Abstract
Farnesoid X receptor (FXR) functions as a regulator of bile acid and lipid homeostasis and is recognized as a promising therapeutic target for metabolic diseases. The biologic function of FXR is mediated in part by a small heterodimer partner (SHP); ligand-activated FXR enhances SHP expression, and SHP in turn represses the activity of multiple transcription factors. This study aimed to investigate the effect of FXR activation on expression of the major drug-metabolizing enzyme CYP3A4. The effects of 3-(2,6-dichlorophenyl)-4-(3′-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole (GW4064), a synthetic agonist of FXR, on the expression and activity of CYP3A4 were examined in primary human hepatocytes by using quantitative real-time polymerase chain reaction and S9 phenotyping. In human hepatocytes, treatment of GW4064 (1 μM) for 48 hours resulted in a 75% decrease in CYP3A4 mRNA expression and a 25% decrease in CYP3A4 activity, accompanied by ∼3-fold increase in SHP mRNA expression. In HepG2 cells, SHP repressed transactivation of CYP3A4 promoter by pregnane X receptor (PXR), constitutive androstane receptor (CAR), and glucocorticoid receptor. Interestingly, GW4064 did not repress expression of CYP2B6, another target gene of PXR and CAR; GW4064 enhanced CYP2B6 promoter activity. In conclusion, GW4064 represses CYP3A4 expression in human hepatocytes, potentially through upregulation of SHP expression and subsequent repression of CYP3A4 promoter activity. Clinically significant drug-drug interaction involving FXR agonists and CYP3A4 substrates may occur.
Introduction
Farnesoid X receptor (FXR, NRIH4) is a ligand-activated nuclear receptor and a member of the steroid/thyroid hormone receptors. FXR is highly expressed in the liver, intestine, kidney, and adrenal gland (Forman et al., 1995; Parks et al., 1999; Wang et al., 1999). Endogenous bile acids are ligands for FXR, and binding of bile acids to FXR leads to regulation of genes involved in bile acid homeostasis and glucose metabolism (Wang et al., 1999; Eloranta and Kullak-Ublick, 2008). Based on the biologic function of FXR as a regulator of lipid and glucose metabolism, many FXR agonists are currently being investigated as potential therapeutic agents for the treatment of metabolic disorders, including hypercholesterolemia (Thomas et al., 2008; Fiorucci et al., 2012). FXR is known to cause both transactivation and transrepression of its target gene promoters (Hollman et al., 2012). For example, ligand-activated FXR transrepresses the promoter of a drug-metabolizing enzyme UGT2B7 in Caco-2 cells (Lu et al., 2005), and FXR transactivates the promoter of a small heterodimer partner (SHP) by direct binding to –333/−320 of SHP (Goodwin et al., 2000). SHP is a nuclear receptor that lacks a DNA-binding domain, and it represses the expression of genes involved in bile acid synthesis by interfering with action of other transcriptional factors (Lee et al., 2000; Wang et al., 2002).
Cytochrome P450s (P450s) are the major drug-metabolizing enzymes responsible for the oxidative biotransformation of drugs, and CYP3A4 is the most prevalent among all P450 enzymes. Expression of P450 enzymes is highly inducible by endobiotics and xenobiotics, mainly via actions of ligand-activated transcription factors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) (Gao and Xie, 2010). PXR and CAR, on binding to respective ligands, transactivate CYP3A4 and CYP2B6 promoters and increase their expression (Zhou, 2008), which subsequently leads to clinically significant drug-drug interactions such that the doses of CYP3A4 substrate drugs need to be adjusted to prevent a lack of drug efficacy. Additionally, corticosteroids (at physiologic concentrations) enhance PXR transactivation of CYP3A4 promoter by increasing the expression of PXR and its binding partner retinoid X receptor (RXR), as well as the transcriptional activity of PXR, via glucocorticoid receptor (GR) (Pascussi et al., 2001).
Previously, FXR was shown to transactivate CYP3A4 promoter in HepG2 cells by binding to response elements located in the distal regulatory region of CYP3A4 (Gnerre et al., 2004). Also, in mice, 3-(2,6-dichlorophenyl)-4-(3′-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole (GW4064, a synthetic agonist of FXR) was shown to enhance expression of Cyp3a11, the murine homolog of human CYP3A4 (Gnerre et al., 2004). Together, these results suggest that FXR activators that are currently under development likely would increase CYP3A4-mediated drug metabolism in humans; however, extrapolation of the results to humans appears difficult because of the minimal expression of key transcription factors involved in P450 regulation (e.g., PXR or CAR) in HepG2 cells (Hart et al., 2010; Guo et al., 2011) and well-known interspecies differences in the regulation of hepatic drug-metabolizing enzymes between humans and rodents (Martignoni et al., 2006). Taken together, the effects of FXR activation on CYP3A4 expression and the underlying regulatory mechanisms remain unclear.
