Abstract
Central nervous system infections can underlie the development of epilepsy, and Theiler’s murine encephalomyelitis virus (TMEV) infection in C57BL/6J mice provides a novel model of infection-induced epilepsy. Approximately 50–65% of infected mice develop acute, handling-induced seizures during the infection. Brains display acute neuropathology, and a high number of mice develop spontaneous, recurrent seizures and behavioral comorbidities weeks later. This study characterized the utility of this model for drug testing by assessing whether antiseizure drug treatment during the acute infection period attenuates handling-induced seizures, and whether such treatment modifies associated comorbidities. Male C57BL/6J mice infected with TMEV received twice-daily valproic acid (VPA; 200 mg/kg), carbamazepine (CBZ; 20 mg/kg), or vehicle during the infection (days 0–7). Mice were assessed twice daily during the infection period for handling-induced seizures. Relative to vehicle-treated mice, more CBZ-treated mice presented with acute seizures; VPA conferred no change. In mice displaying seizures, VPA, but not CBZ, reduced seizure burden. Animals were then randomly assigned to acute and long-term follow-up. VPA was associated with significant elevations in acute (day 8) glial fibrillary acidic protein (astrocytes) immunoreactivity, but did not affect NeuN (neurons) immunoreactivity. Additionally, VPA-treated mice showed improved motor performance 15 days postinfection (DPI). At 36 DPI, CBZ-treated mice traveled significantly less distance through the center of an open field, indicative of anxiety-like behavior. CBZ-treated mice also presented with significant astrogliosis 36 DPI. Neither CBZ nor VPA prevented long-term reductions in NeuN immunoreactivity. The TMEV model thus provides an etiologically relevant platform to evaluate potential treatments for acute seizures and disease modification.
Introduction
Viral infections of the central nervous system can underlie the development of chronic epilepsy due to an increased expression of inflammatory cytokines, lowered seizure threshold, and increased risk of status epilepticus. For example, infection with human herpes 6B virus is associated with the development of encephalitis, seizures, and epilepsy (Caserta et al., 1998; Solomon et al., 2007). Human patients with viral infection–induced encephalitis who present with seizures during the acute infection period are up to 22 times more likely to develop spontaneous, unprovoked seizures than the general population (Annegers et al., 1988). More importantly, inflammation represents a significant risk factor for seizure induction and maintenance, with proinflammatory cytokines being highly expressed in various animal seizure models (Pernot et al., 2011; Vezzani et al., 2011; Vezzani and Friedman, 2011) and patients with epilepsy (Kan et al., 2012; He et al., 2013; Hu et al., 2014). In fact, some seizure-induced proinflammatory signaling molecules remain upregulated during epileptogenesis (Voutsinos-Porche et al., 2004; Ravizza et al., 2008; Maroso et al., 2010) and may be essential to the establishment of spontaneous recurrent seizures associated with temporal lobe epilepsy (TLE) (Ravizza et al., 2011). Thus, models that recapitulate the clinical symptoms of encephalitis may ultimately provide a useful platform to identify novel compounds that may modify or prevent acute seizures, as well as epileptogenesis, in this and other acquired epilepsies.
Theiler’s murine encephalomyelitis virus (TMEV) infection–induced epilepsy in the C57BL/6J mouse represents a useful model of TLE (Libbey et al., 2008, 2011a; Stewart et al., 2010a,b). Animals develop subsequent behavioral comorbidities (Umpierre et al., 2014), which are also associated with human TLE (Brooks-Kayal et al., 2013). Moreover, TMEV-infected mice present with many characteristics associated with human viral encephalitis–associated seizures (Misra et al., 2008). Approximately 50–65% of TMEV-infected animals develop acute handling-induced seizures and show significant elevations in inflammatory cytokines and hippocampal cell death (Stewart et al., 2010a,b). Nearly 50% of the animals that survive the initial infection then develop spontaneous, recurrent seizures weeks later. However, it is currently unknown whether acute therapeutic intervention during the infection can reduce the severity or block development of acute handling-induced seizures in TMEV-infected animals. Moreover, it is unknown whether acute treatment confers any long-term effects (positive or negative) on the comorbidities associated with TMEV infection (Umpierre et al., 2014), a question that was one goal of these studies.
Traditional antiseizure drugs (ASDs) may control TMEV-induced seizures through direct suppression of seizure propagation via ion-channel modulation or effects on neurotransmission. Thus, the ASDs, valproic acid (VPA) and carbamazepine (CBZ), were subchronically administered during the acute TMEV seizure phase to determine whether these agents could suppress acute behavioral seizures and whether pharmacological inhibition of seizures is sufficient to alter the development of associated long-term behavioral deficits (Umpierre et al., 2014). VPA can reduce seizure frequency and severity through multiple ion channel–centric mechanisms (White et al., 2007; Barker-Haliski et al., 2014b). Additionally, VPA can induce brain-derived neurotrophic factor activation (Yasuda et al., 2009), as well as potently inhibit histone deactylase and glycogen synthase kinase-3 activity (Rosenberg, 2007; Hoffmann et al., 2008; Chiu et al., 2013), which may altogether contribute to network remodeling underlying epileptogenesis (Cantley and Haynes, 2013; Liu et al., 2013; Vezzani et al., 2013). Moreover, VPA is neuroprotective in models of Alzheimer’s disease (Kilgore et al., 2010), traumatic brain injury (TBI) (Dash et al., 2010), and septic encephalopathy (Wu et al., 2013). Conversely, CBZ possess notable antiseizure efficacy most likely mediated by sodium channel inhibition (White et al., 2007; Barker-Haliski et al., 2014b); however, it has yet to be associated with any direct anti-inflammatory effects or modulatory effects on signaling pathways. Thus, VPA and CBZ represent two very diverse strategies to prevent acute TMEV-induced seizures.
The present investigation therefore sought to determine whether pharmacological treatment during the acute infection phase of the TMEV model of acquired epilepsy could modify the acute behavioral seizure incidence and severity. Additionally, the long-term effects of acute therapeutic intervention on associated anxiety-like behavior (Umpierre et al., 2014) and neuropathology (Stewart et al., 2010a,b) known to develop after the acute viral infection were evaluated. Novel and symptom-specific models are of high value to translational research endeavors (Barker-Haliski et al., 2014a); therefore, characterizing the pharmacological profile of these models with known ASDs is necessary before any novel therapy is likely to advance to the clinic. The TMEV model of viral encephalitis–induced epilepsy thus provides an etiologically relevant platform to evaluate compounds with the potential for acute seizure-suppressive, and possibly disease-modifying, effects.
Materials and Methods
Animal Handling and Drug Dosing.
Male, C57BL/6J mice (4–5 weeks old; The Jackson Laboratory, Bar Harbor, ME) were randomly divided into three treatment groups (n = 28/treatment group). ASD-treated or vehicle-treated control groups were subchronically dosed (b.i.d.) for days 0–7 of the acute viral infection based on the study design detailed in Fig. 1. Twice-daily doses of VPA (200 mg/kg) and CBZ (20 mg/kg) were based on the ED50 of these drugs in male mice in the maximal electroshock seizure test (Bialer et al., 2004; Rowley and White, 2010). For reference, a correlative analysis between mouse maximal electroshock ED50 values and human steady state plasma levels has previously been described (Bialer et al., 2004). Investigational compounds were made fresh daily in vehicle (0.5% methylcellulose in water). Body weights were recorded daily during the acute infection period (Fig. 1), and then weekly until behavioral testing (data not shown). Mice were evaluated twice per day for days 3–7 for the presence and severity of handling-induced behavioral seizures by experimenter-conducted visual observation during each observation session (Libbey et al., 2008). Mice were group-housed (9–11 mice/cage) for the duration of the subchronic dosing and behavioral testing periods, that is, up to 36 days postinfection (DPI). Following the acute infection period on day 8, randomly selected cohorts of mice from each treatment group that presented with and without seizures (n = 4/seizure condition/treatment condition; n = 8 total/treatment group) were euthanized for brain collection for immunohistochemical assessment of glial fibrillary acidic protein (GFAP) and NeuN immunoreactivity. All of the surviving animals from each treatment group were then allowed to recover for an additional 4 weeks to assess long-term changes in behavioral performance in the open field (OF) activity monitor. Those mice were then euthanized 36 DPI, and brains were collected for immunohistochemical assessment of GFAP and NeuN immunoreactivity.
