Abstract
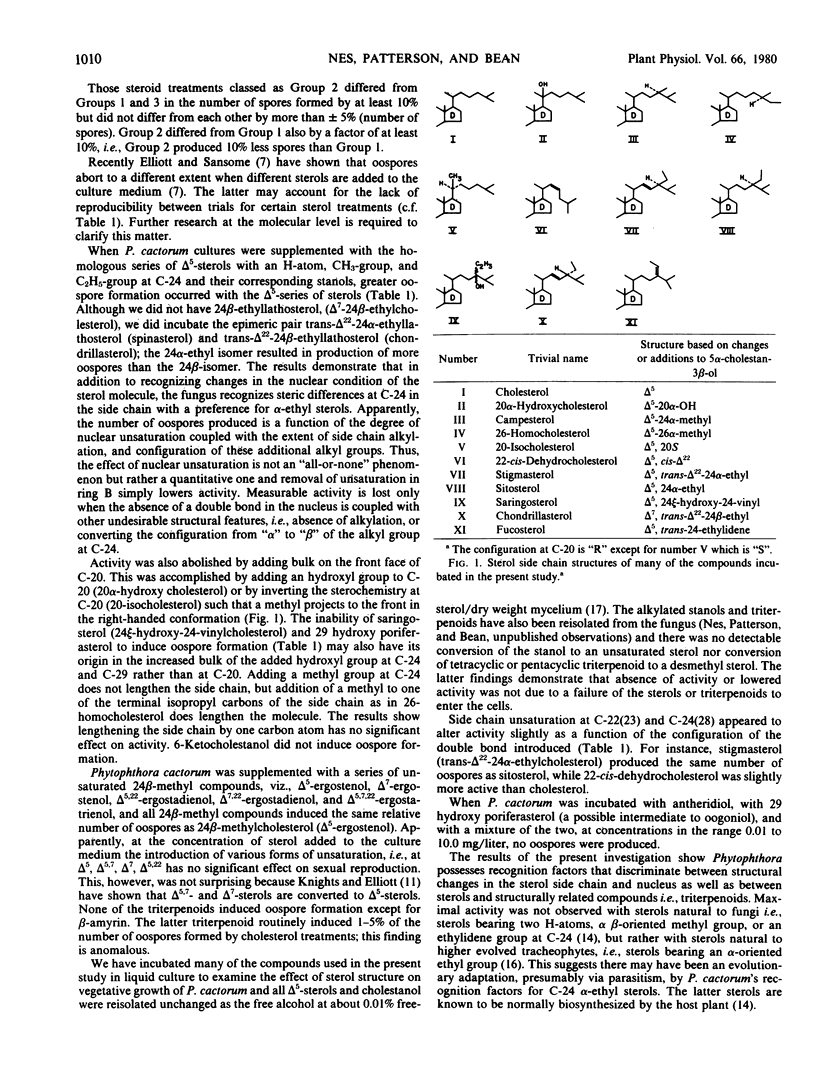
The comparative biological activity of 21 naturally occurring or synthetically derived steroids, 7 tetracyclic and pentacylic triterpenoids, and antheridiol incubated with cultures of Phytophthora cactorum has been examined. There was greater dependence on precise steric features of the sterol side chain than on the extent of nuclear unsaturation in inducing oospore formation. There was no significant effect on oospore formation by changing nuclear unsaturation in ring B from Δ5 to Δ7 or to Δ5,7. Converting the unsaturated sterol to its corresponding stanol resulted in a significant reduction in the number of oospores produced. The effectiveness of sterols bearing different side chains in inducing oospores was found to be in the following relative order: 24α-ethyl = trans-Δ22-24α-ethyl > trans-Δ22-24β-ethyl = 24α-E-ethylidene = 24α-methyl > 24β-methyl = trans-Δ22-24β-methyl = 26-methyl = saturated C7 side chain and C-20 R (17-αH, 20-αH, right-handed conformer) = cis-Δ22-C7 side chain and C-20 R > saturated C7 side chain and C-20 S (17-αH, 20-βH, right-handed conformer) > no sterol = 29-hydroxyporiferasterol = 20α-hydroxycholesterol = 24ξ-hydroxy-24-vinylcholesterol. Of the sterols examined the most significant stereochemical criterion for the induction of oospore formation was absence of bulk on the front face of C-20. This follows from the observation that 20-isocholesterol and 20α-hydroxycholesterol, in which a methyl and hydroxy group, respectively, project to the front in the right handed conformation, were inactive in stimulating production of oospores. None of the triterpenoids studied induced oospore formation to any significant degree. Oospore formation was not induced by antheridiol nor 29-hydroxyporiferasterol in combination or added separately to growing cultures of P. cactorum in the concentration range 0.01 - 10.0 milligrams per liter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott C. G., Hendrie M. R., Knights B. A. The sterol requirement of Phytophthora cactorum. J Gen Microbiol. 1966 Mar;42(3):425–435. doi: 10.1099/00221287-42-3-425. [DOI] [PubMed] [Google Scholar]
- Elliott C. G., Sansome E. The influence of sterols on meiosis in Phytophthora cactorum. J Gen Microbiol. 1977 Jan;98(1):141–145. doi: 10.1099/00221287-98-1-141. [DOI] [PubMed] [Google Scholar]
- Elliott C. G. Sterols in fungi: their functions in growth and reproduction. Adv Microb Physiol. 1977;15:121–173. doi: 10.1016/s0065-2911(08)60316-1. [DOI] [PubMed] [Google Scholar]
- Knights B. A., Elliott C. G. Metabolism of delta7- and delta5,7-sterols by Phytophthora cactorum. Biochim Biophys Acta. 1976 Aug 23;441(2):341–346. doi: 10.1016/0005-2760(76)90178-8. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E., Krevitz K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J Am Chem Soc. 1977 Jan 5;99(1):260–262. doi: 10.1021/ja00443a054. [DOI] [PubMed] [Google Scholar]
- Nordby H. E., Nagy S. An evaluation of recent gas-liquid chromatographic liquid phases for resolution of acetylated plant sterols. J Chromatogr. 1973 Jan 17;75(2):187–193. doi: 10.1016/s0021-9673(00)85547-8. [DOI] [PubMed] [Google Scholar]
- Patterson G. W., Karlander E. P. Fucosterol Reduction to Clionasterol in vivo by Chlorella ellipsoidea. Plant Physiol. 1967 Nov;42(11):1651–1652. doi: 10.1104/pp.42.11.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G. W. Sterols of Laminaria. Comp Biochem Physiol. 1968 Feb;24(2):501–505. doi: 10.1016/0010-406x(68)91001-3. [DOI] [PubMed] [Google Scholar]



