms evolved host defense mechanisms that combat bacterial infections. These include endogenous antimicrobial peptides, a major mechanism of host defense, capable of both killing microbes and modulating the host's immune response. Granulysin is an antimicrobial peptide of the saposin-like family, a family of peptides that has been conserved from amoebas to humans and has been shown to be important in host defense of the skin (Ochoa et al, 2001). Our analysis of acne lesions demonstrated the presence of granulysin in inflammatory T cells, yet not in the pilosebaceous units where P. acnes resides. Other studies have described the presence of T cells in both early and late acne lesions, demonstrating the importance of T cells in the biology of acne.
We hypothesized that granulysin, representing a natural form of host defense in cutaneous infection, may be useful as a topical agent in the treatment of acne. We sought to explore the effectiveness of granulysin and granulysin-derived peptides in killing P. acnes in vitro, both in standard microbiologic assays and in human-derived microcomedones. Moreover, since P. acnes has been shown to trigger pro-inflammatory responses (Vowels et al, 1995; Kim et al, 2002) and since current antibiotics are thought to exert both antimicrobial and anti-inflammatory effects, we also sought to determine whether granulysin-derived peptides had the ability to inhibit P. acnes-induced cytokine release.
Results
Granulysin and granulysin peptides have antimicrobial activity against P. acnes
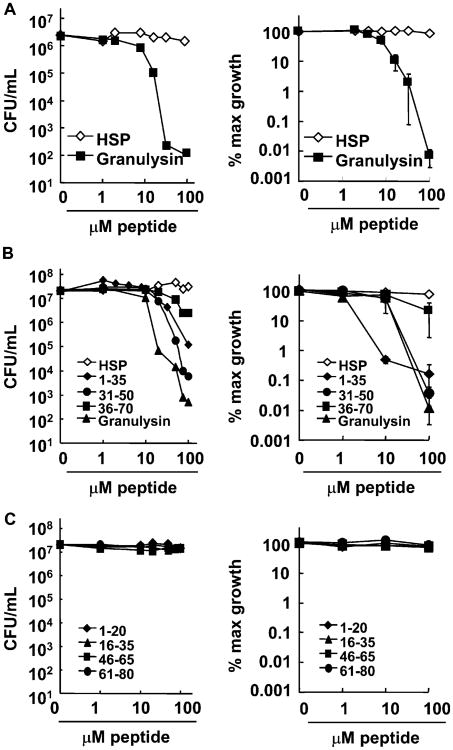
We hypothesized that antimicrobial proteins, having a broad spectrum of activity and being naturally present during acne, might be effective in combating P. acnes, a major therapeutic target in acne. One such protein is granulysin, which is localized in cytotoxic granules of NK and T cells and kills both Gram-positive and Gram-negative bacteria (Stenger et al, 1998). To determine whether the antimicrobial protein granulysin kills P. acnes, we tested the activity of recombinant human granulysin against P. acnes using the CFU assay. Granulysin exhibited antimicrobial activity in a concentration-dependent manner, reducing the number of P. acnes CFU by approximately 4 logs (to less than 5% maximum growth) at a concentration of 32 μM, whereas a control peptide derived from HSP70 had no effect (Fig 1A).
Figure 1. Granulysin and granulysin peptides have antimicrobial activity against Propionibacterium acnes.
(A) Various concentrations (0–100 μM) of recombinant granulysin were incubated with P. acnes for 4 h and tested for antimicrobial activity using the CFU assay (left, representative experiment; right, average % maximum growth ± SEM, n = 8). (B) Synthetic peptides of granulysin predicted to conform to a helix–loop–helix motif (residues 1–35, 36–70, and 31–50) and the 9-kDa granulysin were incubated with P. acnes at various concentrations (0–100 μM) for 4 h and the antimicrobial activity was determined by CFU assay (left, representative experiment; right, average % maximum growth ± SEM, n = 3). (C) Additional synthetic granulysin peptides (residues 1–20, 16–35, 46–65, and 61–80) were compared with the 9-kDa granulysin for antimicrobial activity by CFU assay (left, representative experiment; right, average % maximum growth ± SEM, n = 3).
The crystal structure of granulysin reveals that it contains five alpha helical domains, and the ability of granulysin to kill Salmonella typhimurium and Escherichia coli has been localized to regions of granulysin that include helices 2 or 3 (Ernst et al, 2000; Wang et al, 2000; Anderson et al, 2003). Therefore, we synthesized peptides based on the sequence of granulysin, some of which included these regions. Two peptides containing helix–loop–helix domains and corresponding to amino acid residues 1–35 and 31–50 were found to reduce the number of viable P. acnes; these peptides killed P. acnes in a concentration-dependent manner with 100 μM of peptide reducing the number of viable P. acnes by 2 and 4 logs, respectively, to less than 1% of maximum growth (Fig 1B). In contrast, four non-helix–loop–helix peptides (peptides 1–20, 16–35, 46–65, and 61–80) had little or no antimicrobial activity and reduced the number of viable P. acnes by <1 log without effecting the % maximum growth (Fig 1C). In whole, these data indicate that granulysin can kill P. acnes, and its antimicrobial activity can be localized to a smaller region of the molecule with a defined structural motif, the helix–loop–helix.
