Summary
The tumor suppressor protein Merlin inhibits cell proliferation upon establishing cell-cell contacts. Because Merlin has high sequence similarity to the Ezrin-Radixin-Moesin (ERM) family of proteins, the structural model of ERM protein autoinhibition and cycling between closed/resting and open/active conformational states is often employed to explain Merlin function. However, recent biochemical studies suggest alternative molecular models of Merlin function. Here, we have determined the low resolution molecular structure and binding activity of Merlin and a Merlin(S518D) mutant that mimics the inactivating phosphorylation at S518 using small angle neutron scattering (SANS) and binding experiments. SANS shows that in solution both Merlin and Merlin(S518D) adopt a closed conformation, but binding experiments indicate that a significant fraction of either Merlin or Merlin(S518D) is capable of binding to the target protein NHERF1. Upon binding to the phosphatidylinositol 4,5-bisphosphate lipid, the wild-type Merlin adopts a more open conformation than in solution, but Merlin(S518D) remains in a closed conformation. This study supports a rheostat model of Merlin in NHERF1 binding, and contributes to resolve a controversy about the molecular conformation and binding activity of Merlin.
Keywords: Merlin, Neurofibromatosis type-2, phosphatidylinositol 4, 5-bisphosphate, Ezrin, Radixin, Moesin, NHERF1, membrane-cytoskeleton, adapter proteins, scaffolding proteins, tumor suppressor, small angle neutron scattering
Introduction
Merlin is a tumor suppressor protein that is encoded by the Neurofibromatosis type-2 (NF2) gene. Merlin regulates the growth and development of tissues and organs [1]. Malfunctioned mutations in the NF2 gene cause the genetic cancerous syndromes of neurofibromatosis type 2, schwannomas, meningiomas, and ependymomas tumors in the peripheral nervous system [2-4]. Merlin malfunction mutations are also implicated in other types of mammalian cancers, for reviewed see ref [5].
Merlin is activated to suppress cell proliferation upon establishing cell-cell contacts [6-8]. Cell contact-dependent inhibition is essential for normal tissue organization, and loss of cell contact-dependent inhibition is the hallmark of invasive cancer [9]. Merlin mediates contact-dependent inhibition by recruiting cell-adhesion and cell polarity complexes to the cell junctions [1, 10]. Upon establishing cell-cell contacts, Merlin also negatively regulates growth signals from the epidermal growth factor receptors [6, 11, 12]. Merlin regulates a number of mitogenic signaling pathways including the Rac–PAK, PI3K–Akt, FAK–Src, as well as the Hippo tumor-suppressor signaling pathways (reviewed in [5]). Recent studies find that Merlin can migrate to the nucleus, and induce the growth-suppressive program by inhibiting the CRL4DCAF1 E3 ubiquitin ligase [13].
The growth suppressor function of Merlin is modulated by phosphorylation [14, 15]. Merlin is phosphorylated at the carboxyl-terminal S518 by the PAK kinase, which deactivates the growth suppression function of Merlin [14, 16-18]. Upon establishing cell–cell contacts, Merlin S518 is dephosphorylated by the phosphatase PP1δ, and the S518 dephosphorylated Merlin becomes active, thereby suppresses cell growth and proliferation [19]. Although phosphorylation/dephosphorylation at S518 is a crucial event that drives the Merlin activation–inactivation cycles, the molecular basis of Merlin S518 phosphorylation on Merlin function is not well understood.
Because Merlin has high sequence similarity to a family of structurally conserved Ezrin-Radixin-Moesin (ERM) proteins [20], the structural model of ERM proteins is often employed to explain Merlin conformation and function. The ERM proteins are membrane-cytoskeleton adapter proteins. The functions of the ERM proteins are regulated by a conformational autoinhibition mechanism, with the inactive protein being held in a closed conformation by head-to-tail intramolecular interactions [21, 22]. Binding to the phosphatidylinositol 4,5-bisphosphate (PIP2) lipid and phosphorylation at a conserved carboxyl-terminal threonine (Ezrin T567, Radixin T564, and Moesin T558) disrupt the autoinhibition and activate ERM proteins [23]. Upon ERM protein activation, the released FERM domain binds to a variety of target proteins including the scaffolding protein NHERF1, whereas the carboxyl-terminal tail binds to the cytoskeletal actin [24, 25]. The ERM protein associated protein complexes are involved in targeting and assembling a number of receptors and ion transport proteins at the interface between the cell membrane and the actin cytoskeleton.
The conformation of Merlin is also considered to be regulated by head-to-tail intramolecular interactions [26-29]. Based on comparisons with ERM proteins, it is thought that that phosphorylation at S518 of Merlin causes the disruption of intramolecular head-to-tail interactions, and that the S518 phosphorylated Merlin adopts an open conformation. According to this model, the closed and unphosphorylated conformation of Merlin is the active form that suppresses growth while the open, S518 phosphorylated Merlin is the inactive form. However, recent biochemical studies suggest alternative models of Merlin activation. These studies imply that either phosphorylation at S518 has minimal effects on Merlin conformation, or that the S518 phosphorylated Merlin adopts a more closed form than the unphosphorylated Merlin [30, 31]. A direct structural determination of Merlin and S518 phosphorylated Merlin together with quantitative binding experiments will help to clarify the relationship between the Merlin molecular conformation and its target binding activity.
Using contrast-matching SANS, we have determined the molecular shapes of Merlin and the phosphomimetic Merlin(S518D) mutant in solution and in PIP2. We find that, in solution, both the wild-type Merlin and the phosphomimetic Merlin(S518D) adopt a closed conformation. However, unlike the closed Ezrin that does not bind to NHERF1 [32], about 30-40% of the wild-type Merlin or Merlin(S518D) molecules are capable of binding to NHERF1 with binding affinity that is comparable to that of the Merlin FERM domain. Moreover, upon binding to PIP2, a fraction of Merlin or Merlin(S518D) forms aggregates. Contrast-matching SANS shows that the soluble fraction of PIP2-bound wild-type Merlin is monomeric and adopts a more open structure, but the soluble fraction of PIP2-bound Merlin(S518D) remains in a closed conformation. This study supports a rheostat model of Merlin, whose conformation and binding activity cannot simply described as open and closed molecular conformation as the ERM proteins.
Results
We have expressed and purified the wild-type full-length Merlin(wt), the phosphomimic Merlin(S518D) and Merlin(S518E) mutants from bacteria, see Figure 1A. The S518D and S518E mutations are chosen as mimetics for the negative electrostatic charges of phosphorylated Merlin at S518 because a number of biochemical and cell biological studies have shown that the Merlin(S518D) mutant is inactive to suppressing cell growth, similar to the S518 phosphorylated Merlin [14, 18, 19, 29]. At room temperature, gel filtration and light scattering show that the purified Merlin(wt), Merlin(S518D), and Merlin(S518E) do not aggregate in solution, see Figure 1B and 1C. Because the gel filtration profiles of Merlin(S518D) and Merlin(S518E) are similar, we focus on Merlin(S518D) in the following studies. Molecular mass measurements by static light scattering indicates that Merlin(wt) and Merlin(S518D) are monomers in solution, see Figure 1C. This result is in agreement with previous fluorescence resonance energy transfer and analytical ultracentrifugation studies that also find Merlin is predominantly a monomer in solution [30].