In this study, we investigated the effect of FXR activation on CYP3A4 expression using primary human hepatocytes. Our results show that GW4064 represses CYP3A4 expression, potentially through upregulating SHP expression and subsequent repression of PXR and CAR transactivation of CYP3A4 promoter.
Materials and Methods
Reagents.
GW4064, midazolam, 1-hydroxymidazolam, cholic acid, rifampin, and dexamethasone were purchased from Sigma-Aldrich (St. Louis, MO).
Plasmids.
A luciferase vector harboring distal and proximal xenobiotic response elements of CYP3A4 (i.e., pGL3-CYP3A4) was obtained from Dr. Hongbing Wang (Faucette et al., 2006). The luciferase vector containing 1.8 kb of CYP2B6 upstream regulatory region (i.e., pGL3-CYP2B6) and the luciferase vector containing 2.2-kb of SHP upstream regulatory region (i.e., pGL2-SHP) (Kim et al., 2003) were kindly provided by Drs. Masahiko Negishi (National Institute of Environmental Health Sciences) and Hueng-Sik Choi (Chonnam National University), respectively. Expression vectors for SHP (Koh et al., 2014), PXR, or CAR (Koh et al., 2012) were previously described. GR and FXR expression vectors were kindly provided by Drs. Alan McLachlan (University of Illinois at Chicago) and Yoon-Kwang Lee (Northeast Ohio Medical University), respectively.
Cell Culture.
HepG2 cells from the American Type Culture Collection (ATCC, Manassas, VA) were cultured in complete Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gemini, Sacramento, CA), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% minimum Eagle’s medium nonessential amino acids. Human embryonic kidney cells (i.e., HEK293T cells) were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (Gemini, West Sacramento, CA), 10 mM HEPES, 100 μM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA).
Primary Hepatocyte Culture.
Freshly isolated human hepatocytes were obtained from Liver Tissue Cell Distribution System (Pittsburgh, PA). Briefly, human hepatocytes were shipped overnight in cold preservation media. On receipt, the media were replaced with serum-free Williams’ E media (without phenol red) containing 0.1 μM dexamethasone, 100 U/ml penicillin, 100 μg/ml streptomycin, 15 mM HEPES, 2 mM l-glutamine, 5.5 μg/ml transferrin, and 5 ng/ml sodium selenite. Cells were allowed to recover from shipping for 18 hours at 37°C in an atmosphere containing 5% CO2 and used for experiments on the next day.
Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was isolated from human hepatocytes using Trizol (Life Technologies) and used as a template for the synthesis of complementary DNA using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). With the cDNA as template, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green reagents and the primers listed in Supplemental Table S1. The fold change in mRNA levels was determined after normalizing the gene expression levels to those of glyceraldehyde-3-phosphate dehydrogenase (2-∆∆Ct method).
Luciferase Reporter Assays.
HepG2 and HEK293T cells were seeded in 24-well plates at a density of 5 × 105 or 1 × 105 cells/well, respectively. On the next day, the cells were transfected with 0.3 μg of luciferase construct, 0.1 μg of expression plasmid (or empty vector as a control) for a transcription factor, and 0.002 μg of Renilla expression plasmid, using Fugene HD transfection reagent (Promega, Madison, WI) according to the manufacturer’s protocol. At 48 hours post-transfection, the transfected cells were harvested for determination of luciferase activities using Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was normalized to the Renilla luciferase activity. At least two independent experiments were performed in triplicate.
Determination of CYP3A4 Activity.
S9 fractions were prepared as described previously (Felmlee et al., 2008). Midazolam (final concentration 3 μM) was mixed with S9 fractions (10 μg) in an NADPH-regenerating system (5 mM isocitric acid, 0.2 unit/ml isocitric acid dehydrogenase, and 5 mM magnesium chloride in 100 mM Tris-HCl buffer, pH 7.4; total volume,100 μl). The reactions were started by the addition of NADP+ (10 mM) and further incubated for 1 hour. The reactions were quenched by adding cold acetonitrile (120 μl) containing phenytoin (0.5 μM) as internal standard. The mixture was kept on ice for 30 minutes, followed by centrifugation at 16,100g for 15 minutes at 4°C to obtain the supernatant. The concentrations of 1-hydroxymidazolam in the supernatants were determined by liquid chromatography-tandem mass spectrometry (MS/MS) (Agilent 1200 HPLC interfaced with Agilent 6410 Triple Quadrupole tandem MS equipped with an electrospray ion source; Agilent Technologies, Santa Clara, CA). Chromatographic separation was carried out by using a Waters XTerra MS C18 column (2.1 × 50 mm, 3.5 μm; Waters Corporation, Milford, MA). Mobile phase was delivered at 250 μl/min, and the gradient was initiated at 90% A and 10% B [A, 0.1% (v/v) formic acid in water; B, acetonitrile]. The proportion of mobile phase B was increased to 90% over 1 minute, held constant for 2 minutes, and then restored to the initial composition. The injection volume was 10 μl. Midazolam was detected by MS/MS (341.9/324.0), and phenytoin was used as the internal standard (253.2/182.2) in positive ion mode. All data were acquired using Agilent 6410 Quantitative Analysis version analyst data processing software.