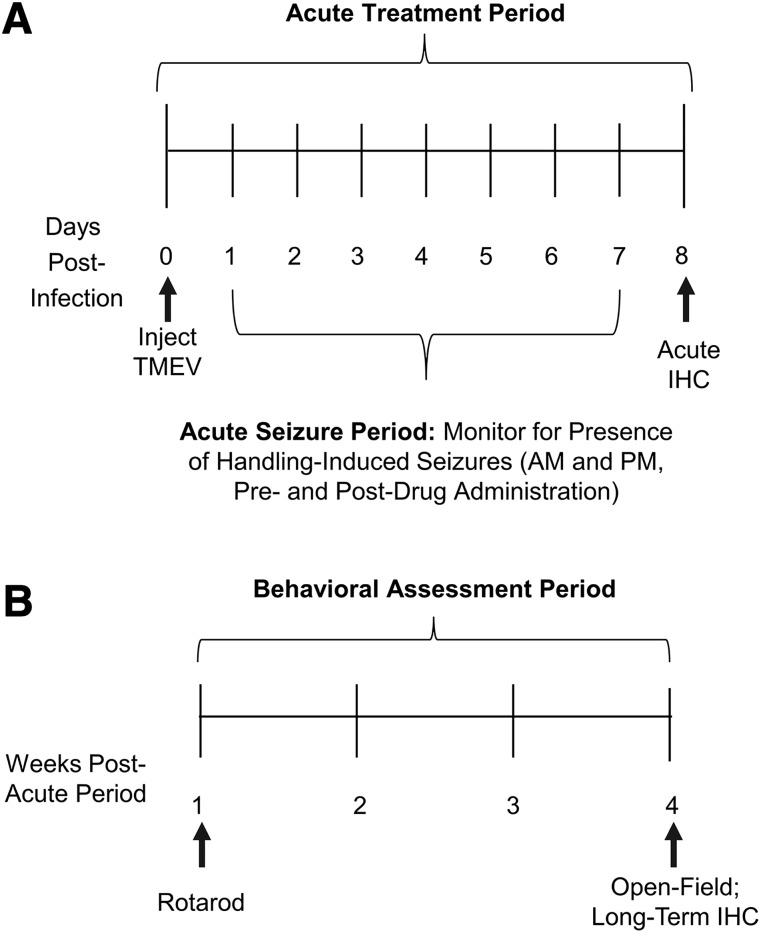
Fig. 1.
Timeline of acute treatment period and long-term behavioral assessment period. (A) Male C57BL/6J mice were randomly assigned to treatment groups of VEH, CBZ, or VPA on day 0. Immediately after the first drug or VEH treatment, mice were injected intracerebrally with Theiler’s murine encephalomyelitis virus (2.5 × 105 plaque-forming units). Animals were then subchronically dosed (b.i.d.) with CBZ, VPA, or VEH for day 0–7 of the acute infection period. On day 8, animals were randomly assigned to acute or long-term follow-up studies. (B) Animals assigned to long-term follow-up studies were evaluated on the rotarod 15 DPI. All animals that survived to 36 DPI were evaluated for anxiety-like behavior in an OF activity monitor, followed by immunohistochemical (IHC) assessment of NeuN and GFAP immunoreactivity.
Surgical Preparation and TMEV Injections.
One hour following the first ASD dose on day 0, mice were injected intracerebrally with 20 µl TMEV (titer concentration of 2.5 × 105 plaque-forming units) under isoflurane anesthesia, as previously described (Libbey et al., 2008, 2011b; Stewart et al., 2010a,b; Umpierre et al., 2014). All injection procedures were performed under sterile conditions. Following TMEV injection, the animals were monitored until they had recovered from anesthesia. All animal care and use was approved by University of Utah Institutional Animal Care and Use Committee.
Assessment of Handling-Induced Seizures.
ASD or vehicle (0.5% methylcellulose) (VEH) was administered each morning and afternoon (b.i.d.). In an effort to demonstrate the utility of this model as a novel drug-testing platform, all attempts were made to maximize the throughput for testing. To address this, video electroencephalogram (vEEG) monitoring was not implemented so as to reduce the time for surgical implantations and increase the number of mice per drug treatment group that were able to be feasibly monitored by experienced technicians. Animals were evaluated twice per day, both before and after drug dosing, on days 0–7 for clinical assessment of TMEV-induced seizures and severity, as well as general assessment of disease score through the experimenter-conducted observation of motor functions by an experimenter blinded to treatment condition (Rauch et al., 1987; Tolley et al., 1999; Tsunoda et al., 2001; Libbey et al., 2008). The presence and severity of handling-induced seizures were scored according to the Racine scale (Racine, 1972). We have previously implemented this experimenter-conducted observation of motor functions to evaluate animals with TMEV-induced acute seizures (Stewart et al., 2010b; Umpierre et al., 2014). Because physical handling was required to perform the twice-daily drug dosing, which could also provoke a handling-induced seizure, animals were also monitored during the drug-dosing session for the presence or absence of any provoked, for example, handling-induced, seizures (i.e., dosing). Twice-daily handling sessions occurred 30 minutes after drug dosing; this time best suited the pharmacokinetic profile of the ASDs under evaluation [in mice, VPA T1/2 = 30 minutes (Ben-Cherif et al., 2013); CBZ T1/2 = 60 minutes (Nishimura et al., 2008)]. Each drug-dosing session was separated by at least 7 hours, occurring daily at 9:00 AM and 4:00 PM. Mice were not evaluated at any other point during the acute infection period, nor any point thereafter, for the presence of spontaneous seizures either by visual assessment or by 24-hour vEEG.
Tissue Collection for Immunohistochemical Analysis.
Twenty four–hour observations were not included in this study to definitively determine which mice experienced behavioral and/or nonconvulsive seizures during the acute infection period. For this reason, it is possible that a mouse that did not present with behavioral seizures during the twice-daily experimenter-performed handling sessions may have presented with spontaneous convulsive or nonconvulsive seizures at another point during the acute infection period. Additionally, 24-hour vEEG recordings were not included to monitor for subconvulsive electrographic seizures in an attempt to develop this moderate-throughput drug-testing platform. Therefore, all mice were candidates for subsequent analyses of both acute and long-term immunohistochemical changes. Immunostaining, image acquisition, and analysis for acute and long-term cohorts were processed separately upon collection of each cohort. For both acute and long-term collections, animals from each treatment group were sacrificed by decapitation, and the brain was quickly removed into ice-cold 4% paraformaldehyde/0.9% phosphate-buffered saline. Brains were fixed for 24 hours, and then dehydrated and cryoprotected in 30% sucrose/0.9% phosphate-buffered saline for 48 hours before processing for downstream analysis (Friend and Keefe, 2013). Coronal sections of dorsal hippocampus (bregma; −3.3 AP) were cut at 40-µm thick on a freezing-stage microtome (Leica, Wetzlar, Germany) and mounted onto slides before immunostaining. Two adjacent dorsal hippocampal sections from each mouse in each treatment group were processed with Cy3-conjugated GFAP (C9205; Sigma-Aldrich, St. Louis, MO) and fluorescein isothiocyanate–conjugated NeuN (MAB377X; Millipore, Billerica, MA), with 4′,6′-diamidino-2-phenylindole nuclear counterstain (Life Technologies, Carlsbad, CA), as previously described (Friend and Keefe, 2013). Our prior work has demonstrated that TMEV infection is associated with acute and long-term neurodegeneration most predominately in area CA1 of dorsal hippocampus (Stewart et al., 2010a); therefore, images from dorsal hippocampus (CA1) from both contra- and ipsilateral sites of viral infection were collected at 20× magnification on an Axiovision A.1 fluorescence microscope (Zeiss, Jena, Germany). Images were analyzed by ImageJ (NIH, Bethesda, MD) denistometric analysis for percent field area with signal, as previously described (Barker-Haliski et al., 2012). An investigator blinded to treatment condition conducted all image analysis.