Modification of a granulysin peptide enhances its antimicrobial activity
We next sought to determine whether modifications to peptide 31–50 could enhance its antimicrobial activity. As amino acid 44 resides in a scissor-like pocket between helices 2 and 3, we hypothesized that substitution at this position to create a hydrophobic anchor for the peptide in the bacterial surface would enhance killing. Our modeling indicated that substituting the valine at position 44 with a tryptophan (31–50v44w) would increase the Fauchere and Pliska hydrophobicity from −0.3 to 0.7. As a positive Fauchere & Pliska hydrophobicity correlates with strong antimicrobial activity, we predicted peptide 31–50v44w would have enhanced activity. Moreover, tryptophans have been found in proteins that cross membranes and have been shown to be important for the activity of other antimicrobial peptides (Qi and Grabowski, 2001; Fimland et al, 2002). We therefore compared the antimicrobial activity of 31–50v44w to 31–50. Incubation with 32 μM of peptide 31–50v44w resulted in 2–3 logs fewer bacteria (with greater than 50 times the maximum growth inhibition) as compared to incubation with the peptide 31–50; the enhanced antimicrobial activity of 31–50v44w was evident over peptide concentrations ranging from 8 to 100 μM and was concentration-dependent (Fig 2A). This demonstrates that modifications to increase peptide hydrophobicity may also increase the antimicrobial activity of the peptide.
Figure 2. Modification of a granulysin peptide to enhance its antimicrobial activity.
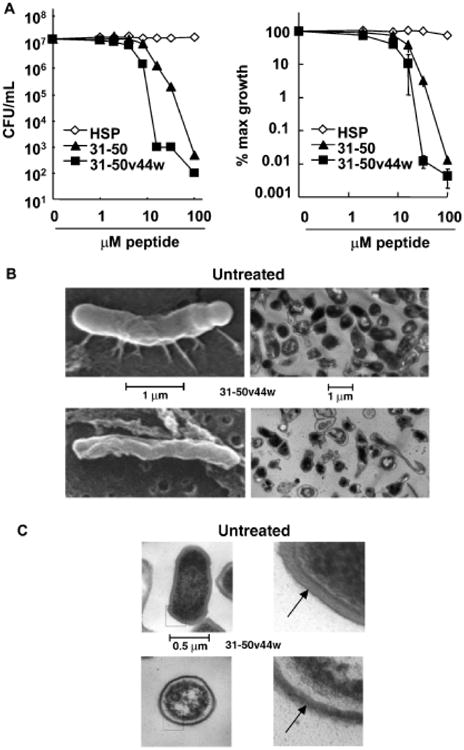
The antimicrobial effect of a structure-function modification to a granulysin peptide was determined. A hydrophobic residue, tryptophan, was added to the granulysin peptide 31–50 to create an anchor for the peptide in the bacterial surface. (A) Peptides 31–50 and 31–50v44w were then incubated with P. acnes and tested for antimicrobial activity by CFU assay (left, representative experiment; right, average % maximum growth ± SEM, n = 5), (B) SEM and low power TEM (10K magnification), demonstrated the effect of 31–50v44w (20 mM) on the surface of P. acnes after incubation for 72 h as compared to the surface of untreated control bacteria. (C) At higher power (36K magnification), peptide treatment (20 mM) was shown to disturb the sharply layered surface architecture of P. acnes, making the cell wall less distinct and more permeable to fluid influx. EM studies represent data from three different experiments.
Granulysin peptide 31–50v44w alters the surface of P. acnes
Granulysin exerts its antimicrobial activity against the Gram-negative bacterium E. coli by disturbing the integrity of the cell membrane (Ernst et al, 2000). To determine if a similar mechanism is employed in the killing of P. acnes, a Gram-positive organism, by granulysin peptides, we examined bacteria that had been treated with 31–50v44w using both scanning EM and transmission EM. Both, scanning and transmission EM micrographs of untreated P. acnes illustrate the bacterium's normal pleomorphic structure. By scanning EM, the surface of the untreated bacteria appeared smooth and rounded with fimbriae present, whereas the peptide-treated bacteria demonstrated a recessed and withered surface with an absence of fimbriae (Fig 2B). Similarly, differences between untreated and peptide-treated bacteria were appreciated by low magnification transmission EM, which demonstrated many “ghost” cells after 72 h of peptide treatment and few surviving bacteria with darker and more condensed cytoplasms compared to untreated bacteria. Higher magnification transmission EM revealed the untreated P. acnes had a cell wall with well-demarcated outer and inner dark, lipophilic layers and a lighter, hydrophilic peptidoglycan layer. In contrast, after only 1 h of incubation with 31–50v44w, P. acnes lost the integrity of this surface architecture as the lighter, more hydrophilic, peptidoglycan layer as well as the darker more lipophilic layers of the cell wall appear disturbed, losing their crisp, well-defined structures (Fig 2C). In addition, we observed a wider, likely edematous, space inside of the cell wall, further suggesting its disruption as well as peripheral clumping of nuclear material within the cell. In whole, these images reveal that peptide 31–50v44w perturbs the surface integrity of P. acnes in a manner that may make it porous, suggesting that this is the likely mechanism by which granulysin peptides kill this organism.
Further modification of 31–50v44w generates a peptide with greater antimicrobial activity
Since naturally occurring peptides exist in l-conformations and are susceptible to proteases, we synthesized a more stable peptide with the sequence 31–50v44w by using only d-type amino acids (d-31–50v44w) as they have been shown to be more protease-resistant (Alvarez-Bravo et al, 1994). We then tested this peptide against a wild-type strain of E. coli (TOP10) and a protease-deficient mutant strain of E. coli (ATCC 55099) to determine if greater peptide stability would enhance antimicrobial activity. Peptide d-31–50v44w was equally effective as l-31–50v44w in killing the protease-deficient strain of E. coli. The minimum bactericidal concentration (MBC) of d-31–50v44w against the wild-type, protease-positive strain of E. coli was, however, only 2 μM whereas that of l-31– 50v44w was 16 μM (Fig 3A). Additional studies demonstrated that d-31–50v44w exhibited great potency against P. acnes in vitro; treatment with as little as 32 μM of this peptide resulted in a 5 log reduction (to less than 0.01% maximum growth) in viable P. acnes by CFU assay (Fig 3B).