Statistical Analysis.
Each experiment with primary human hepatocytes was conducted in triplicate, and data were expressed as mean ± S.D. Student’s t test was performed for statistically analysis.
Results
GW4064 Represses CYP3A4 Expression and Activity.
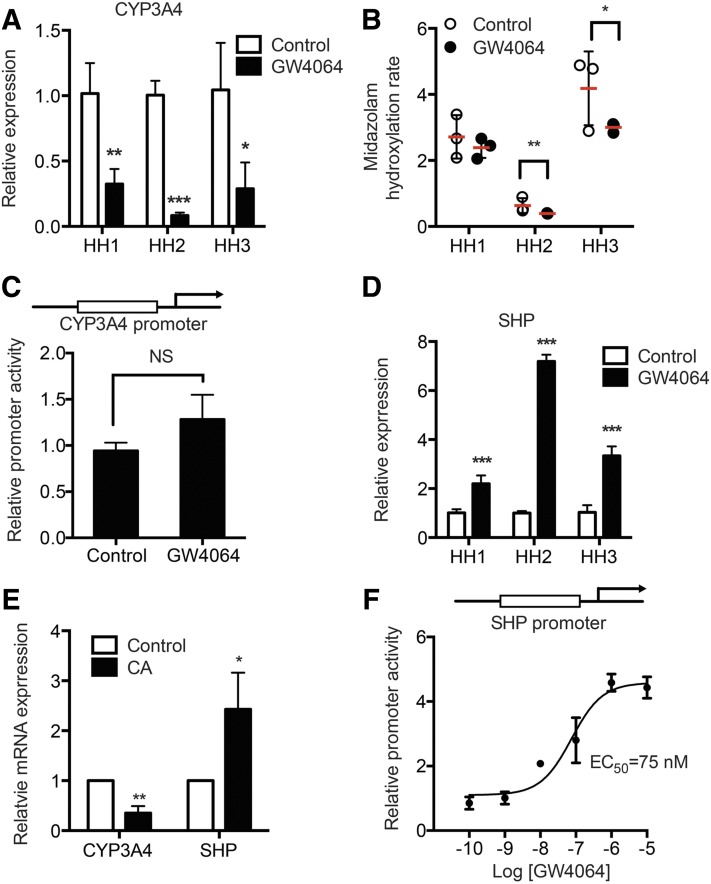
To determine whether FXR activation alters CYP3A4 expression, human hepatocytes from three different donors were treated with GW4064 (a FXR agonist, 1 μM) or DMSO for 48 hours, and then CYP3A4 mRNA expression and enzyme activity levels were examined. GW4064 significantly repressed mRNA expression levels (Fig. 1A) and enzyme activity (Fig. 1B) of CYP3A4.
Fig. 1.
GW4064 represses CYP3A4 expression. Primary human hepatocytes (HH) from three different donors were treated with vehicle (DMSO) or GW4064 (1 μM) for 48 hours in triplicate. RNAs were collected, and mRNA levels of CYP3A4 (A) and SHP (D) were determined by using qRT-PCR. (B) CYP3A4 activity was measured in S9 fractions from the GW4064- or vehicle-treated hepatocytes using midazolam as a probe substrate. Data shown are production rates of hydroxymidazolam in pmol/h per milligram of protein. (C) HepG2 cells were cotransfected with pGL3-CYP3A4 and Renilla luciferase vector. On the next day, the cells were treated with vehicle (DMSO) or GW4064 (1 μM) for 24 hours, and dual luciferase assays were performed. (E) Primary human hepatocytes were treated with vehicle (DMSO) or cholic acid (50 μM) for 48 hours in triplicate. RNAs were collected to measure CYP3A4 and SHP mRNA expression by qRT-PCR. (F) HEK293T cells were cotransfected with pGL2-SHP and Renilla luciferase vectors, along with FXR and RXR expression vectors. On the next day, the cells were treated with vehicle (DMSO) or GW4064 (0.1, 1, 10, 100, 1000, or 10,000 nM) for 24 hours, and dual luciferase assays were performed. NS, not significant. Data shown are mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle-treated group.