Figure 1. Purified Merlin and Merlin(S518D) are monomeric.
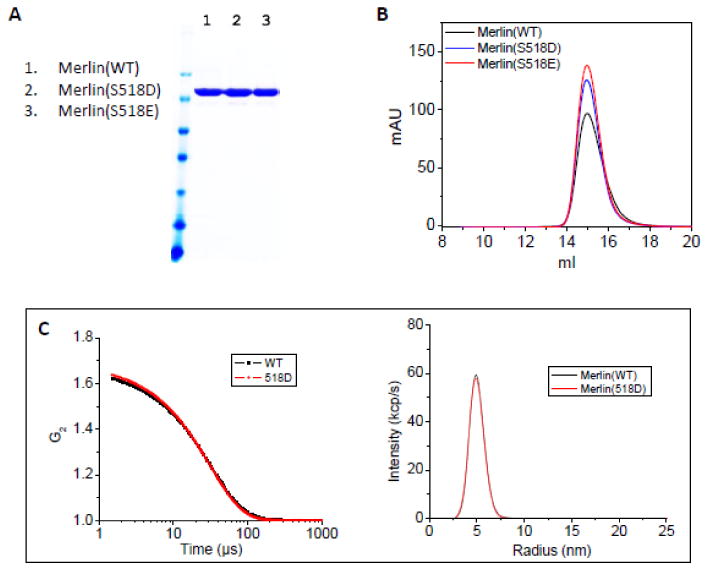
(A) SDS-PAGE of purified Merlin(wt), Merlin(S518D), and Merlin(S518E). (B) Gel filtration of Merlin(wt), Merlin(S518D), and Merlin(S518E). The gel filtration was performed at room temperature. (C) Dynamic light scattering of Merlin(wt) and Merlin(S518D). Left panel: auto-correlation function. Right panel: size distribution. Molecular mass measurements by static light scattering indicate that Merlin and Merlin(S518D) are monomers. The hydrodynamic radius Rh=48.4±0.5 Å for Merlin(wt), and Rh=47.9±0.1 Å for Merlin(S518D). The light scattering experiments were performed at 25 °C.
Circular dichroism (CD) experiments on Merlin(wt) and Merlin(S518D) yielded spectra with a strong positive peak at wavelength λ ∼195 nm and two strong negative peaks at ∼208 nm and ∼222 nm, which are characteristic of α-helical structure (Figure S1). Deconvolution of the CD spectra yields the fractions of secondary structures. For the full-length Merlin(wt) and Merlin(S518D), the CD spectra suggest a comparable helical and beta sheet structure around 40-50 % and 20% respectively. The helical structures are slightly enhanced upon binding to PIP2 (Figure S1).
The full-length Merlin and Merlin(S518D) are capable of binding to NHERF1 but with lower activity than the Merlin FERM domain
The FERM domain of Ezrin (ezFERM) binds to the carboxyl-terminal tail of the scaffolding protein NHERF1 with high affinity [33-37]. The ezFERM domain is considered as a mimetic to the active form of Ezrin because the autoinhibiting carboxyl-terminal tail is removed [38]. We have previously shown that the closed, full-length wild-type Ezrin does not bind to the scaffolding protein NHERF1, while 16.2% of the phosphomimic Ezrin(T567D) mutant is active to bind to NHERF1 [32], see Table 1. The results of Ezrin to NHERF1 binding are consistent with our SANS results that the full-length Ezrin(wt) adopts a closed conformation, and Ezrin(T567D) adopts a slightly more open conformation [32].
Table 1. Comparing Rg and Dmax of dMerlin(WT) and dMerlin(S518D) mutants from SANS performed at 20°C.
| In 0% D2O | In 20% D2O | In 20% D2O in PIP2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Rg | Dmax | c | I(0)abs/c | Rg | Dmax | c | I(0)abs/c | Rg | Dmax | c | I(0)abs/c | |
| (Å) | (Å) | (mg/ml) | (cm2 mg-1) | (Å) | (Å) | (mg/ml) | (cm2 mg-1) | (Å) | (Å) | (mg/ml) | (cm2 mg-1) | |
| dezrin(WT)+ | 40.7±0.5 | 140 | 1.8 | 40.7±0.5 | 140±5 | 1.75 | 0.31±0.01 | 67.4±1.2 | 240±5 | 2.3 | 0.28±0.01 | |
| dezrin(T567D)+ | 41.0±0.8 | 140 | 1.8 | 41.0±0.8 | 140±5 | 1.57 | 0.30±0.01 | 68.0±1.0 | 240±5 | 1.4 | 0.33±0.01 | |
| dMerlin(wt) | 40.1±0.4 | 145±5 | 2.20 | 0.32±0.01 | 40.0±0.7 | 145±5 | 1.27 | 0.268±0.002 | 59.4±2.2 | 210±5 | 0.77 | 0.222±0.017 |
| dMerlin(S518D) | 39.7±0.6 | 145±5 | 1.68 | 0.30±0.01 | 39.0±0.9 | 140±5 | 1.07 | 0.249±0.004 | 42.2±0.9 | 140±5 | 0.70 | 0.244±0.005 |
SANS results are from ref. (Jayasundar et al., 2012).
Studies have shown that the FERM domain of Merlin (mFERM) also binds to the carboxyl-end of NHERF1 [27, 39]. Likewise, the binding of mFERM domain to NHERF1 may also be used as a control to compare with that of the full-length Merlin, in order to assess the degree of openness of the full-length Merlin or Merlin(S518D). Using surface plasmon resonance (SPR), we have first compared the binding affinities of NHERF1 to mFERM and ezFERM, respectively (Figure 3A and Table 1). The ezFERM domain binds to NHERF1 with high affinity with dissociation constant Kd=25.8±1.8 nM, which is close to previously reported [33, 37, 40], while mFERM binds NHERF1 with Kd=459±38 nM. The binding affinity of NHERF1 for mFERM is thus about 18 fold lower than that for ezFERM domain. Moreover, SPR shows that NHERF1 binds to the full-length Merlin(wt) with Kd=956±70 nM, and to Merlin(S518D) with Kd=1240±220 nM, see Figure 2A and Table 2.
Figure 3. Comparing the SANS data of dMerlin(wt), dMerlin(S518D) and dEzrin(wt0 in solution.