Figure 3. Antimicrobial effects of granulysin peptides with d- and l-type amino acids.
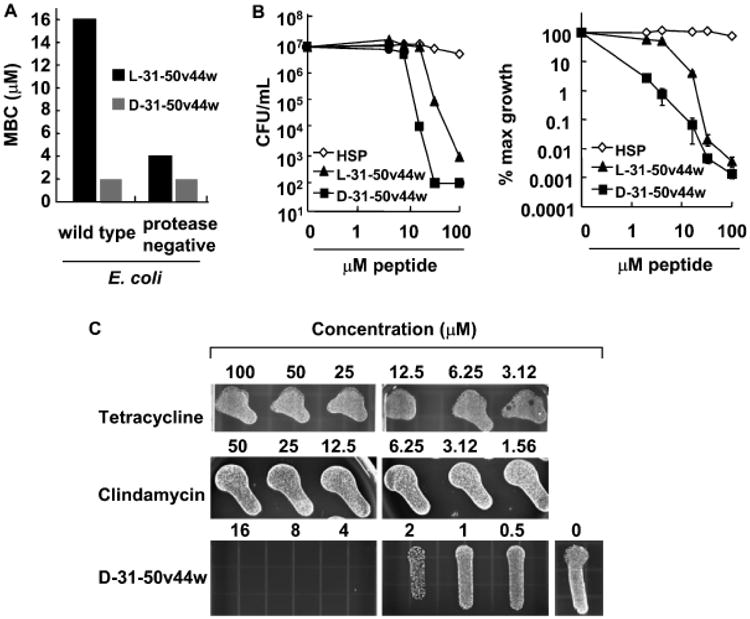
(A) Granulysin peptides with the sequence 31–50v44w were synthesized using only d-type amino acids (d-31–50v44w) or l-type amino acids (l-31–50v44w) and incubated with a wild-type strain of Escherichia coli or a mutant strain of E coli, and the minimum bactericidal concentration was determined. (B) Granulysin peptides with d-type amino acids (d-31–50v44w) or l-type amino acids (l-31–50v44w) were incubated with P. acnes and the antimicrobial activity was determined using CFU assay (left, representative experiment; right, average % maximum growth ± SEM, n = 4). (C) The antimicrobial activity of granulysin peptide d-31–50v44w was compared to tetracycline and clindamycin in a qualitative CFU assay which involved plating antibiotics and peptides with P. acnes on solid media and counting individual colonies to determine the CFU. Experiments in (A) and (C) are representative of two or more experiments.
Granulysin peptides are superior antimicrobials compared to antibiotics currently used to treat acne
In comparing the antimicrobial activity of the granulysin peptides against that of widely used antibiotics, we found that tetracycline and clindamycin were bacteriostatic, preventing the further growth of P. acnes. On the other hand, peptide d-31–50v44w was bactericidal, capable of potently decreasing the number of viable P. acnes (Fig 3C). Thus, granulysin peptides have an efficacy advantage over standard antibiotics in the control of P. acnes.
d-31–50v44w demonstrates antimicrobial activity in human microcomedone isolates
The earliest subclinical acne “lesion” is a microcomedone, which consists of keratinized material, lipids such as squalene, cholesterol, cholesterol esters, wax esters, and triglycerides, and P. acnes. In order to demonstrate that granulysin peptides maintain their antimicrobial activity within this lipid-rich environment and against strains of P. acnes present within the community, we have isolated microcomedones from human donors as an in vitro model for the efficacy of antimicrobial peptides. We tested the antimicrobial activity of d-31–50v44w against clinical isolates of P. acnes using microcomedone isolates. Indeed, d-31–50v44w was effective in killing P. acnes in sebaceous microcomedome extracts (Fig 4). Importantly, this demonstrates not only that this peptide is an effective bactericidal agent within sebaceous environments, but also that strains of P. acnes present in the community are susceptible to this peptide.
Figure 4. Antimicrobial effects of a granulysin peptide within the sebaceous micro-comedone environment.
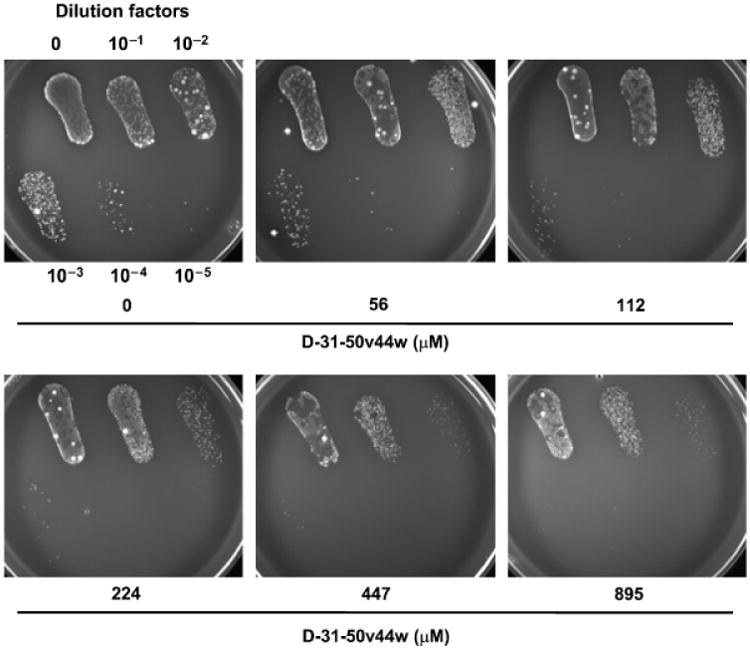
Granulysin peptide d-31–50v44w was incubated with Propionibacterium acnes in a collection of lipid-rich microcomedones isolated from donors' faces using pore cleasing strips, and the antimicrobial activity was determined using the CFU assay.