FXR is capable of causing transactivation or transrepression of its target gene promoters. To determine whether FXR directly transrepresses CYP3A4 promoter, luciferase reporter assays were performed in HepG2 cells. The cells were transfected with a CYP3A4 promoter vector and treated with GW4064 (or vehicle control), after which luciferase activities were measured. GW4064 did not decrease CYP3A4 promoter activity (Fig. 1C), suggesting that direct FXR transrepression of CYP3A4 promoter is an unlikely mechanism for CYP3A4 repression by GW4064. On the other hand, in human hepatocytes, CYP3A4 repression by GW4064 was accompanied by increased SHP expression (Fig. 1D). Downregulation of CYP3A4 and upregulation of SHP were also observed in human hepatocytes treated with cholic acid, a natural ligand of FXR (Fig. 1E), suggesting that FXR activation leads to CYP3A4 repression. In HEK293T cells cotransfected with a luciferase vector where luc expression is driven by 2.2 kb of SHP promoter (along with FXR and RXR expression plasmids), GW4064 enhanced SHP promoter activity in a concentration-dependent manner with an estimated EC50 of 75 nM.
SHP Represses PXR and CAR Transactivation of CYP3A4 Promoter.
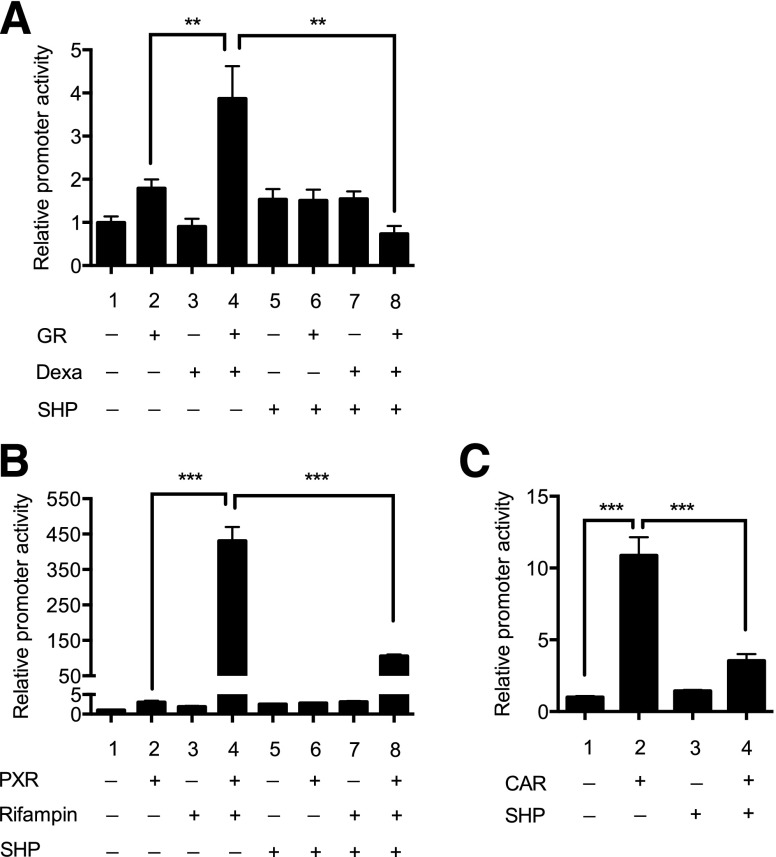
SHP is known to repress activity of multiple transcription factors, which potentially include PXR, CAR, and GR, which are involved in the regulation of CYP3A4 expression. Considering the absence of prominent PXR or CAR ligands, but the presence of dexamethasone, in the culture media for human hepatocytes, we first examined whether SHP represses regulation of CYP3A4 promoter activity via GR, by using promoter reporter assays. HepG2 cells were cotransfected with a luciferase vector where luc expression is driven by xenobiotic response element of CYP3A4 that binds directly to PXR or CAR (Faucette et al., 2006), along with expression vectors of GR and/or SHP. The transfected cells were treated with vehicle control or dexamethasone, and luciferase activity was measured. The results showed that activation of GR (by dexamethasone treatment) led to a significant increase in CYP3A4 promoter activity (Fig. 2A, lane 2 vs. 4), consistent with the previously reported data (Pascussi et al., 2001). The enhanced CYP3A4 promoter activity, however, was abrogated by SHP (Fig. 2A, lane 4 vs. 8).
Fig. 2.
SHP represses PXR and CAR transactivation of CYP3A4 promoter. (A) HepG2 cells were cotransfected with pGL3-CYP3A4 and Renilla luciferase vector, along with expression vectors for GR or SHP. On the next day, the cells were treated with vehicle (DMSO) or dexamethasone (1 μM) for 24 hours, and dual luciferase assays were performed. (B) HepG2 cells were cotransfected with pGL3-CYP3A4 and Renilla luciferase vector, along with expression vectors for PXR or SHP. On the next day, the cells were treated with vehicle (DMSO) or rifampin (5 μM) for 24 hours, and dual luciferase assays were performed. (C) HepG2 cells were cotransfected with pGL3-CYP3A4 and Renilla luciferase vector, along with expression vectors for CAR or SHP. The cell lysates were collected after 24 hours, and dual luciferase assays were performed. **P < 0.01; ***P < 0.001.
