Abstract
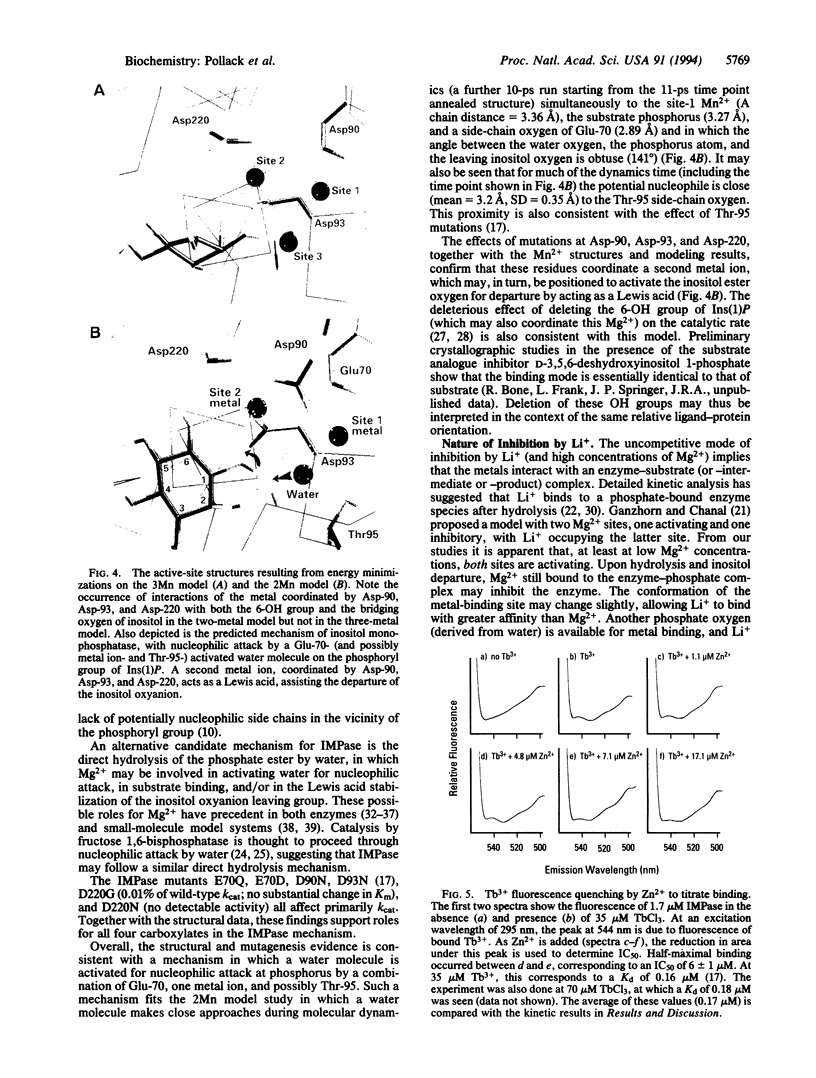
myo-Inositol monophosphatase (myo-inositol-1-phosphate phosphohydrolase, EC 3.1.3.25) is an attractive target for mechanistic investigation due to its critical role in the phosphatidylinositol signaling pathway and the possible relevance of its inhibition by Li+ to manic depression therapy. The x-ray crystallographic structure of human inositol monophosphatase in the presence of the inhibitory metal Gd3+ showed only one metal bound per active site, whereas in the presence of Mn2+, three ions were present with one being displaced upon phosphate binding. We report here modeling, kinetic, and mutagenesis studies on the enzyme, which reveal the requirement for two metal ions in the catalytic mechanism. Activity titration curves with Zn2+ or Mn2+ in the presence or absence of Mg2+ are consistent with a two-metal mechanism. Modeling studies based on the various x-ray crystallographic structures (including those with Gd3+ and substrate bound) further support a two-metal mechanism and define the positions of the two metal ions relative to substrate. While the first metal ion may activate water for nucleophilic attack, a second metal ion, coordinated by three aspartate residues, appears to act as a Lewis acid, stabilizing the leaving inositol oxyanion. In this model, the 6-OH group of substrate acts as a ligand for this second metal ion, consistent with the reduced catalytic activity observed with substrate analogues lacking the 6-OH. Evidence from Tb3+ fluorescence quenching and the two-metal kinetic titration curves suggests that Li+ binds at the site of this second metal ion.