Anti-inflammatory effects of an antimicrobial granulysin peptide
An important component of acne is the inflammatory response elicited by P. acnes. Primary human monocytes stimulated with P. acnes secreted a wide variety of inflammatory cytokines and chemokines including IL-12p40, IL-12p70, IL-6, IL-8, MCP-1, IP-10, MIP-1α, MIP-3α, MDC, and ITAC (data not shown). The production of these cytokines and chemokines may play important roles in the formation of inflammatory acne lesions (Vowels et al, 1995; Kim et al, 2002). For instance, the induction of IL-8 by P. acnes may help recruit neutrophils to the skin, promoting pustule formation.
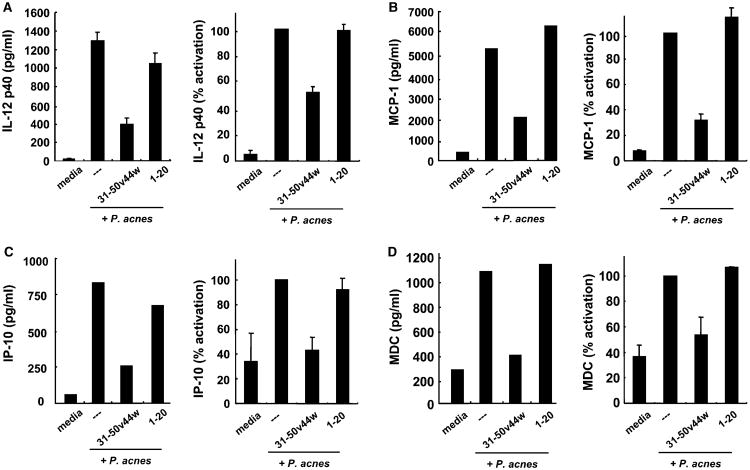
Given that antimicrobial peptides have been shown to have immunomodulatory effects (Territo et al, 1989; Van Wetering et al, 1997; Lentsch et al, 1999; Lillard et al, 1999; Yang et al, 1999; Scott et al, 2000, 2002), we determined whether peptide 31–50v44w could alter P. acnes-stimulated production of cytokines and chemokines from primary human monocytes. Importantly, 31–50v44w decreased P. acnes-stimulated production of IL-12p40, as compared to the non-helix-loop-helix peptides 1–20 and 16–35 (Fig 5A). In addition, peptide 31–50v44w inhibited the production of several chemokines, including MCP-1, IP-10, and MDC, from P. acnes-stimulated monocytes (Figs 5B–D). 31–50v44w, however, demonstrated little to no inhibition of IL-6 and IL-8 by P. acnes stimulated monocytes (data not shown). Furthermore, granulysin peptide d-31–50v44w also decreased P. acnes-stimulated production of cytokines and chemokines (data not shown).
Figure 5. The immunomodulatory effect of granulysin peptides.
Granulysin peptide 31–50v44w (5 μM) was incubated with primary human monocytes which were subsequently stimulated with Propionibacterium acnes sonicate. (A) IL-12p40 levels were determined by ELISA using triplicates (left, representative experiment; right, average % activation of IL-12p40 by P. acnes ± SEM, n = 12). The chemokines (B) MCP-1, (C) IP-10, and (D) MDC levels were determined by cytokine array (Pierce SearchLight Multiplex). (left, representative experiment; right, average % activation ± SEM of two independent experiments each assayed by array twice, n = 4).
Discussion
The increased incidence of drug resistance in clinical isolates of bacteria has underscored the need to identify new therapeutic agents. This issue is of particular importance in the treatment of P. acnes, a bacteria implicated in the pathogenesis of acne, since the increase in frequency of resistant strains is becoming a major clinical issue (Cooper, 1998; Coates et al, 2002; Ross et al, 2003). Since our immune system utilizes antimicrobial peptides to effectively combat infection, we hypothesized that these molecules may be suitable drug candidates. The ideal therapy for acne would be a potent antimicrobial agent with anti-inflammatory properties that maintains stability in the lipid-rich cutaneous environment. In this paper, we provide evidence that one such candidate, a modified granulysin peptide, effectively kills P. acnes in vitro, both in the growth media and in sebaceous units. Moreover, our data demonstrate that in contrast to tetracycline and clindamycin, bacteriostatic agents currently used to treat acne, granulysin peptides are bactericidal. Finally, these peptides demonstrate anti-inflammatory properties through their reduction of P. acnes-stimulated production of cytokines and chemokines. The combined antimicrobial and anti-inflammatory activity of granulysin-derived peptides make them logical therapeutic agents for the treatment of acne.