GR activation leads to increased CYP3A4 expression via enhancing both the expression and activity of PXR and CAR (Pascussi et al., 2001). To determine whether GW4064 alters the GR-mediated regulation of PXR or CAR expression, mRNA expression levels of PXR and CAR were measured in human hepatocytes treated with GW4064 (or vehicle control) by using qRT-PCR. The result showed that PXR or CAR expression did not differ between the groups (data not shown), suggesting that GW4064 does not repress GR action on PXR or CAR expression. Then, to examine whether SHP decreases PXR or CAR transactivation of CYP3A4, the promoter reporter assay was performed. The results showed that rifampin-activated PXR dramatically enhanced CYP3A4 promoter activity as expected (Fig. 2B, lane 2 and 4), and this was significantly repressed by SHP (Fig. 2B, lane 4 vs. 8). CAR transfection led to increases in CYP3A4 promoter activity (Fig. 2C, lane 1 vs. 2), consistent with the previous report that CAR is constitutively active even in the absence of ligands when transfected into immortalized cells (Baes et al., 1994). The enhanced CYP3A4 promoter activity was decreased on coexpression of SHP (Fig. 2C, lane 4 vs. 8). Together, these results indicate that SHP represses PXR/CAR transactivation of CYP3A4 promoter.
GW4064 Enhances CYP2B6 Promoter Activity.
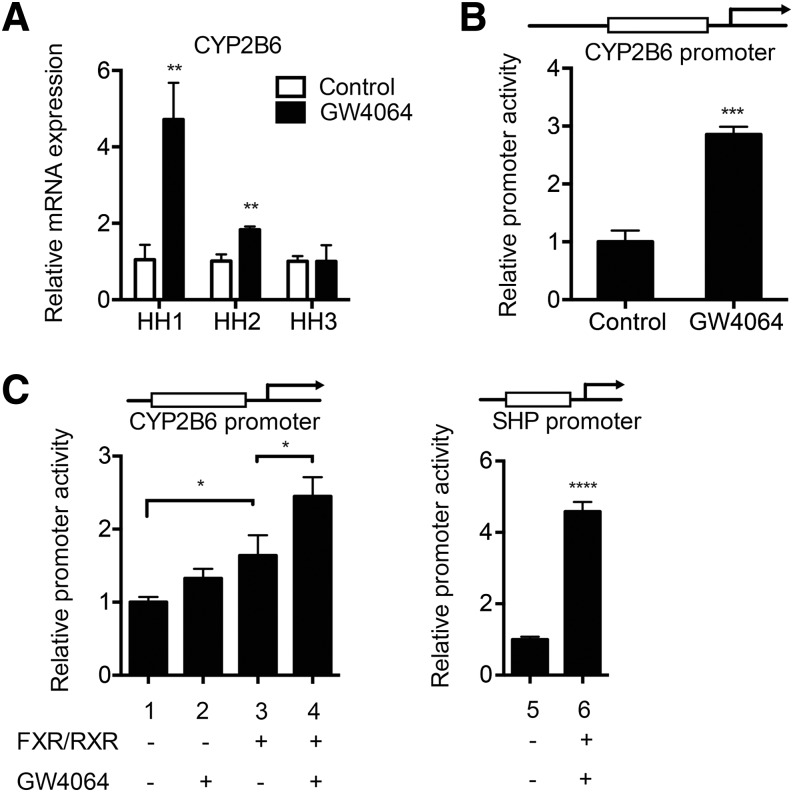
PXR and CAR enhance expression of many hepatic drug-metabolizing enzymes, including CYP2B6 (Faucette et al., 2006). To determine whether the SHP-mediated repression of PXR and CAR action alters expression of other target genes, we examined the expression of CYP2B6 in human hepatocytes treated with GW4064. Interestingly, CYP2B6 mRNA levels were significantly increased (rather than decreased) in two of three different batches of hepatocytes (Fig. 3A). To examine whether GW4064 can directly activate CYP2B6 promoter, we examined promoter reporter assays. HepG2 cells were transfected with a luciferase vector where luc expression is driven by 1.8-kb upstream regulatory region of CYP2B6 and treated with vehicle control or GW4064, and luciferase activity was then measured. The results showed that GW4064 treatment led to ∼3-fold increase in CYP2B6 promoter activity (Fig. 3B).
Fig. 3.