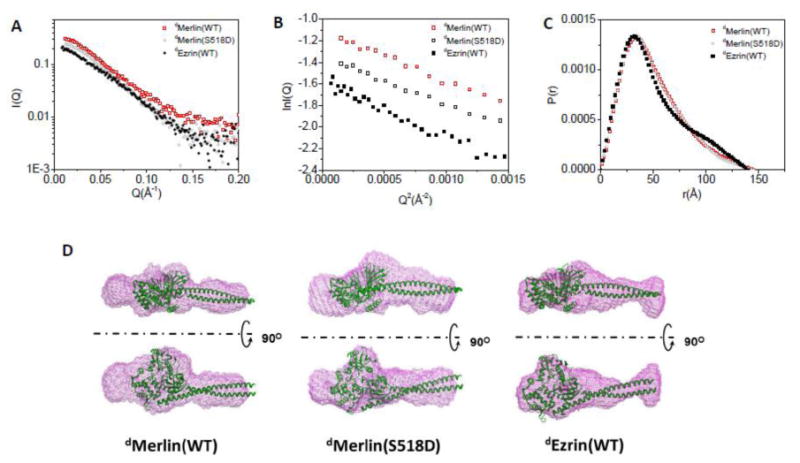
(A) SANS data of dMerlin, dMerlin(S518D), and dEzrin in H2O buffer solution. (B) Guinier plots of the data shown in (A). (C) P(r) functions of dMerlin(wt), dMerlin(S518D), and dEzrin. (D) 3-D shapes of dMerlin(wt), dMerlin(S518D), and dEzrin reconstructed from SANS. The crystal structure Moesin (PDB code: 2I1K)[22] is docked in the 3-D shapes as a comparison. The SANS experiments were performed on 2.2 mg/ml dMerlin and 1.7 mg/ml dMerlin(S518D), and on 1.8 mg/ml dEzrin(wt) in solution [32].
Figure 2. Comparing binding affinities of NHERF1 for ezFERM, mFERM, full-length Merlin(wt), and Merlin(S518D), respectively.
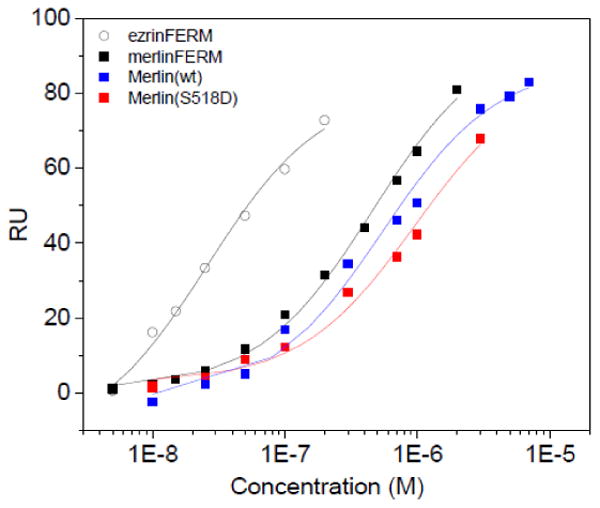
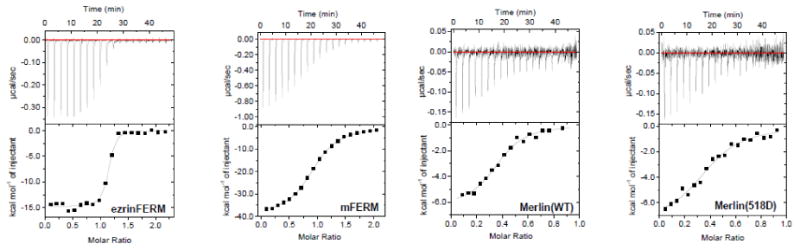
(A) SPR experiments were performed with NHERF1 immobilized on a CM5 sensor chip. Merlin constructs and ezFERM at different concentrations were flown on the NHERF1 immobilized sensor chip. (B) ITC experiments to determine the binding of NHERF1 to ezFERM, mFERM, Merlin(wt), and Melrin(S518D). The binding results are summarized in Table 2.
Table 2. Comparing NHERF1 binding to ezFERM and to Merlin constructs.
| Kd (nM) (SPR) | Kd(nM) ITC | N (ITC) | |
|---|---|---|---|
| ezFERM | 25.8±1.8 | 17.8±6.8 | 1.06 |
| mFERM | 459±38 | 649±18 | 0.93 |
| Merlin(wt) | 956±70 | 855±160 | 0.37 |
| Merlin(S518D) | 1240±220 | 1570±322 | 0.39 |
We have also performed isothermal titration calorimetry (ITC) experiments to compare the binding of NHERF1 to ezFERM, mFERM, Merlin(wt) and Merlin(S518D), see Figure 2B and Table 2. ITC experiments show that NHERF1 binds to ezFERM with higher affinity than to mFERM. ITC also indicates that the binding affinities of NHERF1 for mFERM is higher than for full-length Merlin(wt), whereas the binding affinity of NHERF1 for Merlin(wt) is about twice that for Merlin(S518), Figure 2A, 2B and Table 2. Thus, both SPR and ITC experiments indicate that the full-length Merlin(wt) and Merlin(S518D) are capable of binding to NHERF1, even though SANS shows that Merlin(wt) adopts a closed conformation. Comparing the binding results shows that Merlin(S518D) has a slightly weaker affinity for NHERF1 than Merlin(wt).
Furthermore, one can use ITC to judge the fraction of active Merlin molecules that is involved in binding to NHERF1 by examining the “N” values. For ezFERM and mFERM, the N value is close to 1 suggesting that the NHERF1-binding sites in the FERM domains are unobstructed, and that NHERF1 binds to either mFERM or ezFERM with a 1:1 stoichiometry. However, for the full-length Merlin(wt) and Merlin(S518D), the N value indicate that less than 40% of Merlin or Merlin(S518D) are active or competent in binding to NHERF1, see Table 2, whereas we have shown previously that a closed Ezrin is essentially incapable of binding to NHERF1 [32]. These results indicate that, unlike Ezrin that is completely autoinhibited from binding to NHERF1 [32], the NHERF1-binding sites in either Merlin(wt) or Merlin(S518D) are not totally masked by the carboxyl-terminal tail of Merlin. The ITC and SPR results thus imply a graded binding capability of Merlin to NHERF1, with the binding affinity mFERM>Merlin(wt)>Merlin(S518D).
Both the full-length Merlin(wt) and Merlin(S518D) adopt a closed conformation
The SANS experiments were performed at 2.2 mg/ml dMerlin(wt) and 1.68 mg/ml dMerlin(S518D) in 0% D2O. Figure 3 shows the SANS data of dMerlin(wt), and dMerlin(S518D) in 0% D2O. As a comparison, the SANS results of a closed deuterated Ezrin (dEzrin) and an open dEzrin(wt) bound to PIP2 from our previous study [32] are also shown. The radius of gyration Rg and the maximum dimension Dmax values of dMerlin(wt) and dMerlin(S518D) obtained from the SANS experiments at these two D2O buffer conditions are summarized in Table 1. As a comparison, the Rg and Dmax values of wild-type dEzrin and dEzrin(T567D) from our previous publication are also listed in Table 1.
The length distribution function P(r), Rg, and Dmax of dMerlin(wt) are very similar to that of dMerlin(S518D), indicating that these dMerlin(wt) and dMerlin(S518D) have similar conformation (Figure 3C and Table 1). P(r) and Dmax of the closed form of dEzrin(wt) are also quite similar to those of dMerlin(wt), and dMerlin(S518D) in solution (Figure 3C). However, there are differences in the details of P(r)s between Merlin and Ezrin. In the P(r) function of dMerlin or dMerlin(S518D), the peak at r=32 Å is broader than that of dEzrin(wt) (Figure 3C), suggesting that the overall structure of dMerlin(wt) or dMerlin(S518D) is less compact than dEzrin.