Assessment of Motor Function by Rotarod.
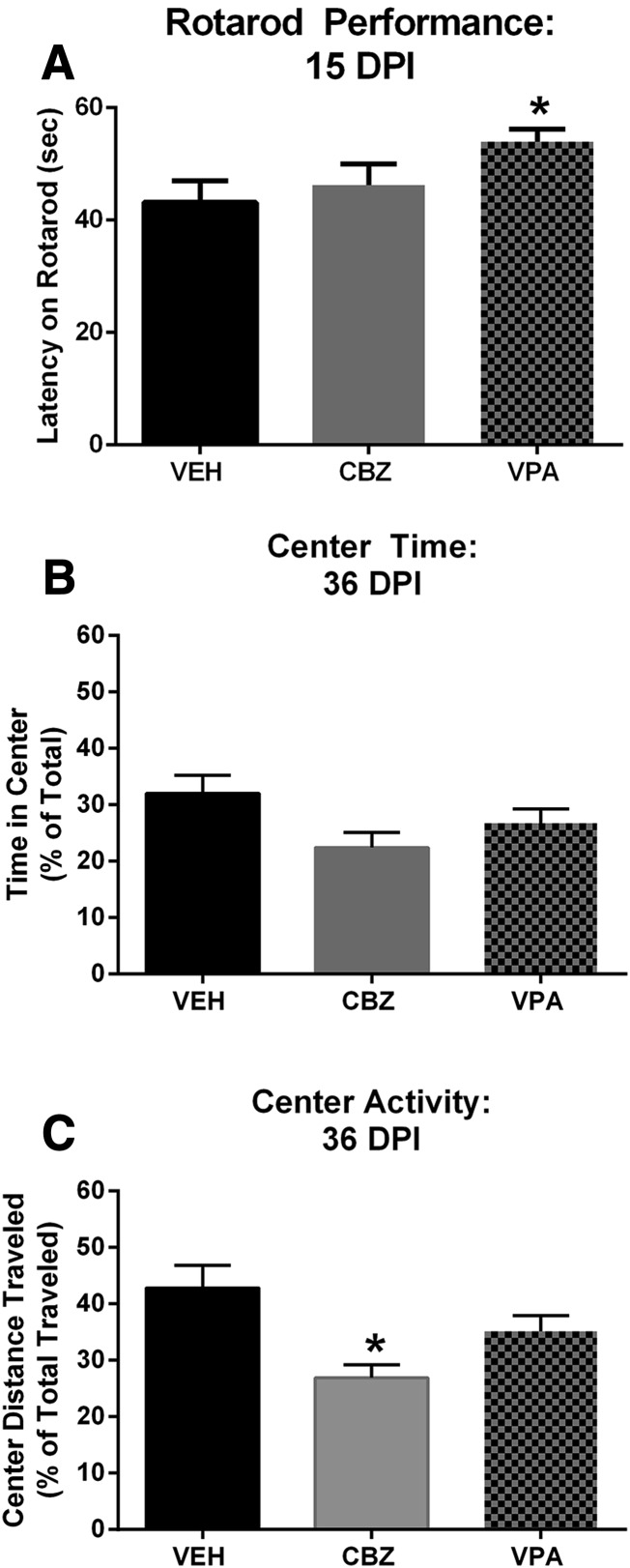
There was no further drug administration (VEH or ASD) after 7 DPI. Animals were tested 15 DPI, 8 days after the last administration of VEH or ASD, for their ability to maintain balance on a rotating rod (6 rpm) for 1 minute over three consecutive trials, as previously described (Dunham and Miya, 1957; Rowley and White, 2010; Umpierre et al., 2014). The average latency (seconds) to fall off the rotarod was calculated from the three trials, with the group means calculated for each study under investigation.
OF Assessment of Anxiety-Like Behavior 4 Weeks Postinfection.
As detailed above, following the acute infection period (days 0–8; see Table 1 for study timeline), all animals that were not euthanized for acute immunohistochemical assessments were allowed to recover for 4 weeks before testing in the OF activity monitor (5 weeks postinfection), a known platform to evaluate anxiety-like behavior in rodents (Prut and Belzung, 2003). As stated above, prior research has only monitored vEEG with cortical electrodes (Stewart et al., 2010a); therefore, it remains possible that subconvulsive electrographic seizures originating in other structures may occur in mice infected with TMEV (Stewart et al., 2010a). For these reasons, all mice were candidates for subsequent behavioral testing. On the testing day, one animal from each treatment group (n = 17 CBZ; n = 21 VPA; n = 13 VEH) was randomly monitored in an OF Plexiglas chamber measuring 40L × 40W × 30H cm (AccuScan, Salt Lake City, UT), with a total of eight mice run in parallel for each OF monitoring session (7 sessions total). Animals were allowed to explore the OF for 10 minutes, with measurements of total distance traveled, vertical, horizontal activity, rest time, and stereotypy time recorded in the center and periphery of the OF, adapted from testing methods described previously (Umpierre et al., 2014). This testing window is appropriate to identify anxiogenic effects of the OF (Prut and Belzung, 2003).
TABLE 1.
Summary of whole (center + perimeter) open field activity measures: 36 DPI
| Output Measure: Center + Perimeter | VEH (0.5% Methylcellulose; n = 12) | VPA (200 mg/kg b.i.d.; n = 19) | CBZ (20 m/kg b.i.d.; n = 17) | Overall Analysis of Variance P Value |
|---|---|---|---|---|
| Total distance (cm) | 1265.1 ± 161.5 | 1361.0 ± 231.7 | 1600.8 ± 233.1 | 0.56 |
| Horizontal activity counts | 1889.5 ± 216.6 | 1736.0 ± 222.4 | 2124.9 ± 235.8* | 0.046 |
| Vertical activity counts | 254.0 ± 49.3 | 180.5 ± 30.1 | 209.3 ± 30.6 | 0.35 |
| Rest time (s) | 216 ± 15.3 | 229.9 ± 20.3 | 195.0 ± 17.1 | 0.37 |
| Stereotypy time (s) | 13.9 ± 1.1 | 12.5 ± 0.9 | 14.9 ± 1.1 | 0.22 |
Significantly different from VEH by one-way analysis of variance and Tukey’s post hoc test.
Statistical Analysis.