The secondary structure of an antimicrobial peptide has been shown to be essential for maintenance of its antimicrobial activity. Previous studies by Wang et al (2000) demonstrated that peptides based on the sequence of granulysin that contained segments of either helix 2 (amino acids 23–36) or helix 3 (amino acids 42–51) were capable of lysing bacteria, identifying these regions as important for antimicrobial activity against S. typhimurium (Wang et al, 2000). Furthermore, our earlier study demonstrated that peptides corresponding to amino acid residues 1–35, 31–50, and 36–70, which contain helix–loop–helix structures were capable of killing both E. coli and Mycobacterium tuberculosis, whereas those peptides that did not contain this secondary structure were incapable of bacterial killing (Ernst et al, 2000). Similarly, here we provide evidence that granulysin peptides with helix–loop–helix structures, peptides 1– 35 and 31–50, are effective at killing P. acnes. Additionally, it may be possible to design even shorter granulysin peptides with enhanced antimicrobial activity (Hamamoto et al, 2002).
An ideal topical antimicrobial therapy must be both potent in activity and stable to the cutaneous environment. We have modified the granulysin peptide 31–50 in two different ways to address these characteristics. First, we substituted the valine at amino acid position 44 for a tryptophan and created a peptide (31–50v44w) with enhanced antimicrobial activity against P. acnes. Since tryptophan residues have the ability to penetrate membranes, their presence in antimicrobial peptides has been correlated with antimicrobial activity (Fimland et al, 2002). For instance, NK-lysin, a member of the saposin-like protein family along with granulysin, has antimicrobial activity and has been shown to partially insert its tryptophan in membranes (Qi and Grabowski, 2001). Similarly, the native granulysin protein has one tryptophan at amino acid position 41 on helix 3, a highly lytic segment. Our EM studies document the ability of 31–50v44w to disrupt bacterial surface architecture. Although we do not show that there is a direct interaction between the tryptophan residues and the bacteria, the potent antimicrobial activity of this modified peptide suggests that tryptophans are indeed important for antimicrobial activity against P. acnes.
We next made an additional modification to granulysin peptide 31–50 to enhance its stability to proteases from both bacteria and human skin. We synthesized 31–50v44w entirely with d-type amino acids (d-31–50v44w) and found this peptide exhibited even greater potency in vitro as compared to 31–50v44w. Our experiments with E. coli demonstrated that the d versus l form of 31–50v44w exhibited increased antimicrobial potency due to its protease-resistance. This corroborates the findings in the study of Alvarez-Bravo et al (1994), which found that the d-form of a sapecin B-derived peptide was able to avoid degradation by trypsin. Based on the enhanced activity and stability of these modified peptides, it is possible that additional modifications may even further improve their antimicrobial utility.
A key feature of effective anti-acne agents is their ability to maintain antimicrobial activity within the lipid-rich environment of the pilosebaceous unit. Since the earliest acne “lesion” is a microcomedone, sampling of microcomedones has been used for assessment of microbial colonization (Holland and Roberts, 1974) and the efficacy of antimicrobial therapy in acne (Pierard-Franchimont et al, 2002). Microcomedones removed with cyanoacrylate strips are “follicular casts” containing a mixture of keratinized material, cell debris, lipids, and bacteria (Thielitz et al, 2001). Therefore, in order to test the efficacy of granulysin peptides against clinical isolates of P. acnes and within lipid-rich environments, we used pore strips to extract P. acnes and other follicular contents from human donors for use in an in vitro assay. Our results of this assay demonstrate peptide d-31–50v44w maintains its antimicrobial activity against clinical isolates and within sebaceous environments, suggesting its promise in acne therapy. Furthermore, experiments in our lab indicate these peptides have minimal effect on the viability of both HaCaT and human monocytes (unpublished data), suggesting that they may not only be efficacious antimicrobials but also safe topical agents.
Antimicrobial peptides have been shown to have immunomodulatory functions. Here we show that granulysin peptides 31–50v44w and d-31–50v44w inhibit the production of inflammatory cytokines and chemokines by P. acnes-stimulated monocytes. P. acnes is a potent stimulator of host immune responses, and since inflammation is a key clinical feature of acne, the anti-inflammatory nature of these peptides increases their therapeutic potential. Previously, it has been shown that LL-37 decreases cytokine production in response to LPS, a Toll-like receptor 4 (TLR4) ligand (Scott et al, 2002). Here, we show that granulysin peptides decrease cytokine production in response to P. acnes, which stimulates cells through TLR2 (Kim et al, 2002). Although the precise anti-inflammatory mechanism is not known, it is possible that the peptides are interfering with aspects of the TLR2 pathway, such as the activation of NFκB. On the other hand, the peptide may be interacting with and binding up TLR ligands present in P. acnes sonicates, preventing some of these components from activating TLR. Furthermore, since P. acnes contain a unique peptidoglycan, N-acetylmuramic acid-l-alanine-d-glutamate-l, l-diaminopimelic acid within the cell wall (Kamisango et al, 1982), it is possible that cytokine production may occur through the intracellular peptidoglycan receptors Nod1 or Nod2 which recognize similar motifs. Since P. acnes may contain ligands for Nods as well as TLR, blocking of either of these pathways may explain the diminution of cytokine and chemokine production. Our current studies are exploring these potential mechanisms.
In this paper, we have demonstrated that an endogenous antimicrobial peptide, granulysin, can be a novel therapeutic agent in treating acne, one of the most common dermatologic conditions affecting over 85% of the population. The bactericidal and anti-inflammatory properties of granulysin peptides make them ideal candidates for the treatment of acne. Furthermore, we have demonstrated that modification of granulysin peptides can lead to more stable peptides that maintain their antimicrobial activity in the lipid-rich environment of microcomedones.