GW4064 upregulates CYP2B6 expression via FXR. (A) Primary human hepatocytes from three different donors were treated with vehicle (DMSO) or GW4064 (1 μM) for 48 hours in triplicate. RNA was collected, and CYP2B6 mRNA expression level was measured by using qRT-PCR. (B) HepG2 cells were cotransfected with pGL3-CYP2B6 and Renilla vector and treated with vehicle or GW4064 (1 μM), and dual luciferase assay was performed. (C) HEK293T cells were cotransfected with pGL3-CYP2B6 (or pGL2-SHP) and Renilla vector, along with FXR and RXR expression vectors. On the next day, the cells were treated with vehicle (DMSO) or GW4064 (1 μM). The cell lysates were collected after 24 hours, and dual luciferase assays were performed. **P < 0.01; ***P < 0.001.
To determine whether the enhanced CYP2B6 promoter activity is through FXR transactivation of CYP2B6 promoter, promoter reporter assays were performed in HEK293T cells cotransfected with a CYP2B6-promoter driven luciferase vector and expression vector for FXR and RXR. The cells were treated with GW4064 (or vehicle control), and luciferase activity was measured. A luciferase vector harboring 2.2-kb SHP promoter was included as a control. Results showed that GW4064 enhanced CYP2B6 promoter activity ∼2.5-fold (Fig. 3C, lanes 1 and 4). This induction was of a similar magnitude as the increase in SHP promoter activity by GW4064 (Fig. 3C, lane 5 and 6). Collectively, these data indicate that the enhanced CYP2B6 expression could be due to increased CYP2B6 promoter activity by FXR activation.
Discussion
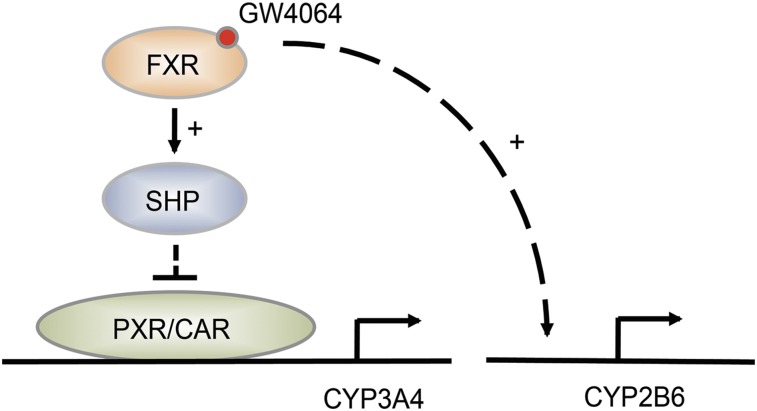
In this study, we examined the effects of FXR activation on expression of CYP3A4 by using freshly isolated primary human hepatocytes. Our results showed that GW4064 significantly decreases the expression of the major drug-metabolizing enzyme CYP3A4 in human hepatocytes and that this is in part attributable to increased SHP expression upon FXR activation. We found that a different inducer of SHP, all-trans retinoic acid (Koh et al., 2014), also repressed CYP3A4 expression in human hepatocytes (data not shown), further supporting the important role of SHP in CYP3A4 regulation. CYP3A4 expression is regulated by nuclear receptors, including PXR and CAR. Whereas PXR and CAR are activated to the fullest extent on binding of respective ligands to the receptors, both transcription factors appear activated at basal levels in human hepatocytes (Zamule et al., 2008; Hariparsad et al., 2009). This could be due in part to the presence of corticosteroids in culture media that are known to enhance the expression as well as transcriptional activity of PXR and CAR via GR (Pascussi et al., 2000, 2001). Our results from luciferase reporter assays showed that SHP represses actions of GR, as well as those of PXR and CAR on CYP3A4 promoter, suggesting that repression of these transcriptional activators on CYP3A4 promoter by SHP may potentially trigger CYP3A4 repression by GW4064. Interestingly, such repressive action of SHP on PXR/CAR target gene promoter was not shown for CYP2B6, in part because GW4064 activates CYP2B6 promoter. The model for differential regulation of CYP3A4 and CYP2B6 by GW4064 is shown in Fig. 4.
Fig. 4.
Differential regulation of CYP2B6 and CYP3A4 by GW4064. FXR activation by GW4046 leads to decreased CYP3A4 expression, potentially through SHP upregulation and subsequent repression of PXR and CAR transactivation of CYP3A4 promoter. On the other hand, GW4064 enhances CYP2B6 promoter activity.