The 3-D shapes of dMerlin(wt) and dMerlin(S518D), and that of dEzrin reconstructed from SANS using the program DAMMIN [41] are shown in Figure 3D. As a comparison, the available crystal structure of a closed insect Moesin [22], which is a member of ERM family proteins, is docked in the 3-D shapes of dEzrin, dMerlin(wt), and dMerlin(S518D), respectively. The overall 3-D shapes of dEzrin, dMerlin(wt), and dMerlin(S518D) are quite similar. However, Figure 3D shows that the crystal structure of Moesin fits better in the map of dEzrin envelope than in that of dMerlin or dMerlin(S518D). This is not surprising considering that Moesin has higher sequence homology to Ezrin than to Merlin, especially in the carboxyl-terminal half. These analyses show that both dMerlin(wt) and dMerlin(S518D) adopt an overall closed conformation.
To compare the flexibility of Merlin(wt) and Merlin(S518D), we have analyzed the SANS data by the ensemble optimization method using the program EOM [42], see Figure 5D. By allowing the coexistence of different conformations of the protein contributing to the experimental scattering pattern, EOM can reveal the flexibility of a protein by determine the optimum number of conformers in the ensemble. This method can distinguish between rigid and flexible proteins, and can also give a distribution profile of Rg and Dmax. Figure 5D shows that the distributions of Rg and Dmax of the dMerlin(wt) is slightly broader than those of dMerlin(S518D), and that dMerlin(S518D) is slightly more compact than dMerlin(wt).
Figure 5. PIP2-binding induces the dMerlin(wt) to adopt a more open conformation than in solution, but dMerlin(S518D) is retained in a closed conformation.
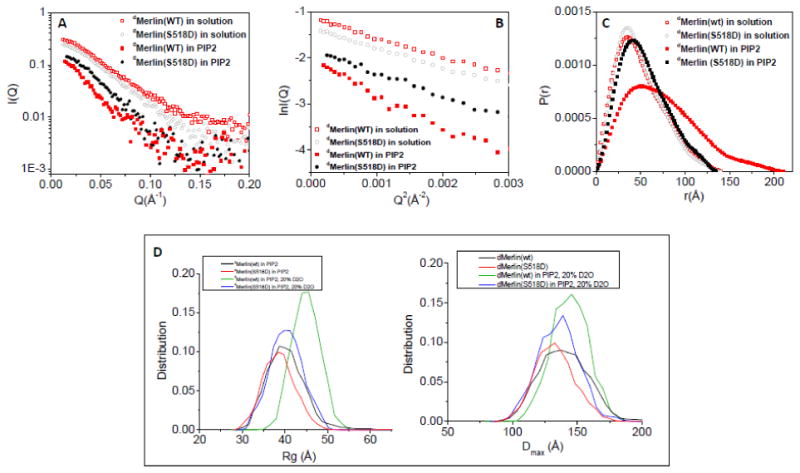
(A) SANS data of the dMerlin(wt) at 1.3 mg/ml and dMerlin(S518D) at 1.1 mg/ml, and the PIP2-bound dMerlin(wt) at 0.8 mg/ml and dMerlin(S518D) at 0.7 mg/ml in 20% D2O at the contrast-matching point of PIP2. (B) Guinier plots of SANS data shown in (A). (C) Comparing P(r) functions of dMerlin(wt) and dMerlin(S518D) in solution and in PIP2. (D) Comparing the Rg and Dmax distribution of dMerlin(wt) and dMerlin(S518D) in solution and in PIP2.
To summarize the SANS results in solution, both Merlin(wt) and Merlin(S518D) adopt a monomeric and head-to-tail like folded conformation. Although Merlin(S518D) is slightly more compact and less flexible than the wild-type Merlin, the differences between Merlin(wt) and Merlin(S518D) are subtle. These results are thus consistent with previous findings that either there are subtle conformational changes between the wild-type Merlin and the Merlin(S518D) [30] or that Merlin(S518D) is slightly more compact than Merlin(wt) [31]. We also find that about a non-insignificant fraction of either Merlin(wt) or Melin(S518D) is capable of binding to NHERF1 with similar binding affinity as the fully exposed mFERM domain. Altogether these results suggest that although Merlin(wt) or Merlin(S518D) adopts a closed form, the intramolecular interaction within Merlin(wt) or Merlin(S518D) is weaker than in Ezrin.
Upon binding to PIP2, Merlin adopts an expanded conformation, but Merlin(S518D) remains in a compact conformation
We have used ITC to determine the binding of PIP2 to Merlin(wt), Merlin(S518D), mFERM and ezFERM, see Figure 4. The results show that ezFERM, mFERM, Merlin(wt), and Merlin(S518D) have similar magnitude binding affinities for PIP2, with the full-length Merlin(wt) and Merlin(S518D) having slightly higher affinity for PIP2 (Table 3). It is interesting to note that ezFERM and mFERM show distinct PIP2-binding behaviors. The binding of PIP2 to mFERM or to full-length Merlin is endothermic, while the binding of PIP2 to ezFERM is exothermic (Figure 4). The exothermic PIP2-binding behavior of ezFERM has also been reported before [43].
Figure 4. ITC experiments to determine the binding of PIP2 to ezFERM, mFERM, Merlin(wt), and Merlin(S518D).
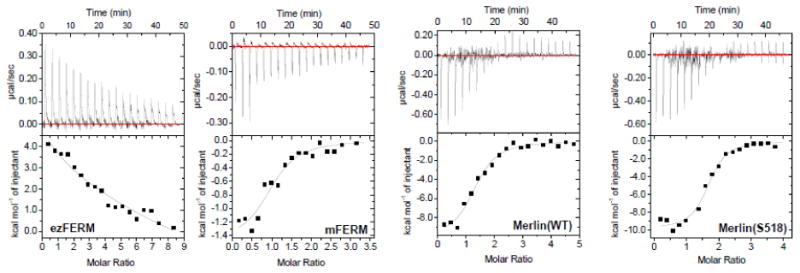
PIP2 solution of 900 μM was titrated into the protein solutions of 30 μM. The binding data are summarized in Table 3.
Table 3. Comparing PIP2 binding to ezFERM and to Merlin constructs.
| Kd (nM) | N | |
|---|---|---|
| ezFERM | 3311±888 | 1.86 |
| mFERM | 3380±799 | 1.01 |
| Merlin(WT) | 650±169 | 1.83 |
| Merlin(518D) | 961±248 | 1.63 |
We then performed SANS experiments on dMerlin(wt) and dMerlin(S518D) in PIP2 in 20% D2O buffer solution at the contrast-matching point of PIP2. Contrast-matching SANS in 20% D2O thus determines the conformational changes in dMerlin(wt) and dMerlin(S518D) in PIP2 without the interference of the scattering from PIP2. For preparing samples for the SANS experiments, we dissolved 1.1 mg/ml (15.8 μM) dMerlin(wt) and 1.0 mg/ml (14.3 μM) dMerlin(S518D) in 230 μM PIP2, respectively. We found that both dMerlin and dMerlin(S518D) form precipitate upon the addition of PIP2. This is in contrast to our previous study that shows that dEzrin or dEzrin(T567D) does not aggregate, and that dEzrin or dEzrin(T567D) is a monomer in PIP2 solution [32]. The dMerlin/PIP2 or dMerlin(S518D)/PIP2 precipitates were removed at 14,000 rpm using a table top centrifuge. After removal of the precipitates, the dMerlin/PIP2 and dMerlin(S518D)/PIP2 complexes were dialyzed against 20% D2O buffer, respectively. The final protein concentrations were 0.8 mg/ml (11.0 μM) dMerlin(wt) and 0.7 mg/ml (10.5 μM) dMerlin(S518D). SANS experiments were performed on these two samples in 20% D2O buffer.