Whether granulysin peptides will be effective in clinical settings is not yet certain as there are no animal models for acne. Furthermore, it is possible that novel peptides produced from significant modifications to endogenous peptides may activate the host immune response. Therefore, it may be wise for us to use natural peptides for clinical applications. Future clinical trials in humans will determine the outcome of their use. Certainly, the broad spectrum of activity increases the utility of granulysin peptides as they may be effective in treating other cutaneous infections caused by resistant bacteria.
Methods
Production and purification of recombinant granulysin
Granulysin was produced in E. coli BL21 (DE3) transformed with the kanamycin-selective vector, pET28, containing a hexahistidine fusion tag (Novagen, Madison, Wisconsin) as previously described (Pena et al, 1997; Ernst et al, 2000). The transformed E. coli were grown in 2 × YT broth and induced with 1 mM isopropyl-β-d-thiogalactoside (Fisher Scientific, Pittsburgh, Pennsylvania). The bacteria were harvested and denatured in 6 M guanidine HCl/0.05 mM Tris HCl pH 7.4. Granulysin was purified via nickel affinity chromatography according to the manufacturer's recommendation (Qiagen, Valencia, California) and eluted with 0.2 M imidazole, then reduced with 10 mM DTT. The denatured granulysin was re-natured in 0.75 M arginine, 0.05 M Tris HCl pH 8.0, 0.05 M KCl, 0.1 mM EDTA, and 10 mM oxidized DTT at a 1:5 dilution with constant stirring for 48 h at 4°C. The re-natured protein was then dialyzed against a buffer containing 2 mM sodium phosphate and 13 mM sodium chloride pH 7.2, then lyophilized. The granulysin pellet was rehydrated and treated with thrombin for 16 h to cleave the hexahistidine tag. Following thrombin cleavage, the protein was loaded onto a Rainin C18 reverse phase chromatography column (Braintree, Massachusetts) and eluted by a linear gradient of 10%–60% aqueous acetonitrile in 0.1% trifluoroacetic acid. The fractions containing granulysin, as determined by Coomassie staining of a 15% SDS-PAGE gel, were lyophilized then hydrated in 10 mM sodium phosphate pH 7.2 unless otherwise noted. The final protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, Illinois) with bovine serum albumin as a standard. Protein purity was assessed by Coomassie staining of 15% SDS-PAGE gels and was >95%. Additionally, purified granulysin was analyzed by matrix-assisted laser desorption ionization mass spectrometry and shown to contain one species at 9081 Da that corresponded to the calculated mol wt, which is 9070.4 assuming that four of the five cysteines are involved in disulfide bridges.
Peptide synthesis
Peptides encompassing the entire amino acid sequence of granulysin were synthesized using F-moc chemistry by either an Applied Biosystems (Foster City, California) or UCLA Peptide Core (Los Angeles, California) automatic peptide synthesizer and were purified to >95% homogeneity by reverse phase HPLC, and peptide composition was confirmed by mass spectrometry and amino acid analysis. Stock peptide solutions were prepared at 20 mg per mL in DMSO or at 2 mM in water. To construct a peptide containing amino acid residues 62–74, additional sequence from the 15–kDa form of granulysin was used to generate the 61–80 peptide so the length of this peptide would be similar to the others in our study. Serine residues were also substituted for the protein's native cysteine residues to avoid formation of disulfide linkages between peptides, these substitutions are marked with italics. The sequences for the resulting peptides are as follows: 1–35, GRDYRTSLTIVQKLKKMVDKPTQRSVSNAATRVSR; 36–70, TGRSRWRDVSRNFMRRYQSRVIQGLVAGETAQQIS; 1–20, GRDYRTSLTIVQKLKKMVDK; 31–50, TRVSRTGRSRWRDVSRNFMR; 16–35, KMVDKPTQRSVS NAATR VSR; 46–65, RNFMRRYQSRVIQGLVAGET; 61–80, VAGETAQQISEDLR. Additionally, the valine residue at position 44 of peptide 31–50 was substituted with a tryptophan, resulting in peptide 31–50v44w with the sequence of TRVSRTGRSRWRD WSRNFMR. Also, this same peptide sequence was synthesized with all d-type amino acids to generate peptide d-31–50v44w.
Granulysin peptide derivatives were assembled via solid phase peptide synthesis, using either an Applied Biosystems 433A or Symphony (Rainin/Protein Technologies) automated peptide synthesizer. Standard methods of Fmoc-based solid phase peptide synthesis were applied using modules in the software packages provided with the automated instrument. Rink amide resin and all amino acid derivatives (d- and l-) were purchased from EMD Biosciences/Novabiochem (San Diego, California). Peptide couplings were achieved via activation as the HOBT ester using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU, American Bioanalytical, Natick, Massachusetts). Cleavage of the peptide from the solid phase resin was achieved with a cocktail consisting of 90% TFA and 2% each of water, triisopropylsilane, ethanedithiol, thioanisole, and phenol (all from Sigma-Aldrich, Milwaukee, Wisconsin) for 2.5 h. Crude peptides were purified to greater than 95% purity via C18 reversed phase HPLC using acetonitrile–water–TFA eluant systems and lyophilized to dryness. Peptide identity was confirmed by MALDI-TOF mass spectrometry (Bruker Autoflex mass spectrometer, Bruker Daltonics, Billerica, Massachusetts) in a matrix of α-cyanohydroxycinnamic acid (CHCA, Sigma-Aldrich). Amino acid analysis (Molecular Structure Facility at the University of California, Davis) was used to confirm peptide composition and content.