Daily body weights were analyzed by repeat measures analysis of variance and post hoc Tukey’s t test. Latency to first seizure Kaplan-Meier curves was determined with a log-rank (Mantel-Cox) test. Effect of treatment on seizure burden and average number of stage 4/5 seizures were evaluated by Kruskal-Wallis test. Latency to fall off the rotarod, OF measures, and immunohistochemical densitometric results were analyzed using one-way analysis of variance and post hoc Tukey’s t test. The proportion of severe seizures in ASD-treated and vehicle-treated animals was determined with a standard score analysis. All statistical analyses were conducted with Prism v.6.0 (GraphPad Software, La Jolla, CA), with P < 0.05 considered statistically significant.
Results
Overall Animal Health Is Reduced during the Acute Viral Infection Period.
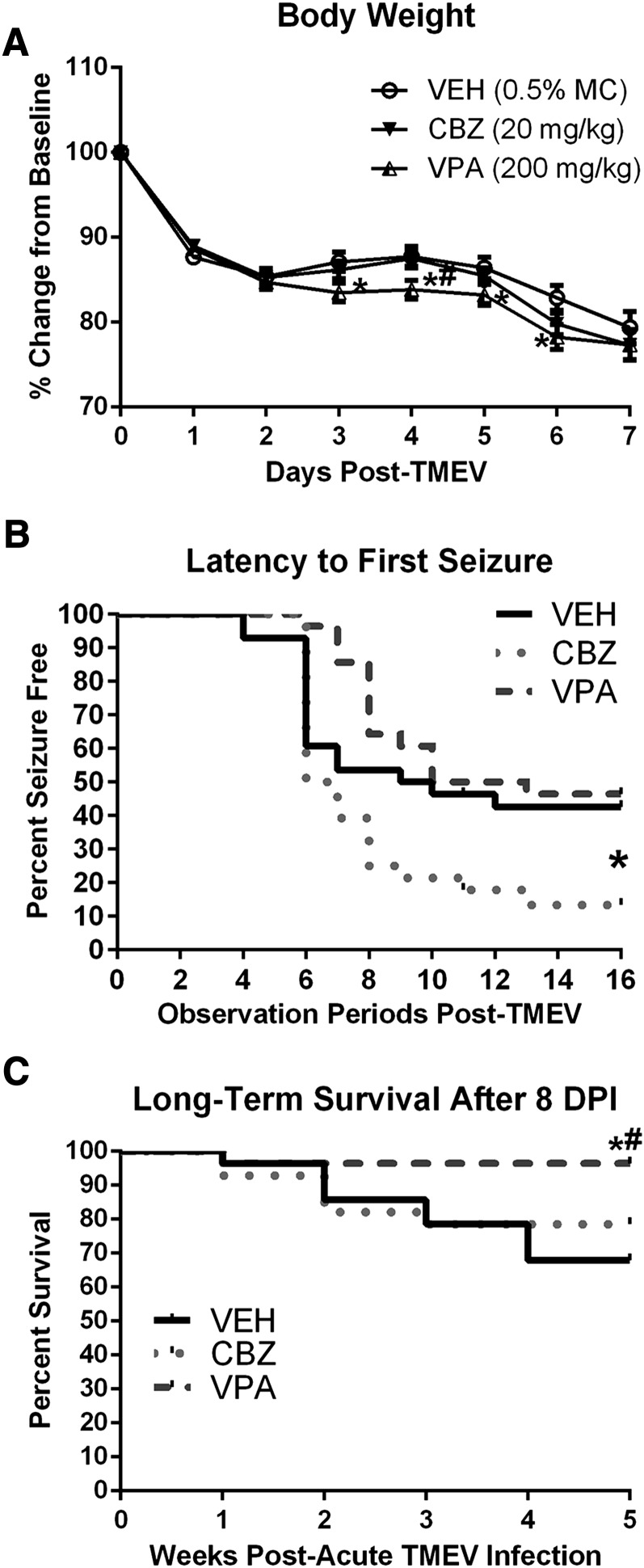
Body weights were recorded daily throughout the acute infection period (days 0–7; Fig. 2A), then weekly until completion of the long-term behavioral studies (data not shown). During the acute seizure period (day 0–7), there was a significant main effect of time postinfection on the percent change in body weight for all TMEV-infected animals, regardless of treatment [Fig. 2A; F(7,546) = 276.3, P < 0.0001]. Post hoc analysis, however, revealed no significant differences between VEH- and CBZ-treated mice at any time point. Conversely, VPA- (200 mg/kg) treated mice lost significantly more weight than VEH-treated mice 3–6 DPI (Fig. 2A; day 3, P < 0.03; day 4, P < 0.02; day 5, P < 0.05; day 6, P < 0.01), and than CBZ-treated mice on day 5 (P < 0.03). There were no other observable or significant health differences between treatment groups during the acute viral infection period. However, there was some attrition in the long-term survival for all treatment groups after 8 DPI (period after 1 week postinfection; Fig. 2C). There were four mice from the CBZ treatment group, one mouse from the VPA-treatment group, and eight mice from the VEH treatment group failing to survive to 36 DPI (Fig. 2C). VPA-treated mice demonstrated significantly improved long-term survival relative to VEH- (Fig. 2C; χ2 = 7.35, P = 0.007) and CBZ- (χ2 = 3.90, P < 0.05) treated mice, but VEH- and CBZ-treated mice were no different in their long-term survival (χ2 = 0.57, P > 0.4). Importantly, when body weights from surviving animals were measured weekly after the viral infection period, there was no significant time × treatment interaction in the percent weight change from day 0 values [F(6, 138) = 1.19, P > 0.3; data not shown]. All surviving animals recovered from the viral infection with similar rates of weight gain relative to day 0 weights, completing the study 36 DPI at similar body weights relative to each other (VEH, 115% ± 2.0; CBZ, 111.5% ± 1.1; VPA, 114% ± 2.2; data not shown). Thus, the acute effects of viral infection did not significantly alter long-term health outcomes for the surviving mice in each treatment group, despite some adverse effects on overall long-term survival.
Fig. 2.
Body weight change during the acute infection period, latency to first seizure curve, and long-term group survival. (A) Acute TMEV infection leads to significant weight loss in all treatment groups (P < 0.0001). There were no significant differences between VEH- and CBZ-treated mice at any time point. Conversely, VPA- (200 mg/kg) treated mice were significantly different from VEH-treated mice 3–6 DPI (day 3, P < 0.03; day 4, P < 0.02; day 5, P < 0.05; day 6, P < 0.01), and significantly different from CBZ-treated mice on day 5 (P < 0.03). *Significantly different from VEH-treated, TMEV-infected control mice, P < 0.05; #significantly different from CBZ-treated, TMEV-infected mice, P < 0.05. (B) The latency to first seizure was compared for each treatment group. Interestingly, there was no significant difference between the latency to seizure onset in VPA-treated versus VEH-treated mice (χ2 = 0.804, P > 0.3). Relative to VEH-treated mice, however, CBZ-treated mice showed a profound decrease in latency to first seizure (χ2 = 4.094, P < 0.05). *Significantly different from VEH-treated, TMEV-infected mice, P < 0.05. (C) TMEV-infected mice not selected for acute immunohistochemistry were monitored weekly for 5 weeks (36 DPI) for any effects of acute treatment on long-term survival rates. All mice selected for acute immunohistochemistry were omitted from the Kaplan-Meier plot. Long-term survival beyond 8 DPI (1 week postinfection) is only significantly improved by acute treatment of TMEV-infected mice with VPA. Long-term survival was no different between CBZ- and VEH-treated mice after 8 DPI (1 week postinfection). *Significantly different from VEH-treated, TMEV-infected control mice, P < 0.05; #significantly different from CBZ-treated, TMEV-infected mice, P < 0.05.