Figure 5 shows the contrast-matching SANS results performed on the soluble fraction of the PIP2-bound dMerlin(wt) or dMerlin(S518D). The protein concentration normalized forward scattering intensity Iabs(0)/c of protein is proportional to the molecular mass of a protein [44-46]. The Iabs(0)/c dMerlin(wt) or dMerlin(S518D) in PIP2 is the same as that of dMerlin(wt) or dMerlin(S518D) in 20% D2O solution (Table 1). This result indicates that the soluble fraction of dMerlin(wt) or dMerlin(S518D) is a monomer in PIP2.
Figure 5B, 5C and Table 1 show that in PIP2, the size of dMerlin(wt) in the soluble fraction becomes apparently larger than that of dMerlin(wt) in solution, with Rg=59.4±2.2 Å and Dmax=210 Å. However, for the soluble fraction of dMerlin(S518D) in PIP2, the change in the overall size is insignificant, with Rg=42.2±0.9 Å and Dmax=140 Å, indicating that, upon binding to PIP2, dMerlin(S518D) becomes slightly swollen but the whole molecule is retained in a closed conformation. Comparing the SANS results on Merlin with our previous results on Ezrin [32] indicates that in PIP2, the changes in Merlin(wt) conformation and in the overall size are not as dramatic as Ezrin in PIP2, see Figure S2 and Table 1.
The distributions of Rg and Dmax of the PIP2-bound dMerlin(wt) and dMerlin(S518D) are calculated using the program EOM [42]. The data are shown together with those of dMerlin(wt) and dMerlin(S518D) in solution (Figure 5D). Comparing the 3-D shape also shows that the PIP2-bound dMerlin(wt) becomes more expanded, whereas the conformational change in PIP2-bound dMerlin(S518D) is minimal.
Figure 6 shows the 3-D shape of dMerlin(wt) and dMerlin(S518D) in PIP2 reconstructed from SANS using the program DAMMIN [41]. Docking the crystal structure of Moesin in the 3D shape of dMerlin(wt) shows the opening or unwinding of the helical coiled-coiled region, and the separation between the FERM domain and the carboxyl-terminal tail. By contrast, although the 3-D shape of dMerlin(S518D) becomes slightly more swollen in PIP2 than in solution, the overall molecule remains folded, and the head-to-tail intramolecular interactions in dMerlin(S518D) between the FERM domain and the carboxyl-terminal tail remain intact. These results indicate that unlike dMerlin(wt), dMerlin(S518D) remains a compact conformation in PIP2.
Figure 6.
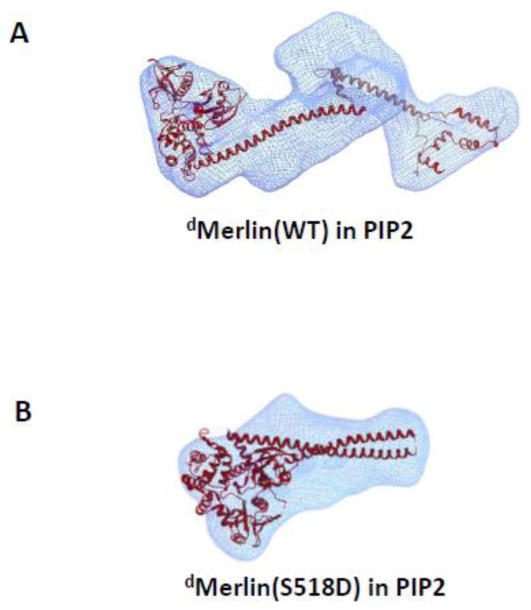
Comparing the changes in 3-D shapes of (A) dMerlin(wt) (A) and (B) dMerlin(S518D) in PIP2 (lower panel). The 3-D images are reconstructed from the SANS data using DAMMIN [41]. Note that the fit to the envelope in (A) is a simplification, as the C-terminal tail domain is not necessarily folded in absence of interaction with FERM domain.
To summarize, both Merlin and Merlin(S518D) bind to PIP2 with similar binding affinities. Binding to PIP2 causes Merlin(wt) and Merlin(S518D) to form an insoluble fraction and a soluble fraction. In the soluble fraction, Merlin(wt) undergoes conformational changes with apparent increase in the molecular size, and opening of the molecular structure. By contrast, the soluble fraction of Merlin(S518D) remains in a closed conformation in PIP2, with the head-to-tail association between FERM and the carboxyl-terminal tail intact.
Discussion
Recent biochemical studies indicate that the molecular conformation of Merlin may not be the generally accepted model that is borrowed from the structural and functional relationship of the ERM proteins. These studies suggest that phosphorylation at S518 either has minimal effects on the conformation [30], or the S518 phosphorylated Merlin adopts a more closed form than the unphosphorylated Merlin [31]. These studies underscores the importance to determine the structure of the full-length Merlin in order to understand the mechanisms of Merlin activation and inactivation. Currently, only the high-resolution structures of several versions of the Merlin FERM domain are available [47-50], despite intensive effort to determine the full-length Merlin structure.
Although the SANS study is of low resolution, SANS provides a direct structural comparison of the molecular shapes of Merlin and Merlin(S518D). Our results show that in solution both Merlin(wt) and Merlin(S518D) are monomers, and that both Merlin(wt) and the phosphomimetic Merlin(S518D) adopt a folded and compact conformation, similar to that of a closed Ezrin. Additionally, Merlin(S518D) is slightly more compact than Merlin(wt). These results support the recently proposed view that phoshorylation at S518 stabilizes the closed form rather than the open form of merlin [30, 31].
Nevertheless, the ITC experiments indicate that only less than 40% of the full-length Merlin(wt) or Merlin(S518D) is capable of binding to NHERF1. Our previous SPR studies find that an insignificant 0.4% Ezrin binds to NHERF1 in solution, suggesting that Ezrin is closed and inactive. Thus, unlike Ezrin, the NHERF1 binding sites in Merlin or Merlin(S518D) are not completely masked. In the full-length Merlin, the interaction between FERM and the carboxyl-terminal end is likely to be quite dynamic. Small amplitude inter-domain motions [40, 51-53] in Merlin are likely to be responsible for such graded NHERF1-binding behavior. The results that NHERF1 binds to both Merlin(wt) and Merlin(S518D) may support a view that the interaction of NHERF1 with Merlin is not involved in growth suppression.