Full text
PDF
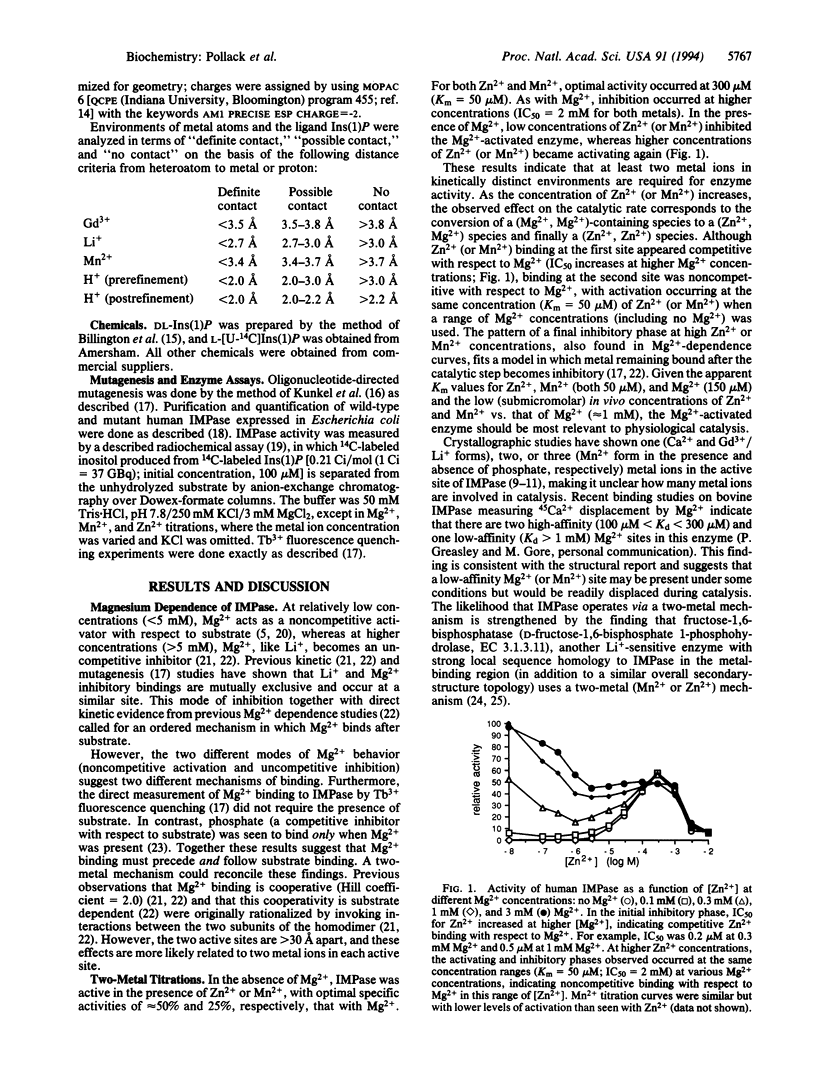
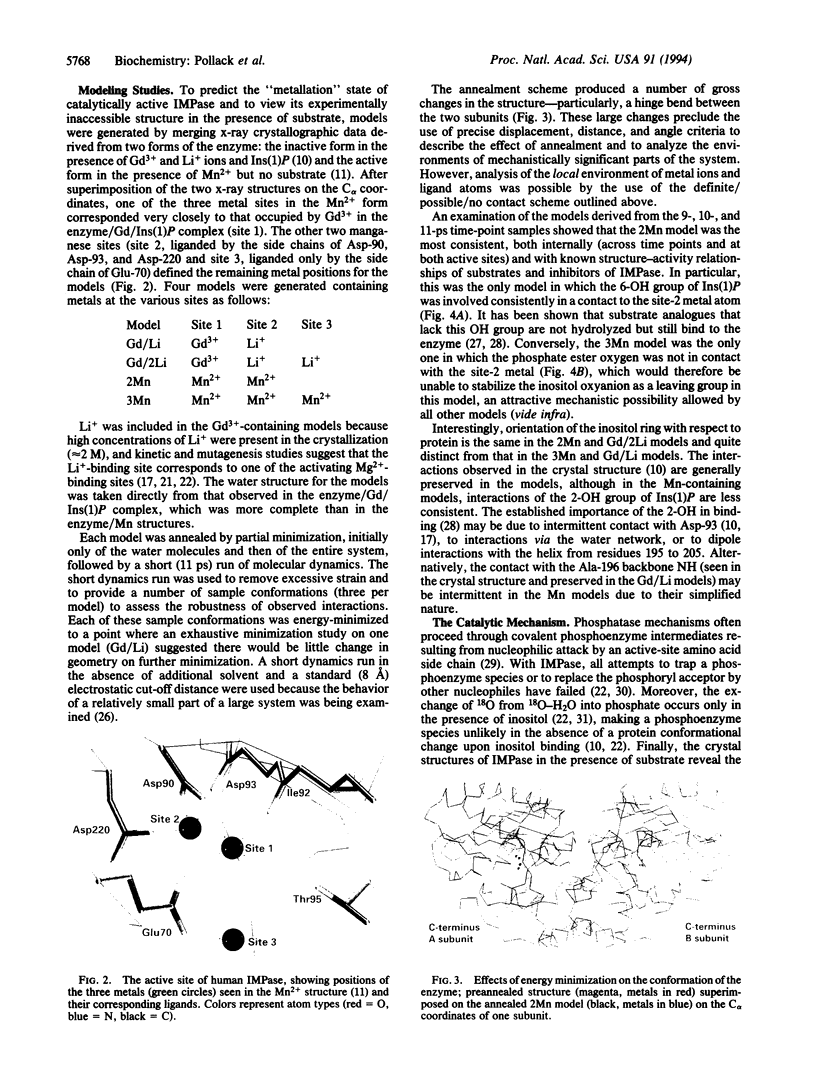

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beese L. S., Steitz T. A. Structural basis for the 3'-5' exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 1991 Jan;10(1):25–33. doi: 10.1002/j.1460-2075.1991.tb07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bone R., Springer J. P., Atack J. R. Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10031–10035. doi: 10.1073/pnas.89.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. Why is uncompetitive inhibition so rare? A possible explanation, with implications for the design of drugs and pesticides. FEBS Lett. 1986 Jul 14;203(1):3–6. doi: 10.1016/0014-5793(86)81424-7. [DOI] [PubMed] [Google Scholar]
- Derbyshire V., Freemont P. S., Sanderson M. R., Beese L., Friedman J. M., Joyce C. M., Steitz T. A. Genetic and crystallographic studies of the 3',5'-exonucleolytic site of DNA polymerase I. Science. 1988 Apr 8;240(4849):199–201. doi: 10.1126/science.2832946. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Friedman J. M., Beese L. S., Sanderson M. R., Steitz T. A. Cocrystal structure of an editing complex of Klenow fragment with DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8924–8928. doi: 10.1073/pnas.85.23.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzhorn A. J., Chanal M. C. Kinetic studies with myo-inositol monophosphatase from bovine brain. Biochemistry. 1990 Jun 26;29(25):6065–6071. doi: 10.1021/bi00477a026. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greasley P. J., Gore M. G. Bovine inositol monophosphatase. Studies on the binding interactions with magnesium, lithium and phosphate ions. FEBS Lett. 1993 Sep 27;331(1-2):114–118. doi: 10.1016/0014-5793(93)80308-h. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Herschlag D., Jencks W. P. Catalysis of the hydrolysis of phosphorylated pyridines by Mg(OH)+: a possible model for enzymatic phosphoryl transfer. Biochemistry. 1990 May 29;29(21):5172–5179. doi: 10.1021/bi00473a025. [DOI] [PubMed] [Google Scholar]
- Jackson R. G., Gee N. S., Ragan C. I. Modification of myo-inositol monophosphatase by the arginine-specific reagent phenylglyoxal. Biochem J. 1989 Dec 1;264(2):419–422. doi: 10.1042/bj2640419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. E., Wyckoff H. W. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol. 1991 Mar 20;218(2):449–464. doi: 10.1016/0022-2836(91)90724-k. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Leech A. P., Baker G. R., Shute J. K., Cohen M. A., Gani D. Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+. Eur J Biochem. 1993 Mar 15;212(3):693–704. doi: 10.1111/j.1432-1033.1993.tb17707.x. [DOI] [PubMed] [Google Scholar]