Acute Treatment with Prototype Antiseizure Drugs Produces Varied Effects on Viral-Induced Behavioral Seizures.
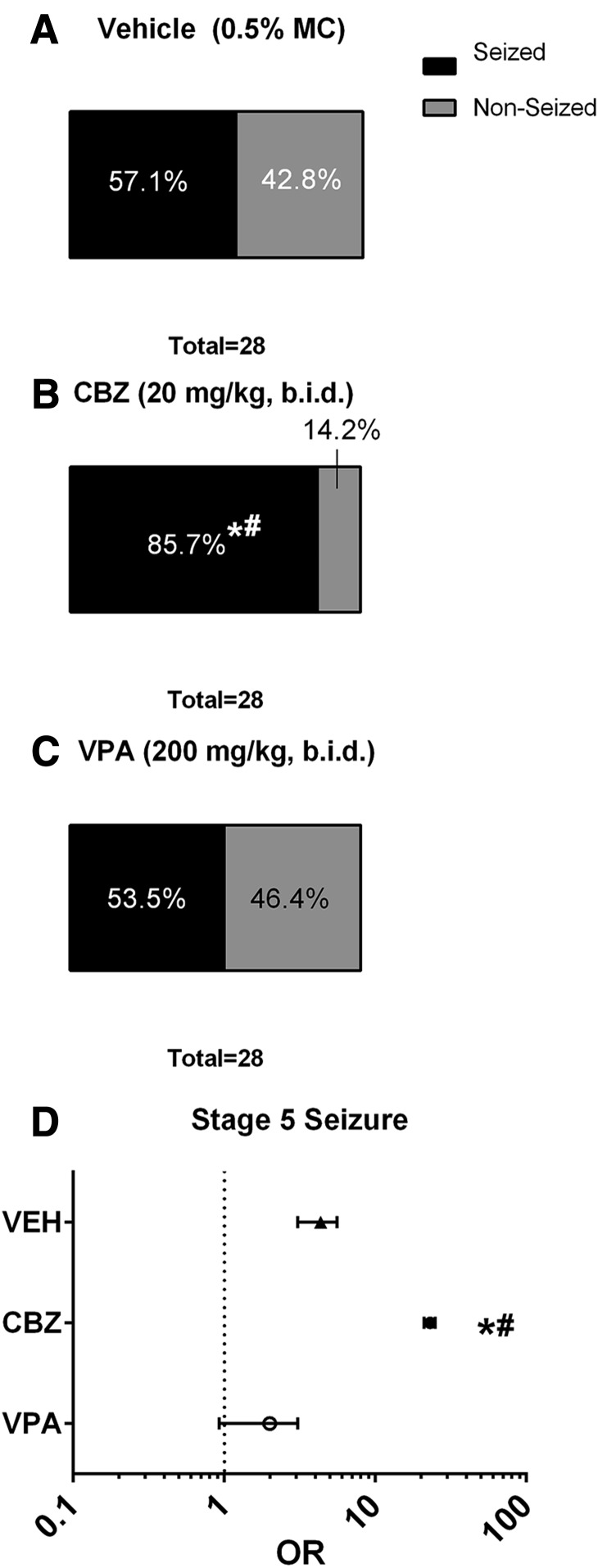
The overall latency to first seizure was evaluated using the Kaplan-Meier survival curve, wherein the outcome measure evaluated was the time in which an animal enrolled in the study remained free from seizures of any Racine stage (Fig. 2B). The proportion of animals that presented with and without seizures of any Racine stage was also determined (Fig. 3, A–C).
Fig. 3.
Acute CBZ treatment (20 mg/kg b.i.d.) increases the proportion of mice infected with TMEV that present with handling-induced seizures and increases the likelihood that mice with seizure will experience a stage 5 seizure. (A–C) The overall proportion of animals presenting with and without seizures was no different between VEH- and VPA-treated mice (z = 0.26, P > 0.5), whereas significantly more CBZ-treated mice presented with a seizure than either VEH (z = 2.36, P < 0.01) or VPA (z = 2.61, P < 0.5) treatment groups. *Significantly different from VEH-treated, TMEV-infected control mice, P < 0.01; #significantly different from VPA-treated, TMEV-infected mice, P < 0.05. (D) OR assessment of all animals that presented with a seizure at any point in the study (dosing or handling) demonstrates that the likelihood of presenting with a stage 5 seizure, if any seizure is observed, is significantly reduced with VPA treatment relative to both VEH- and CBZ-treated mice. Conversely, CBZ treatment concurrent with acute TMEV infection actually increased the likelihood that any CBZ-treated mouse with seizure would present with a stage 5 seizure relative to VEH-treated, TMEV-infected mice. *Significantly different from VEH-treated, TMEV-infected mice; #significantly different from VPA-treated, TMEV-infected mice.
VPA and CBZ were administered at therapeutically relevant doses (Bialer et al., 2004) to determine whether standard ASDs can affect postsymptomatic seizures associated with TMEV infection. Interestingly, the latency to first seizure of any Racine stage was found to be no different between VEH- and VPA-treated mice (Fig. 2B; χ2 = 0.804, P > 0.3), whereas CBZ-treated mice showed a significant decrease in latency to first seizure versus VEH-treated mice (χ2 = 4.094, P < 0.05). Additionally, the overall proportion or animals presenting with and without seizures (Fig. 3, A–C) was no different between VEH- and VPA-treated mice (z = 0.26, P > 0.5), whereas significantly more CBZ-treated mice presented with seizures than either VEH (z = 2.36, P < 0.01) or VPA (z = 2.61, P < 0.5) treatment groups. Thus, acute CBZ treatment resulted in a substantially greater proportion of animals presenting with at least one handling-induced seizure, whereas VPA treatment did not significantly alter the proportion of animals with seizures relative to VEH-treated mice.
The Likelihood of Presenting with a Stage 5 Seizure Is Increased by Acute CBZ Treatment in Mice with Handling-Induced Seizures.
In addition to analysis of the effect of treatment on the average seizure burden and seizure severity in mice that presented with at least one handling-induced seizure, we sought to determine the likelihood that an animal that presented with a seizure of any score would present with a stage 5 seizure at any point during the acute TMEV infection period (both dosing and handling sessions; Fig. 3D). Odds ratio (OR) calculation revealed that there was a significant difference between VEH- and VPA-treated, TMEV-infected mice that presented with at least one handling-induced seizure. VPA treatment significantly reduced the OR that an animal, which had a seizure of any scale, would present with stage 5 seizures [OR = 2.0, 95% confidence interval (CI) = 0.92–3.05] relative to VEH-treated mice (OR = 4.33, 95% CI = 5.59–3.08). CBZ treatment, however, led to a profound increase (OR = 23.0, 95% CI = 21.00–25.00) in the likelihood that an animal would have a stage 5 seizure relative to both VEH- and VPA-treated, TMEV-infected mice with seizures. Thus, VPA treatment significantly reduced the likelihood that TMEV-infected mice, which presented with a seizure at any point, would present with a stage 5 seizure. Conversely, CBZ treatment significantly increased the likelihood that an animal, which presented with a seizure, would have a stage 5 seizure at any observation point during the acute TMEV infection period.
Drug Dosing Affects Seizure Response Profile Regardless of ASD Treatment.