The aggregation fraction of Merlin in PIP2 are likely to be functionally important because clustering of Merlin can sequester EGFR into a membrane compartment that prevent growth factor receptor signaling [11]. The aggregation of Merlin in PIP2 is likely due to ionic interactions between the negatively charged phosphate groups in PIP2, and the polybasic residues in Merlin. Studies have shown that electrostatic interactions between such polybasic residues and phosphatidylinositol lipids are necessary for protein targeting to cell membranes, and are responsible for sequestering membrane proteins into protein/phosphatidylinositol clusters in the cell membrane [54-57]. Three patches of polybasic residues exist in the amino-terminal half of Merlin, including the 79-KKVLDHDVSK-88 and at 269-KEFTIKPLDKK-280 in the mFERM domain [58], which contain the PIP2 binding motif KK(X)nK/RK that is also identified in the FERM domain of ERM proteins [59], and an additional amino-terminal 17-residue extension that is independent of the mFERM domain and is absent in ERM proteins [12]. These polybasic residues in the mFERM domain are necessary for the FERM domain of Merlin to bind to phosphatidylinositol lipids, for Merlin targeting to cell membrane, and for the growth suppressive function of Merlin [11, 12, 58]. It is likely that the basic residues in the 17-residue amino-terminal extension causes Merlin to aggregate in the presence of PIP2.
Interestingly, a latest study finds that neither the full-length Merlin(wt) nor Merlin(S518D) binds to the C-terminal peptide of the E3 ubiquitin ligase substrate adaptor DCAF1, but the mFERM domain binds to this C-terminal peptide of DCAF1 [50]. Thus, NHERF1 is likely to bind to the Merlin FERM domain at a site that is different from the DCAF1-binding sites. The inability of PIP2-bound Merlin(S518D) to adopt an open conformation may affect Merlin binding to target proteins other than NHERF1, such as DCAF1 [13, 50]. The fact that the isolated FERM domain does not suppress CRL4DCAF1 activity indicates that the C-terminal half of Merlin is important in growth suppression [13]. The relatively weak binding of the DCAF1 C-terminal peptide to the FERM domain of Merlin [50] raises an important question whether other portions of DCAF1 interact with Merlin at sites other than the FERM domain.
Experimental Procedures
Protein expression and purification
The human cDNA encoding the full-length Merlin isoform 1 was subcloned into the pET32a vector (EMD Biosciences, Inc), with a 6XHis plus thioredoxin tag at the amino-terminal end of the expressed proteins. The Merlin(S518D) and Merlin(S518E) mutants were generated with the QuikChange II site-directed mutagenesis kit (Agilent Technologies). The expression plasmids were transformed into the Rosetta2(DE3) competent cells (EMD Biosciences, Inc). The bacterial cells were grown in LB medium in the presence of 0.100 mg/ml ampicillin and 0.034 mg/ml chloramphenicol. At cell optical density at 600 nm of about 0.8, the cells were induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside, and the cells were grown at 20 °C for overnight. The cells were harvested and pelleted.
To purify protein, the cell pellets were suspended in 50-80 ml of lysis buffer (50 mM sodium phosphate, pH 7.5, 300 mM NaCl, 20 mM Imidazole, 0.1 mM PMSF) and incubated at 4 °C with agitation for 30 min. The homogenized lysate was sonicated on ice for 11 cycles of 30 s on and 30 s off, followed by centrifuge at 10,000 rpm for 30 min at 4°C and the supernatant saved for next step. The supernatant ran through a 5 ml HiTrap Chelating Sepharose™ High Performance (GE healthcare) and washed extensively with 200 ml of lysis buffer to remove impurities and non-specific bindings. Next, the bound Merlin eluted with adequate amount of elution buffer (50mM NaH2PO4, 300 mM NaCl, pH 7.5, 500 mM Imidazole, 0.1 mM PMSF) and then the best fractions were pooled and dialyzed in Tris 20 mM, 300 mM NaCl, 0.5 mM DTT and 0.5 mM EDTA. The thioredoxin tag of the purified proteins was cleaved by homemade tobacco etch virus protease. Again a second run of Ni2+-chelating column purification was performed to remove the cleaved tag, residual un-cleaved proteins, and the His-tagged TEV. Further purification followed by gel filtration chromatography using a Superdex 200 10/300 GL column (GE Healthcare). An extinction coefficient of 55350 M-1.cm-1 was used to determine protein concentration of wild type merlin in reduced condition and finally, the purity of the proteins estimated by SDS- PAGE (Fig. 1). The concentration of a purified Merlin or Merlin mutant was determined using a UV spectrometer at 280 nm using an extinction coefficient of 55350 M-1cm-1.
For SANS experiments, the deuterated Merlin were expressed in M9/D2O medium using our published protocols [32, 37, 51]. The procedure for purifying the deuterated proteins are the same as the undeuterated proteins.
Static and dynamic light scattering
The light scattering measurements were performed using DynaPro NanoStar (Wyatt Technology Corporation). Before measurements, the purified proteins in solution were centrifuged at 14000 rpm at room temperature for 15 minutes. Protein concentrations were kept constant at 5 μM in the same buffer as that in gel filtration experiments. DYNAMIC software (V7.1.4) was used to obtain the normalized time autocorrelation function, polydispersity, and the absolute molecular mass.
Circular dichroism spectroscopy
For CD measurements, proteins were dialyzed against 20 mM Potassium phosphate buffer pH 7.5, 0.1 mM EDTA, 150 mM NaCl and 1 mM DTT after purification. CD experiments were performed on a J-180 JASCO circular dichroism spectropolarimeter equipped with a temperature controlled cell holder using the Spectra Manager control (V1. 17.00) and application (V1.3.00) software package (JASCO). The instrument was calibrated with (+)-10-camphorsulfonic acid. Far-UV CD spectra recorded in a 1 mm quartz at 25 °C in a wavelength range of 190-250 nm with 1.0 nm bandwidth, using the continuous-mode setting, with 1.0 s response and a scan speed of 50 nm/min. Two spectra were averaged, and the background was corrected against buffer. The molar ellipticity [θ] was calculated from the formula: [θ]= (θ×100 Mw)/(cl), where c is the protein concentration in mg/ml, Mw is the molecular mass, l is the light path length in centimeters, and θ is the measured ellipticity in degrees at wavelength λ. Data deconvolution and normalization were carried out using DichroWeb[60].
Surface plasmon resonance
Surface plasmon resonance (SRP) experiments were performed on a Biacore X100 (GE Life Sciences, NJ) at 25.0 °C for determining Merlin binding to NHERF1. Before the binding experiments, the hydrogel matrix of the CM5 Biosensor chip (GE Life Sciences, NJ) was activated by N-hydroxysuccinimide and N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (GE Life Sciences, NJ). The ligand, which was 5 μg/ml NHERF1 was dissolved in 10 mM sodium acetate, pH 5.2, was injected to coat the activated surface. Uncross-linked ligand was washed away, and uncoated sites were blocked by 1 M ethanolamine, pH 8.5. The analyte, Merlin or Merlin(S518D), was dissolved in HBS-EP buffer containing 10 mM HEPES buffer, pH 7.4, 300 mM NaCl, 3 mM EDTA, and 0.005% surfactant polysorbate 20. The analyte was injected at a series of concentrations over the NHERF1-coated surfaces at 10 μl/min for 3 min. At the end of each injection, the sensor chip was regenerated with 4.0 M MgCl2, 50 mM triethylamine, pH 9.15, and HBS-EP buffer.