An HSP70 peptide was synthesized and used as a control peptide as previously described (Stenger et al, 1998; Ernst et al, 2000). HSP70 peptide is a good control since as it is of similar length compared to our granulysin-derived peptides, and it is also a self-peptide.
CFU assay
The CFU assay was performed as described previously (Ernst et al, 2000). P. acnes strain ATCC 6919 (American Type Cell Culture (ATCC), Manassas, Virginia) was grown under anaerobic conditions in Reinforced Clostridial Medium (Oxoid, Basingstroke, England) for 2 d and collected in mid-log phase. The bacteria were washed three times with the assay buffer, 10 mM sodium phosphate, pH 7.2, supplemented with 0.03% trypticase soy broth (TSB, Becton-Dickinson, Cockeysville, Maryland), and enumerated by applying a conversion factor of 7.5 × 107 bacteria per mL = 1 OD unit at 600 nm. Various concentrations of granulysin or granulysin peptides were incubated with 3.75 × 105 bacteria in a final volume of 30 μL at 37°C for 4 h. After incubation, 10-fold dilutions were prepared and plated on solid media comprised of Brucella broth (BD Biosciences, San Diego, California) with 5% sheep red blood cells (Remel, Lenexa, Kansas), 0.5 mg per liter vitamin K, and 5.0 mg per liter hemin (Remel). Plates were incubated for 4 d at 37°C under anaerobic conditions, then individual colonies were counted and the number of CFU per tube was calculated.
Electron microscopy
P. acnes at a concentration of 2.08 × 107 bacteria per mL was suspended in 10 mM sodium phosphate buffer pH 7.2 supplemented with 0.03% TSB and incubated with 20 mM granulysin peptides. Samples were then fixed for 30 min with 2% gluteraldehyde in 1 × PBS, pH = 7.35, room temperature, then washed three times and suspended in 1 mL 1 × PBS. Scanning EM samples were filtered onto a micropore filter. The filters with the sample on top were dehydrated in graded ethanol; 50%, 75%, 95%, and 100%–15 min in each, followed by similar transfers in hexa-methyl-disilazane reagent; 50%, 75%, 95%–30 min each followed by 100% hexa-methyl-disilazane reagent overnight. The scanning EM samples were gold coated and then viewed and photographed on a Cambridge Scanning electron microscope. Transmission EM samples were dehydrated in graded ethanol as above, embedded in Epon, and sectioned on Sorvall MT6000 (RMC, Tucson Arizona). Thin sections (75 μm) were stained with uranyl acetate, viewed and photographed on Jeol XC100 at 80-kV.
E. coli killing assays
Log phase cultures of E. coli strains TOP10 (Invitrogen, Carlsbad, California) and ATCC 55099 (negative for proteases OmpT, DegP, and protease III) (ATCC) were diluted to 2 × 107 CFU per mL (= 2 × test inoculum) in assay buffer (20% trypic soy broth, 1 mM MgCl2, 50 mM NaHCO3, pH 7.4). A serial dilution (1–64 μM final assay concentration) of the indicated granulysin peptides were prepared in 96-well microtiter plates in 50 μL assay buffer. After addition of 50 μL 2 × test inoculum the plates were incubated for 24 h at 37°C in an ambient air incubator. The sample was diluted 1:35 with PBS. To determine the MBC, 10 μL of the diluted sample was spotted on a tryptic soy broth agar plate and incubated at 37°C overnight. MBC was defined as the lowest peptide concentration without any visible growth on the typtic soy broth agar plate (equals >log 4 reduction of the initial test inoculum).
Antibiotics
The effect of both Clindamycin and Tetracyclin (Sigma, St Louis, Missouri) against P. acnes was determined with a qualitative CFU assay which involved plating 20 μL of each sample (P. acnes with antibiotics or granulysin peptide) on the solid media and quantifying existing colonies using CFU assay as described above in detail.
Microcomedone assay
After informed consent was obtained, comedones were collected from human volunteers using pore cleansing strips (Jergens, Cincinnati, Ohio) according to the Declaration of Helsinki Principles and the Institutional Review Board at UCLA. Individual plugs were removed from the strips with a fine point tweezer and pooled in a microfuge tube. These were then resuspended in 200 μL of assay medium (20% tryptic soy broth, BD Microbiology Systems, Sparks, Maryland) until the particulate material was broken up into a colloidal suspension as determined by light microscopy. Then, 10 μL of this suspension was combined with either 10 μL of peptide solution or 10 μL of distilled water (negative control). These were then incubated for 2.5 h at 37°C in an ambient air incubator. A serial dilution of each sample was prepared and 15 μL of each dilution was spotted on a Brucella blood agar plates and incubated as previously described. Digital images of individual plates were taken using an Alpha Innotech Chemimager 5500 (Alpha Innotech, San Leandro, California) and bright light illumination. The CFU per mL was determined by counting.
Primary human monocyte isolation and stimulation
Peripheral human blood was drawn from normal healthy volunteers into heparinized tubes according to a protocol approved by the Institutional Review Board at UCLA. PBMC were then isolated on Ficoll–Paque gradients (Pharmacia, Piscataway, New Jersey) and plated (5 × 105 per well) in 96-well plates for 2 h in 1% FCS (Omega Scientific, Tarzana, California). Non-adherent cells were removed by washing three times with RPMI media, leaving adherent cells. Adherent monocytes were cultured in 10% FCS with or without granulysin peptides for 1 h then P. acnes sonicate was added to culture for 24 h at 37°C.