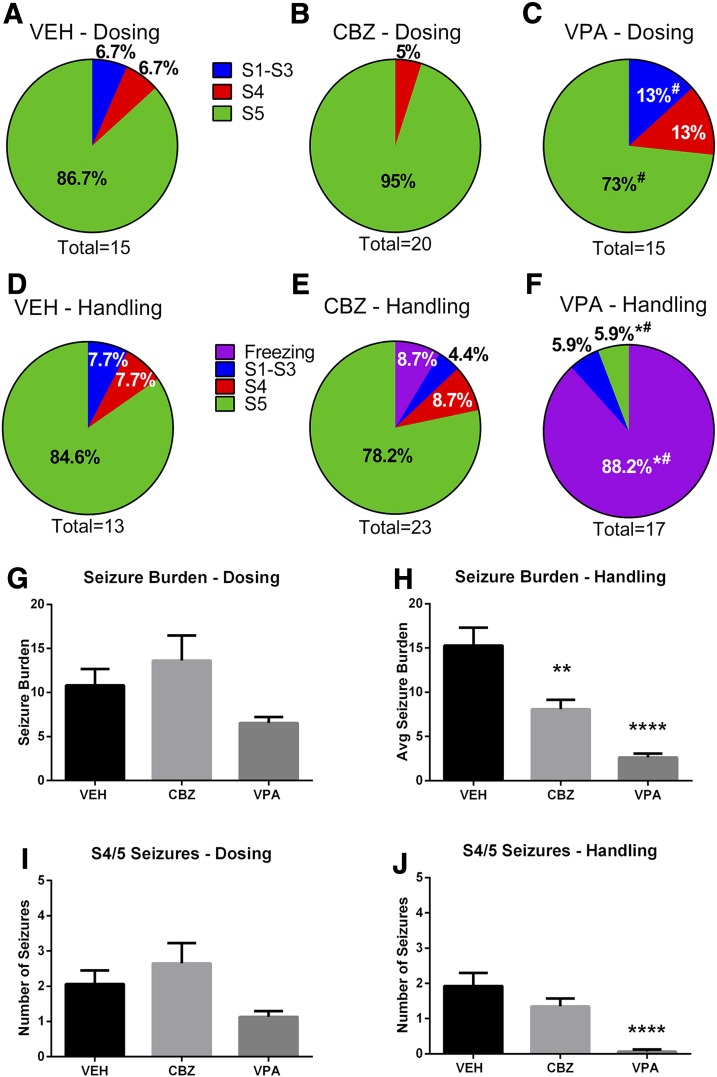
In addition to assessment of number of animals in each study presenting with and without seizures, the average seizure burden and average number of stage 4/5 seizures for any animal that experienced at least one handling-induced seizure during the dosing or handling sessions were determined (Fig. 4). Seizure burden, which represents the sum seizure score of all observed seizures throughout the specific observation session for any animal that presented with a seizure, provides an important evaluation of frequency and severity of seizures. We conducted this in-depth evaluation of the seizure burden and severity during the observation sessions pre- and postdrug administration (dosing and handling, Fig. 4, G and H, respectively), theoretically when the ASDs under investigation were at minimal and peak plasma concentrations, respectively (Nishimura et al., 2008; Ben-Cherif et al., 2013). This analysis sought to determine whether the therapeutic doses of these ASDs could alter seizure burden before and after drug administration (see Fig. 1A for study protocol). Assessments of the maximum observed seizure stage of each animal that experienced a seizure were made for morning and afternoon sessions (9:00 AM; 4:00 PM) prior to drug dosing (Fig. 4, A–C, dosing), and then 30 minutes after drug administration (Fig. 4, D–F, handling). For all studies, the maximum observed Racine seizure score was determined for each animal during the study days 0–7 and represented as the proportional distribution of all maximum seizure scores observed. Thus, the dosing and handling sessions were separated to determine the types of seizures in each session throughout the acute seizure period.
Fig. 4.
Time-dependent effect of drug dosing on seizure severity and seizure burden. Male C57BL/6J mice (4–5 weeks old) were randomly divided into treatment groups (n = 28/treatment group) and subchronically dosed for days 0–7 following TMEV infection. The presence and severity of handling-induced seizures and seizure severity were scored according to the Racine scale. Twice-daily handling (Handling) sessions occurred 30 minutes after drug dosing (Dosing) to accommodate the pharmacokinetic profile of VPA [T1/2 = 30 minutes (Ben-Cherif et al., 2013)] and CBZ [T1/2 = 60 minutes (Nishimura et al., 2008)]. Each dosing session was separated by at least 7 hours, occurring daily at 9:00 AM and 4:00 PM. (A–C) The maximum seizure severity recorded for any animal that presented with a seizure at any point in the dosing period. #Significantly different from CBZ, P < 0.05. (D–F) The maximum seizure severity recorded for any animal that presented with a seizure at any point in the handling period. Handling period occurred 30 minutes after administration of (D) VEH (0.5% MC); (E) CBZ (20 mg/kg); or (F) VPA (200 mg/kg). *Significantly different from VEH, P < 0.05; #significantly different from CBZ, P < 0.05. (G) Average seizure burden for any mouse that presented with at least one handling-induced seizure during the dosing session is not significantly different between treatment groups prior to ASD admiration. (H) Average seizure burden in mice that presented with at least one handling-induced seizure during the handling session is significantly reduced by acute ASD treatment 30 minutes prior. Significantly different from VEH, **P < 0.01 and ****P ≤ 0.0001. (I) Average number of stage 4/5 seizures for any mouse that presented with at least one handling-induced seizure during the dosing session is not significantly different between treatment groups prior to ASD administration. (J) Average number of stage 4/5 seizures for any mouse that presented with at least one handling-induced seizure during the handling session is significantly reduced by acute VPA treatment 30 minutes prior. ****Significantly different from VEH, P ≤ 0.0001.
When the predrug administration (dosing) sessions were analyzed, neither the CBZ-treatment group (95%; Fig. 4B) nor the VPA treatment group (73%; Fig. 4C) demonstrated significantly altered stage 5 seizure incidence relative to the VEH treatment group (86.7%; Fig. 4A). However, VPA treatment did significantly reduce the proportion of animals with a maximum stage 5 seizure relative to the CBZ treatment; 95% of CBZ-treated mice exhibiting seizures experienced a stage 5 seizure, whereas only 73% of VPA-treated mice exhibiting seizures presented with stage 5 during the dosing observation windows of day 0–7 (Fig. 4C; z = 1.81, P < 0.05). Simultaneously, none of the CBZ-treated mice presented with stage 1–3 seizures, whereas 13% of VPA-treated mice presented with stage 1–3 seizures during the dosing sessions (z = 1.68, P < 0.05). Thus, all treatment groups were not significantly different from VEH-treated controls when seizures were observed during the dosing session, but there were significant differences in seizure severity between VPA and CBZ treatment groups long after drug administration (i.e., at least 7 hours).
When mice were then examined for handling-induced seizures 30 minutes after drug administration (handling), a significant reduction in seizure severity occurred only in the VPA-treatment group (Fig. 4, D–F). Although over 88% of VPA-treated mice demonstrated sustained freezing behavior in response to handling (>5 seconds) 30 minutes after drug administration, only one VPA-treated mouse (5.9%) demonstrated a Racine stage 5 seizure at any point in the study (day 0–7; Fig. 4F). As such, the proportion of VPA-treated mice with freezing behavior was significantly different from VEH-treated (0%; z = −4.79, P < 0.0001) and CBZ-treated mice (8.7%; z = −5.03, P < 0.0001). These results are in stark contrast to the effect of VEH or CBZ treatment, wherein the majority of animals (>92% VEH; >86% CBZ) that presented with a seizure during the handling sessions experienced at least a stage 4 Racine seizure 30 minutes after intraperitoneal drug administration (Fig. 4, D and E). These results further suggest that VPA treatment truly protected against further seizure expression when drug was on board (handling), and not simply an effect of a refractory period to seizures due to an event 30 minutes prior during the dosing session.