Isothermal Titration Calorimetry
ITC experiments were run in a MicroCal™ Auto-iTC200 system at 25 °C. Before ITC experiments, all samples were dialyzed in buffer containing HEPES 10 mM pH7.5, 300 mM NaCl, 0.5 mM EDTA and 0.5 mM β-ME. For determining NHERF1 binding to FERM or Merlin constructs, the reaction cells were filled with 460 μL of 20 μM FERM or Merlin, and NHERF (100-200 μM) was titrated into the cell in 19 injections of 2 μL each, with 150 s intervals between each injection. The data were analyzed using the manufacturer provided software Bindworks (version 3.1.5, Calorimetry Sciences Corp. Lindon, UT) to yield the stoichiometry (n), association constant (Ka) and observed enthalpy change (ΔHo) for the binding reactions.
For ITC experiments to determine the binding of Merlin to PIP2, one milligram of PIP2 from porcine brain (Avanti Polar Lipids, Inc) was dissolved in solvent CHCl3/MeOH/H2O (20:9:1). The solvent was evaporated using a Rotavap for 30-40 min, and then the formed solid film was dissolved in the ITC buffer containing HEPES 10mM, 300 mM NaCl, 0.5 mM EDTA and 0.5 mM beta Mercaptoethanol (B-ME) and passed through filter 0.2 micron. To remove additional ammonium salts already exists in the solution, the PIP2 solution was dialyzed against ITC buffer overnight in cold room. For ITC experiments, the reaction cell was filled with 460 μL of 30 μM protein, and PIP2 (0.6-0.9 mM) was titrated into the cell in 19 injections of 2 μL each, with constant stirring at 1000 rpm throughout the experiment and 150 s spacing between each injection. The heat of ligand dilution was obtained from the independent injections of PIP2 into ITC buffer. This value was subtracted from all of the injection points during data analysis.
Small angle neutron scattering experiments
SANS experiments were performed on the extended Q-range small-angle neutron scattering (EQ-SANS) instrument at the Spallation Neutron Source (SNS) located at Oak Ridge National Laboratory (ORNL) [61]. All measurements used a sample-to-detector distance of 4m. Two wavelength settings were used: 60 Hz with a wavelength, λ, band of 2.5 Å to 6.1 Å; and 30 Hz (frame-skipping mode) with two wavelength bands of 2.5 Å to 6.1 Å and 9.4 Å to 13.4 Å. The former configuration provides a useful Q-range (the wavevector transfer, Q = 4π sin(θ)/λ, where 2θ is the scattering angle) of ∼0.01 Å-1 to ∼0.22 Å-1, while the latter provides additional low Q data (down to ∼0.005 Å-1) with the same practical upper limit. The choice of configuration was determined by the expected size of the particles being studied and to probe for high molecular weight contaminants, such as protein aggregates. Samples were loaded into 1 mm pathlength circular-shaped quartz cuvettes (Hellma USA, Plainville, NY) and SANS measurements performed at 20 °C. Data reduction followed standard procedures that are implemented in MantidPlot (http://www.mantidproject.org/) to correct for dark current (background radiation and electronic noise), the detector sensitivity and the scattering contribution from the solvent and empty cells before being azimuthally averaged to produce I(Q) vs. Q. The data were scaled into absolute units of cm-1 using a calibrated standard [62].
SANS data analysis and 3-D shape reconstruction
The length distribution function P(r), radius of gyration Rg, the forward scattering intensity I(0), and the maximum dimension Dmax were calculated from the scattering data using the program GNOM [63]. The 3-D “dummy bead” coordinates were generated using the program DAMMIN [41]. Multiple calculations were performed using DAMMIN, and the generated 10 structures were averaged and filtered using the program DAMAVER and DAMFILT [64]. The 3-D density map was generated from the averaged coordinates using the program Situs [65]. The fitting and docking of the high-resolution structure to the density map were performed using Situs or UCS Chimera [66].
Supplementary Material
Highlights.
The conformation of the tumor suppressor protein Merlin is a subject of controversy.
Here we show that in solution both Merlin and Merlin(S518D) adopt a closed conformation. However a significant fraction of either Merlin or Merlin(S518D) is capable of binding to the target protein NHERF1.
Upon binding to the phosphatidylinositol 4,5-bisphosphate lipid, the wild-type Merlin adopts a more open conformation than in solution, but Merlin(S518D) remains in a closed conformation.
This study contributes to resolve a controversy about the molecular conformation and binding activity of Merlin.
Acknowledgments
This work was supported in part by NIH R01HL086496 (ZB), and 2G12 RR003060 from the National Center for Research Resources to CCNY. A portion of the research conducted at ORNL's Spallation Neutron Source was sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. We thank Tong Liang and Farhad Forouhar for kindly granting access to the ITC equipment at Columbia University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–39. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 3.Gutmann DH, Wright DE, Geist RT, Snider WD. Expression of the neurofibromatosis 2 (NF2) gene isoforms during rat embryonic development. Human molecular genetics. 1995;4:471–8. doi: 10.1093/hmg/4.3.471. [DOI] [PubMed] [Google Scholar]
- 4.McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–65. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Cooper J, Karajannis MA, Giancotti FG. Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 2012;13:204–15. doi: 10.1038/embor.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2008;28:854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 7.Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes & Development. 2001;15:968–80. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–71. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–40. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–84. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 16.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin Phosphorylation by p21-activated Kinase 2 and Effects of Phosphorylation on Merlin Localization. Journal of Biological Chemistry. 2002;277:10394–9. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 17.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–6. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 18.Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene. 2004;23:580–7. doi: 10.1038/sj.onc.1207142. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–9. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- 20.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–75. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus PA, et al. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J Mol Biol. 2007;365:1446–59. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–9. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy C, Martin M, Mangeat P. A dual involvement of the amino-terminal domain of ezrin in F- and G-actin binding. J Biol Chem. 1997;272:20088–95. doi: 10.1074/jbc.272.32.20088. [DOI] [PubMed] [Google Scholar]
- 25.Yao X, Cheng L, Forte JG. Biochemical characterization of ezrin-actin interaction. J Biol Chem. 1996;271:7224–9. doi: 10.1074/jbc.271.12.7224. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Agosti C, Wiederhold T, Herndon ME, Gusella J, Ramesh V. Interdomain Interaction of Merlin Isoforms and Its Influence on Intermolecular Binding to NHE-RF. Journal of Biological Chemistry. 1999;274:34438–42. doi: 10.1074/jbc.274.48.34438. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen R, Reczek D, Bretscher A. Hierarchy of Merlin and Ezrin N- and C-terminal Domain Interactions in Homo- and Heterotypic Associations and Their Relationship to Binding of Scaffolding Proteins EBP50 and E3KARP. Journal of Biological Chemistry. 2001;276:7621–9. doi: 10.1074/jbc.M006708200. [DOI] [PubMed] [Google Scholar]