In addition to PXR and CAR, multiple other transcription factors, including peroxisome proliferator-activated receptor (PPAR) α and liver X receptor (LXR), upregulate CYP3A4 expression (Drocourt et al., 2002; Duniec-Dmuchowski et al., 2007; Thomas et al., 2013). However, potential involvement of PPARα and LXR in CYP3A4 repression by GW4064 appears unlikely. Our results from human hepatocytes indicate that GW4064 does not alter the mRNA expression of the representative target genes of these nuclear receptors, including MDR3 (Ghonem et al., 2014), FASN, and SREBF1 (Wagner et al., 2003) (data not shown). Inflammation is also known to alter CYP3A4 expression; CYP3A4 expression is downregulated in the state of inflammation (Aitken et al., 2006). In GW4064-treated human hepatocytes, there were no increases in the expression levels of inflammatory marker tumor necrosis factor-α (data not shown) and no significant changes in the gross morphology of hepatocytes that are indicative of drug toxicity or inflammation. Together, these findings suggest that PPARα, LXR, or inflammation plays minimal roles, if any, in CYP3A4 repression by GW4064.
Previous studies using HepG2 cells have shown that GW4064 increases (not decreases, as shown in our study) CYP3A4 expression (Gnerre et al., 2004); GW4064 (at 1 μM) increased CYP3A4 mRNA levels by 1.8-fold in HepG2 cells, potentially by FXR binding to ∼7.8-kb upstream region of CYP3A4 and transactivating the promoter (Gnerre et al., 2004). Also, GW4064 enhanced expression of Cyp3a11 (a CYP3A4 homolog gene) in mice by ∼2-fold (Gnerre et al., 2004). Although the reason for the discrepancy between the previous and our study is unclear, it appears possible that in HepG2 cells, the expression levels of key hepatic transcription factors, including PXR and CAR, may have been low for SHP to exhibit any repressive action. Also, significant interspecies differences in the regulation of drug-metabolizing enzymes expression hamper the extrapolation of animal data to humans (Lu and Li, 2001; Vignati et al., 2004). Considering that primary human hepatocytes have served as a gold standard tool to study regulation of hepatic drug-metabolizing enzymes, it appears likely that GW4064 decreases CYP3A4-mediated drug metabolism in humans through SHP-mediated repression of CYP3A4 expression.
FXR agonists, including GW4064, are currently under development as potential therapeutic agents for metabolic diseases such as obesity, type 2 diabetes, hypertriglyceridemia, atherosclerosis, and nonalcoholic steatohepatitis (reviewed in Thomas et al., 2008). As CYP3A4 repression by GW4064 is potentially triggered by increased expression of SHP and that SHP induction is a class effect of FXR agonists, other FXR agonists are also expected to repress CYP3A4 expression, potentially causing drug-drug interactions with CYP3A4 substrates. It remains difficult, however, to predict quantitatively the clinical outcome of these interactions based only on the results from human hepatocytes. This is due in part to the long degradation half-lives of CYP3A4 protein [i.e., 36–50 hours (Fromm et al., 1996)] such that CYP3A4 protein levels in human hepatocytes do not reach the steady state after the typical time of drug treatment (e.g., 48 hour) passes. In accordance, CYP3A4 mRNA expression decreased >50% in GW4064-treated hepatocytes in this study, whereas the decrease in catalytic activity of CYP3A4 (as determined in S9 fraction of hepatocytes) was only 10%–30%. The clinical consequences of FXR agonists repressing CYP3A4 expression thus remain to be examined.
In conclusion, we showed that GW4064 represses CYP3A4 expression, potentially through upregulating SHP expression and subsequent repression of PXR and CAR transactivation of CYP3A4 promoter. This finding suggests that drug-drug interactions may occur clinically between CYP3A4 substrates and FXR agonists that are currently under development for the treatment of metabolic diseases.
Supplementary Material
Acknowledgments
The authors thank Drs. Hongbing Wang (University of Maryland), Masahiko Negishi (National Institute of Environmental Health Sciences), Hueng-Sik Choi (Chonnam National University), Alan McLachlan (University of Illinois at Chicago), and Yoon-Kwang Lee (Northeast Ohio Medical University) for the generous gifts of pGL3-CYP3A4, pGL3-CYP2B6, pGL2-SHP, p6R-GR, and pcDNA3-FXRα, respectively.
Abbreviations
- CAR
constitutive androstane receptor
- FXR
farnesoid X receptor
- GR
glucocorticoid receptor
- GW4064
3-(2,6-dichlorophenyl)-4-(3′-carboxy-2-chlorostilben-4-yl)oxymethyl-5-isopropylisoxazole
- LXR
liver X receptor
- P450
cytochrome P450
- PPARα
peroxisome proliferator-activated receptor α
- PXR
pregnane X receptor
- qRT-PCR
quantitative real-time polymerase chain reaction
- SHP
small heterodimer partner
Authorship Contributions
Participated in research design: Zhang, Pan, Jeong.
Conducted experiments: Zhang, Pan.
Performed data analysis: Zhang, Pan, Jeong.
Wrote or contributed to the writing of the manuscript: Zhang, Pan, Jeong.