The seizure burden and average number of stage 4/5 seizures for any animal that experienced at least one handling-induced seizure were determined for the dosing or handling sessions (Fig. 4, G–J). There was no significant effect of any ASD treatment during the dosing session (Fig. 4G; H = 3.22, d.f. = 2, P = 0.2). However, acute ASD treatment significantly affected seizure burden during the handling session (Fig. 4H; H = 28.5, d.f. = 2, P < 0.0001). Post hoc analysis of the handling session revealed that both CBZ and VPA treatment resulted in significant reductions in average seizure burden relative to VEH (Fig. 4H; VPA, P < 0.0001 and CBZ, P < 0.01, respectively). The average number of stage 4/5 seizures in any animal that presented with at least one handling-induced seizure was not significantly altered in the dosing session (Fig. 4I; H = 3.87, d.f. = 2, P = 0.14). However, acute ASD treatment significantly reduced the number of stage 4 or 5 seizures during the handling sessions (Fig. 4J; H = 21.5, d.f. = 2, P < 0.0001). Post hoc analysis revealed, however, that only VPA treatment significantly reduced the number of stage 4 or 5 seizures relative to VEH (P < 0.0001). The number of stage 4/5 seizures in CBZ- and VEH-treated mice was not different from each other (P > 0.05).
Rotarod Assessment of Motor Behavior after Prototypical ASD Treatment.
Our previous work has demonstrated a substantial effect of TMEV-induced seizures on motor coordination on the rotarod 17 DPI (Umpierre et al., 2014). Thus, we performed an evaluation of the effect of acute treatment with prototypical ASDs on motor coordination and performance 15 DPI. Animals did not receive further drug administration after 7 DPI; thus, any effects on the rotarod were due to the acute effects of treatment or disease progression during the TMEV infection period. Because continuous vEEG recordings were not included in this study to identify all mice that presented with behavioral or nonconvulsive seizures during the acute infection period, all mice (seized/nonseized) were subjected to the rotarod testing, as well as other behavioral and immunohistochemical assessments. We have previously demonstrated that VEH-treated, TMEV-infected mice fall off a rotarod after approximately 43 seconds, whereas sham-infected C57BL/6J mice remain on the rotarod for over 57 seconds (Umpierre et al., 2014). Interestingly, ASD treatment during the acute seizure period conferred significant overall improvements in motor coordination relative to VEH-treated, TMEV-infected mice, as measured by latency to fall off a rotarod [Fig. 5A; F(2,53) = 3.16, P = 0.05]. On average, VPA-treated mice remained on the rotarod for 54.0 ± 2.2 seconds, which was significantly improved relative to VEH-treated mice (43.2 ± 3.8 seconds; P < 0.04). Conversely, CBZ-treated mice (44.5 ± 3.8 seconds) were not significantly better on this task than VEH-treated mice (P > 0.5); however, they did not achieve a significant difference from VPA-treated mice (P = 0.09). Thus, our present and previous data would suggest that VPA treatment during the acute infection period can improve motor performance on the rotarod task relative to VEH-treated mice at 15 DPI, further indicative of reduced disease severity in animals acutely treated with VPA. In contrast, acute CBZ treatment did not improve motor coordination relative to VEH-treated mice at 15 DPI, indicating that CBZ treatment during the TMEV infection may not be beneficial to long-term disease outcomes.
Fig. 5.
Rotarod assessment and OF activity after infection with TMEV. (A) When mice were challenged to maintain balance on a rotating rod (6 rpm) for 60 seconds 15 DPI, only VPA-treated mice demonstrated significant improvements in motor coordination relative to VEH-treated, TMEV-infected mice. *Significantly different from VEH, P < 0.05. (B) Long-term (36 DPI) evaluation of anxiety-like behavior in the OF activity monitor demonstrates that no ASD treatment improved total time (seconds) in the center of an OF, a measure of anxiety-like behavior. (C) Conversely, CBZ-treated mice demonstrated further reduced total distance traveled in the center of the OF, suggesting greater anxiety relative to VEH-treated, TMEV-infected mice. *Significantly different from VEH, P < 0.05.
Effect of Treatment on OF Activity, a Measure of Anxiety-Like Behavioral Comorbidity.
Mice were allowed to recover to 36 DPI before assessment of OF behaviors. OF is a useful means to evaluate anxiety-like behaviors in rodents (Prut and Belzung, 2003). We have previously demonstrated that TMEV-infected mice demonstrate substantial reductions in time spent in the center of an OF (Umpierre et al., 2014), an effect that is suggestive of an anxiety-like phenotype. None of the drug treatment regimens conferred any significant improvement on the amount of time spent in the center of the OF environment [Fig. 5B; F(2,48) = 2.56, P = 0.088]. However, when the distance traveled in the center was similarly compared, there was a significant overall effect of treatment [Fig. 5C; F(2,48) = 6.41, P = 0.003]. CBZ-treated mice traveled significantly less total distance through the center of the OF relative to VEH-treated mice (P < 0.01), but were not significantly different from VPA-treated mice (P > 0.05). VPA-treated animals showed robust improvements relative to VEH-treated mice in acute behavioral seizures during day 0–7 (Figs. 3 and 4) and motor coordination as evaluated on the rotarod 15 DPI (Fig. 5A). However, VPA-treated mice were not significantly better in OF behavioral end points than VEH-treated mice (Fig. 5, B and C; P > 0.05). Thus, acute CBZ treatment actually resulted in a greater reduction in the distance traveled in the center of the OF versus VEH-treated mice, with center distance traveled being a long-term measure of anxiety-like behavior in TMEV-infected mice 36 DPI. As summarized in Table 1, there was a significant overall effect of treatment on horizontal activity counts [F(2,47) = 2.06, P = 0.046], with CBZ-treated, TMEV-infected mice having a significant increase in horizontal activity counts throughout the entire OF (center + perimeter; P < 0.05) relative to VEH-treated, TMEV-infected mice. There was no such significant difference between VEH- and VPA-treated mice (P > 0.05), and horizontal activity counts were the only OF measure for center + perimeter zones that had any significant effect of drug treatment (Table 1). Such observations further suggest that acute CBZ treatment during the viral infection is associated with potentiated adverse long-term outcomes in this model.
Assessment of Acute and Long-Term GFAP and NeuN Immunoreactivity in Dorsal CA1.