- 28.Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–9. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 29.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–54. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 30.Hennigan RF, Foster LA, Chaiken MF, Mani T, Gomes MM, Herr AB, et al. Fluorescence resonance energy transfer analysis of merlin conformational changes. Mol Cell Biol. 2010;30:54–67. doi: 10.1128/MCB.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell. 2012;22:703–5. doi: 10.1016/j.devcel.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayasundar JJ, Ju JH, He L, Liu D, Meilleur F, Zhao J, et al. Open Conformation of Ezrin Bound to Phosphatidylinositol 4,5-Bisphosphate and to F-actin Revealed by Neutron Scattering. J Biol Chem. 2012;287:37119–33. doi: 10.1074/jbc.M112.380972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Dai Z, Jana D, Callaway DJ, Bu Z. Ezrin controls the macromolecular complexes formed between an adapter protein Na+/H+ exchanger regulatory factor and the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2005;280:37634–43. doi: 10.1074/jbc.M502305200. [DOI] [PubMed] [Google Scholar]
- 34.Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–79. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terawaki S, Maesaki R, Hakoshima T. Structural basis for NHERF recognition by ERM proteins. Structure. 2006;14:777–89. doi: 10.1016/j.str.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Finnerty CM, Chambers D, Ingraffea J, Faber HR, Karplus PA, Bretscher A. The EBP50-moesin interaction involves a binding site regulated by direct masking on the FERM domain. J Cell Sci. 2004;117:1547–52. doi: 10.1242/jcs.01038. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Callaway DJ, Bu Z. Ezrin induces long-range interdomain allostery in the scaffolding protein NHERF1. J Mol Biol. 2009;392:166–80. doi: 10.1016/j.jmb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–70. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 39.Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, et al. NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem. 1998;273:1273–6. doi: 10.1074/jbc.273.3.1273. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Poulikakos PI, Dai Z, Testa JR, Callaway DJ, Bu Z. Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J Biol Chem. 2007;282:27086–99. doi: 10.1074/jbc.M702019200. [DOI] [PubMed] [Google Scholar]
- 41.Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J. 1999;76:2879–86. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernado P, Mylonas E, Petoukhov MV, Blackledge M, Svergun DI. Structural characterization of flexible proteins using small-angle X-ray scattering. J Am Chem Soc. 2007;129:5656–64. doi: 10.1021/ja069124n. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho K, Khalifat N, Maniti O, Nicolas C, Arold S, Picart C, et al. Phosphatidylinositol 4, 5-bisphosphate-induced conformational change of ezrin and formation of ezrin oligomers. Biochemistry. 2010;49:9318–27. doi: 10.1021/bi101141d. [DOI] [PubMed] [Google Scholar]
- 44.Jacrot B, Zaccai G. Determination of Molecular Weight by Neutron Scattering. Biopolymers. 1981;20:2413–26. [Google Scholar]
- 45.Bu Z, Engelman DM. A method for determining transmembrane helix association and orientation in detergent micelles using small angle x-ray scattering. Biophys J. 1999;77:1064–73. doi: 10.1016/S0006-3495(99)76956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho DL, Byrnes WM, Ma WP, Shi Y, Callaway DJ, Bu Z. Structure-specific DNA-induced conformational changes in Taq polymerase revealed by small angle neutron scattering. J Biol Chem. 2004;279:39146–54. doi: 10.1074/jbc.M404565200. [DOI] [PubMed] [Google Scholar]
- 47.Yogesha S, Sharff AJ, Giovannini M, Bricogne G, Izard T. Unfurling of the band 4.1, ezrin, radixin, moesin (FERM) domain of the merlin tumor suppressor. Protein Science. 2011;20:2113–20. doi: 10.1002/pro.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang BS, Cooper DR, Devedjiev Y, Derewenda U, Derewenda ZS. The structure of the FERM domain of merlin, the neurofibromatosis type 2 gene product. Acta Crystallogr D Biol Crystallogr. 2002;58:381–91. doi: 10.1107/s0907444901021175. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Seto A, Maita N, Hamada K, Tsukita S, Tsukita S, et al. Structural Basis for Neurofibromatosis Type 2: CRYSTAL STRUCTURE OF THE MERLIN FERM DOMAIN. Journal of Biological Chemistry. 2002;277:10332–6. doi: 10.1074/jbc.M109979200. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Wei Z, Zhang J, Yang Z, Zhang M. Structural Basis of the Binding of Merlin FERM Domain to the E3 Ubiquitin Ligase Substrate Adaptor DCAF1. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.551184. jbc. M114. 551184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farago B, Li J, Cornilescu G, Callaway DJ, Bu Z. Activation of nanoscale allosteric protein domain motion revealed by neutron spin echo spectroscopy. Biophys J. 2010;99:3473–82. doi: 10.1016/j.bpj.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bu Z, Callaway DJ. Proteins MOVE! Protein dynamics and long-range allostery in cell signaling. Adv Protein Chem Struct Biol. 2011;83:163–221. doi: 10.1016/B978-0-12-381262-9.00005-7. [DOI] [PubMed] [Google Scholar]
- 53.Bu Z, Biehl R, Monkenbusch M, Richter D, Callaway DJ. Coupled protein domain motion in Taq polymerase revealed by neutron spin-echo spectroscopy. Proc Natl Acad Sci U S A. 2005;102:17646–51. doi: 10.1073/pnas.0503388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–5. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–75. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 56.Gambhir A, Hangyas-Mihalyne G, Zaitseva I, Cafiso DS, Wang J, Murray D, et al. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–61. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mani T, Hennigan RF, Foster LA, Conrady DG, Herr AB, Ip W. FERM domain phosphoinositide binding targets merlin to the membrane and is essential for its growth-suppressive function. Mol Cell Biol. 2011;31:1983–96. doi: 10.1128/MCB.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the Phosphatidylinositol 4,5-Bisphosphate (Pip2) Binding Site in the Nh2-Terminal Domain of Ezrin Correlates with Its Altered Cellular Distribution. The Journal of Cell Biology. 2000;151:1067–80. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Research. 2004;32:W668–W73. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao JK, Gao CY, Liu D. The extended Q-range small-angle neutron scattering diffractometer at the SNS. Journal of Applied Crystallography. 2010;43:1068–77. [Google Scholar]
- 62.Wignall GD, Bates FS. ABSOLUTE CALIBRATION OF SMALL-ANGLE NEUTRON-SCATTERING DATA. Journal of Applied Crystallography. 1987;20:28–40. [Google Scholar]
- 63.Semenyuk AV, Svergun DI. GNOM - A program Package for small-angle scattering data-processing. J Appl Cryst. 1991;24:537–40. [Google Scholar]
- 64.Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. Journal of Applied Crystallography. 2003;36:860–4. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wriggers W, Chacón P. Using Situs for the registration of protein structures with low-resolution bead models from X-ray solution scattering. J Appl Cryst. 2001;34:773–6. [Google Scholar]
- 66.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera - A Visualization System for Exploratory Research and Analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