Monocyte supernatants were harvested and assayed for IL-12p40 using cytokine-specific, commercially available antibody pairs in a standard sandwich ELISA (Biosource International, Camarillo, California, or BD PharMingen, San Diego, California). Capture antibodies were coated to 96-well EIA/RIA plates (Costar, Corning, New York) and detection was achieved by incubating with biotinylated antibodies followed by Immunopure HRP-conjugated streptavidin (Pierce, Rockford, Illinois) and the ABTS Microwell Peroxidase Substrate System (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland). The absorbance at 405 nm was read using a microtiter plate reader, and cytokine concentrations were calculated from a standard curve of recombinant cytokine (Biosource or Pharmingen). Supernatants were assayed for other cytokines using Pierce SearchLight Multiplex Cytokine array services (Pierce Biotechnology, Woburn, Massachusetts). For the Multiplex arrays, for each separate monocyte stimulation experiment, triplicate samples from 96-well plates were pooled to provide sufficient sample volume at appropriate concentration for analysis.
Acknowledgments
This project was funded by the NIH NIAMS K08 grant (AR48551-01). J. McInturff's Howard Hughes Medical Institute Medical Student Research Training Fellowship is gratefully acknowledged. The authors would like to thank Mrs Alicia Thompson of the USC Center for Electron Microscopy and MicroAnalysis and Mrs. Brigitta Sjostrand of the UCLA EM Core Facility, for their technical help.
Abbreviations
- MBC
minimum bactericidal concentration
- TLR
Toll-like receptor
Footnotes
Conflicts of interest: The authors would like to state the following potential conflicts of interest: T. M., T. R. L., S. H., K. Z. are/have been employees of Ansata Therpeutics; R. L. G., R. L. M. and J. K. have been consultants for Ansata Therpeutics, Inc.,; R. L. G. and R. L. M. served on the Scientific Advisory Board for Ansata Therapeutics, Inc., in the past.
References
- Alvarez-Bravo J, Kurata S, Natori S. Novel synthetic antimicrobial peptides effective against methicillin-resistant Staphylococcus aureus. Biochem J. 1994;302(Pt 2):535–538. doi: 10.1042/bj3020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, Eisenberg D. Granulysin crystal structure and a structure-derived lytic mechanism. J Mol Biol. 2003;325:355–365. doi: 10.1016/s0022-2836(02)01234-2. [DOI] [PubMed] [Google Scholar]
- Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002;146:840–848. doi: 10.1046/j.1365-2133.2002.04690.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJ. Systematic review of Propionibacterium acnes resistance to systemic antibiotics. Med J Aust. 1998;169:259–261. doi: 10.5694/j.1326-5377.1998.tb140250.x. [DOI] [PubMed] [Google Scholar]
- Ernst WA, Thoma-Uszynski S, Teitelbaum R, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- Fimland G, Eijsink VG, Nissen-Meyer J. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry. 2002;41:9508–15. doi: 10.1021/bi025856q. 41–9508–9515. [DOI] [PubMed] [Google Scholar]
- Hamamoto K, Kida Y, Zhang Y, Shimizu T, Kuwano K. Antimicrobial activity and stability to proteolysis of small linear cationic peptides with d-amino acid substitutions. Microbiol Immunol. 2002;46:741–749. doi: 10.1111/j.1348-0421.2002.tb02759.x. [DOI] [PubMed] [Google Scholar]
- Holland KT, Roberts CD. A technique for sampling micro-organisms from the pilosebaceous ducts. J Appl Bacteriol. 1974;37:289–296. doi: 10.1111/j.1365-2672.1974.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Kamisango K, Saiki I, Tanio Y, et al. biological activities of peptidoglycans of Listeria monocytogenes and Propionibacterium acnes. J Biochem (Tokyo) 1982;92:23–33. doi: 10.1093/oxfordjournals.jbchem.a133918. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol. 1999;154:239–247. doi: 10.1016/s0002-9440(10)65270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa MT, Stenger S, Sieling PA, et al. T-cell release of granulysin contributes to host defense in leprosy. Nat Med. 2001;7:174–179. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]
- Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late Tcell activation molecule with homology to small, lytic, granule proteins. J Immunol. 1997;158:2680–2688. [PubMed] [Google Scholar]
- Pierard-Franchimont C, Goffin V, Arrese JE, Martalo O, Braham C, Slachmuylders P, Pierard GE. Lymecycline and minocycline in inflammatory acne: A randomized, double-blind intent-to-treat study on clinical and in vivo antibacterial efficacy. Skin Pharmacol Appl Skin Physiol. 2002;15:112–119. doi: 10.1159/000049398. [DOI] [PubMed] [Google Scholar]
- Qi X, Grabowski GA. Differential membrane interactions of saposins A and C: Implications for the functional specificity. J Biol Chem. 2001;276:27010–27017. doi: 10.1074/jbc.M101075200. Epub May 16. [DOI] [PubMed] [Google Scholar]
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: Lessons from Europe. Br J Dermatol. 2003;148:467–478. doi: 10.1046/j.1365-2133.2003.05067.x. [DOI] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549–553. doi: 10.4049/jimmunol.164.2.549. [DOI] [PubMed] [Google Scholar]
- Stenger S, Hanson DA, Teitlebaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielitz A, Helmdach M, Ropke EM, Gollnick H. Lipid analysis of follicular casts from cyanoacrylate strips as a new method for studying therapeutic effects of antiacne agents. Br J Dermatol. 2001;145:19–27. doi: 10.1046/j.1365-2133.2001.04276.x. [DOI] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Choice E, Kaspar A, et al. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J Immunol. 2000;165:1486–1490. doi: 10.4049/jimmunol.165.3.1486. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, et al. Beta-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]