Footnotes
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD065532]. Liver Tissue Cell Distribution System was supported by the National Institutes of Health [Grant N01-DK-7-0004/HHSN267200700004C].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Aitken AE, Richardson TA, Morgan ET. (2006) Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 46:123–149. [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14:1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. (2002) Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277:25125–25132. [DOI] [PubMed] [Google Scholar]
- Duniec-Dmuchowski Z, Ellis E, Strom SC, Kocarek TA. (2007) Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists. Biochem Pharmacol 74:1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta JJ, Kullak-Ublick GA. (2008) The role of FXR in disorders of bile acid homeostasis. Physiology (Bethesda) 23:286–295. [DOI] [PubMed] [Google Scholar]
- Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. (2006) Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther 317:1200–1209. [DOI] [PubMed] [Google Scholar]
- Felmlee MA, Lon HK, Gonzalez FJ, Yu AM. (2008) Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab Dispos 36:435–441. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Zampella A, Distrutti E. (2012) Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr Top Med Chem 12:605–624. [DOI] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, et al. (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693. [DOI] [PubMed] [Google Scholar]
- Fromm MF, Busse D, Kroemer HK, Eichelbaum M. (1996) Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology 24:796–801. [DOI] [PubMed] [Google Scholar]
- Gao J, Xie W. (2010) Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab Dispos 38:2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonem NS, Ananthanarayanan M, Soroka CJ, Boyer JL. (2014) Peroxisome proliferator-activated receptor α activates human multidrug resistance transporter 3/ATP-binding cassette protein subfamily B4 transcription and increases rat biliary phosphatidylcholine secretion. Hepatology 59:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnerre C, Blättler S, Kaufmann MR, Looser R, Meyer UA. (2004) Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics 14:635–645. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526. [DOI] [PubMed] [Google Scholar]
- Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. (2011) Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos 39:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariparsad N, Chu X, Yabut J, Labhart P, Hartley DP, Dai X, Evers R. (2009) Identification of pregnane-X receptor target genes and coactivator and corepressor binding to promoter elements in human hepatocytes. Nucleic Acids Res 37:1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. (2010) A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos 38:988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman DA, Milona A, van Erpecum KJ, van Mil SW. (2012) Anti-inflammatory and metabolic actions of FXR: insights into molecular mechanisms. Biochim Biophys Acta 1821:1443–1452. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim JY, Park YY, Choi HS. (2003) Synergistic activation of the human orphan nuclear receptor SHP gene promoter by basic helix-loop-helix protein E2A and orphan nuclear receptor SF-1. Nucleic Acids Res 31:6860–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Jurkovic S, Yang K, Choi SY, Jung JW, Kim KP, Zhang W, Jeong H. (2012) Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol 84:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Pan X, Shen HW, Arnold SL, Yu AM, Gonzalez FJ, Isoherranen N, Jeong H. (2014) Altered expression of small heterodimer partner governs cytochrome P450 (CYP) 2D6 induction during pregnancy in CYP2D6-humanized mice. J Biol Chem 289:3105–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD. (2000) The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol 20:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Li AP. (2001) Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem Biol Interact 134:271–281. [DOI] [PubMed] [Google Scholar]
- Lu Y, Heydel JM, Li X, Bratton S, Lindblom T, Radominska-Pandya A. (2005) Lithocholic acid decreases expression of UGT2B7 in Caco-2 cells: a potential role for a negative farnesoid X receptor response element. Drug Metab Dispos 33:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. (2006) Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2:875–894. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. (2001) Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem 268:6346–6358. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. (2000) Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol Pharmacol 58:1441–1450. [DOI] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. (2008) Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693. [DOI] [PubMed] [Google Scholar]
- Thomas M, Burk O, Klumpp B, Kandel BA, Damm G, Weiss TS, Klein K, Schwab M, Zanger UM. (2013) Direct transcriptional regulation of human hepatic cytochrome P450 3A4 (CYP3A4) by peroxisome proliferator-activated receptor alpha (PPARα). Mol Pharmacol 83:709–718. [DOI] [PubMed] [Google Scholar]
- Vignati LA, Bogni A, Grossi P, Monshouwer M. (2004) A human and mouse pregnane X receptor reporter gene assay in combination with cytotoxicity measurements as a tool to evaluate species-specific CYP3A induction. Toxicology 199:23–33. [DOI] [PubMed] [Google Scholar]
- Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, et al. (2003) Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol 23:5780–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553. [DOI] [PubMed] [Google Scholar]
- Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, et al. (2002) Redundant pathways for negative feedback regulation of bile acid production. Dev Cell 2:721–731. [DOI] [PubMed] [Google Scholar]
- Zamule SM, Strom SC, Omiecinski CJ. (2008) Preservation of hepatic phenotype in lentiviral-transduced primary human hepatocytes. Chem Biol Interact 173:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF. (2008) Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab 9:310–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.