- McAllister G., Whiting P., Hammond E. A., Knowles M. R., Atack J. R., Bailey F. J., Maigetter R., Ragan C. I. cDNA cloning of human and rat brain myo-inositol monophosphatase. Expression and characterization of the human recombinant enzyme. Biochem J. 1992 Jun 15;284(Pt 3):749–754. doi: 10.1042/bj2840749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Jenkinson S., Challiss R. A. Disruption of phosphoinositide signalling by lithium. Biochem Soc Trans. 1992 May;20(2):430–434. doi: 10.1042/bst0200430. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Ragan C. I., Challiss R. A. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci. 1991 Aug;12(8):297–303. doi: 10.1016/0165-6147(91)90581-c. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pollack S. J., Knowles M. R., Atack J. R., Broughton H. B., Ragan C. I., Osborne S., McAllister G. Probing the role of metal ions in the mechanism of inositol monophosphatase by site-directed mutagenesis. Eur J Biochem. 1993 Oct 1;217(1):281–287. doi: 10.1111/j.1432-1033.1993.tb18244.x. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Watling K. J., Gee N. S., Aspley S., Jackson R. G., Reid G. G., Baker R., Billington D. C., Barnaby R. J., Leeson P. D. The dephosphorylation of inositol 1,4-bisphosphate to inositol in liver and brain involves two distinct Li+-sensitive enzymes and proceeds via inositol 4-phosphate. Biochem J. 1988 Jan 1;249(1):143–148. doi: 10.1042/bj2490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. B., Crowder M. W., Averill B. A. Hydrolysis of phosphate monoesters: a biological problem with multiple chemical solutions. Trends Biochem Sci. 1992 Mar;17(3):105–110. doi: 10.1016/0968-0004(92)90246-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang J. Y., Huang S., Ke H., Lipscomb W. N. Crystallographic studies of the catalytic mechanism of the neutral form of fructose-1,6-bisphosphatase. Biochemistry. 1993 Feb 23;32(7):1844–1857. doi: 10.1021/bi00058a019. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang J. Y., Lipscomb W. N. Structural similarities between fructose-1,6-bisphosphatase and inositol monophosphatase. Biochem Biophys Res Commun. 1993 Feb 15;190(3):1080–1083. doi: 10.1006/bbrc.1993.1159. [DOI] [PubMed] [Google Scholar]