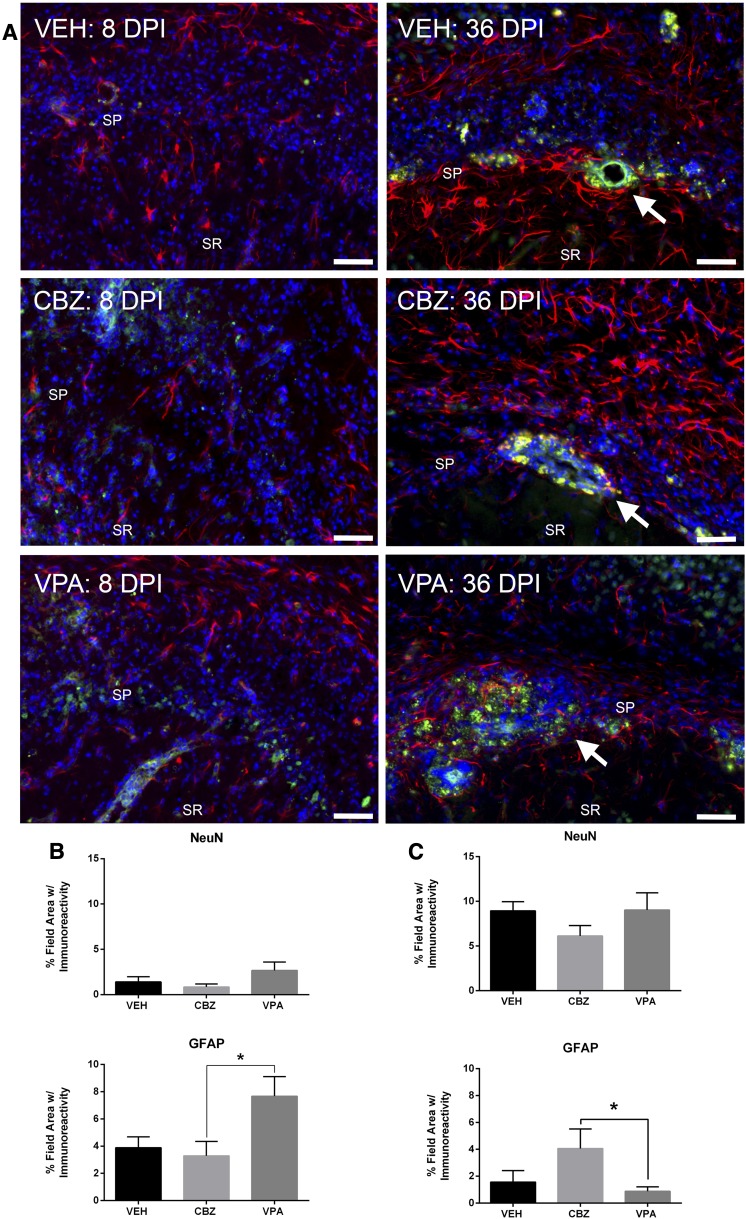
Behavioral differences in both acute seizure severity and long-term postinfection-associated comorbidities provided the rationale to examine the potential for neuroanatomical differences in ASD- versus VEH-treated mice (Fig. 6). TMEV infection results in significant hippocampal pathology and sclerosis, particularly within area CA1 of the dorsal hippocampus (Stewart et al., 2010a; Umpierre et al., 2014). Samples from the long-term cohorts (36 DPI) from this study also demonstrated substantial hippocampal sclerosis (Fig. 6A, white arrows). Thus, we sought to semiquantitatively assess whether pharmacological intervention during the acute infection period would alter short-term (8 DPI; Fig. 6B) or long-term neuropathology (36 DPI; Fig. 6C).
Fig. 6.
Immunohistochemical assessment of acute (8 DPI) and long-term (36 DPI) changes in GFAP and NeuN expression in area CA1 of dorsal hippocampus of TMEV-infected mice treated with and without acute ASD treatment. (A) Representative photomicrographs of GFAP (red) and NeuN (green) immunoreactivity with 4′,6′-diamidino-2-phenylindole nuclear counterstain (blue) from TMEV-infected mice treated with VEH (0.5% methylcellulose) or CBZ or VPA, 8 days postinfection or 36 days postinfection. White arrows in 36 DPI images indicate areas of sclerosis in area CA1 of dorsal hippocampus. The locations of stratum pyramidale (SP) and stratum radiatum (SR) layers are indicated for reference, as best as possible despite notable hippocampal pathology. (Scale bar = 50 µm, applies to all images.) (B) Immunolabeling for NeuN (8 DPI) demonstrates that no acute treatment protected against TMEV-induced neurodegeneration. VPA-treated mice demonstrated significant elevations in GFAP immunoreactivity relative to CBZ-treated mice, but did not achieve a statistically significant difference from VEH by one-way analysis of variance. *Significantly different from CBZ-treated, TMEV-infected mice, P < 0.05. (C) Long-term (36 DPI) immunolabeling for NeuN demonstrates that no drug treatment protected against TMEV-induced neurodegeneration. CBZ-treated mice demonstrated significant elevations in GFAP immunoreactivity relative to VPA-treated mice, but the elevations were not statistically different from VEH by one-way analysis of variance. Acute and long-term cohorts were processed and analyzed separately for immunohistochemical evaluations. *Significantly different from VPA-treated, TMEV-infected mice, P < 0.05.
To maximize the numbers of animals available for subsequent behavioral assessments, analysis of 8 DPI immunoreactivity was limited to eight animals/treatment group, with an equally divided number of mice with and without seizure per treatment condition. As detailed in Materials and Methods, it is possible that mice infected with TMEV may have presented with subconvulsive electrographic seizures during the acute infection period, which would have been missed by an experimenter only performing twice-daily observations of motor seizures. Because continuous vEEG recordings were not included in this study to definitively identify those mice that presented with behavioral or nonconvulsive seizures during the acute infection period, all mice (seized/nonseized) were candidates for subsequent immunohistochemical or behavioral evaluations. Acute treatment with VPA and CBZ during the viral infection period did not result in a significant improvement in NeuN labeling in CA1 [Fig. 6A, left, and Fig. 6B; F(2,15) = 1.96, P < 0.1]. However, there was a significant overall effect of treatment on the extent of GFAP immunoreactivity after TMEV infection [F(2,15) = 4.38, P < 0.04], with a Tukey’s post hoc test revealing that VPA was associated with a significantly greater expression of GFAP when compared with CBZ treatment (P < 0.05). However, VPA treatment did not achieve a statistically significant difference in GFAP expression from VEH-treated mice. To examine the potential effect of a single drug arm versus VEH treatment, a two-tailed t test between VEH- and VPA-treated mice alone revealed a significant difference between treatment groups (t = 2.29, P = 0.045; not graphically illustrated). When this two-tailed t test was similarly used to only compare CBZ- versus VEH-treated mice, there was no such significant difference between groups (t = 0.449, P > 0.6). Thus, acute VPA treatment actually increased the level of reactive astrogliosis 8 DPI (Fig. 6B). Conversely, CBZ treatment did not significantly alter the extent of reactive astrogliosis relative to VEH-treated mice 8 DPI (Fig. 6B).
Following completion of all behavioral testing weeks after the initial infection, the surviving animals were sacrificed for assessment of long-term changes in NeuN and GFAP immunolabeling 36 DPI (Fig. 6A, right panels, and Fig. 6C). In all treatment groups, the mice that survived to 36 DPI consisted of 63–66% of TMEV-infected mice with seizures during the acute infection period. Thus, there were no significant differences in the immunolabeling due to the distribution of mice with and without acute seizure presentation. Importantly, there was notable hippocampal damage observed in some mice that did not present with seizure during a handling-induced observation session. Thus, all animals enrolled in the study were evaluated for changes in NeuN and GFAP immunolabeling 36 DPI. Additionally, as for the acute cohort, all surviving mice were included for evaluation of long-term immunoreactivity after TMEV. Similar to the 8 DPI results, neither VPA nor CBZ treatment resulted in effects on NeuN immunolabeling relative to VEH-treated, TMEV-infected mice 36 DPI [Fig. 6C; F(2,24) = 0.86, P > 0.4]. Interestingly, animals from each treatment group presented with notable CA1 sclerosis (Fig. 6A, right panel, white arrows). However, when GFAP staining was subsequently assessed, there was a significant overall treatment effect [Fig. 6C; F(2,24) = 3.43, P < 0.05]. Post hoc assessment revealed that CBZ-treated mice showed significantly elevated long-term GFAP immunostaining relative to VPA-treated mice (P < 0.05), but were not different from VEH-treated mice (P > 0.05). Moreover, VPA treatment did not result in significant changes relative to VEH-treated, TMEV-infected mice (P > 0.05). When treatment groups were similarly compared by two-tailed t test as in the 8 DPI studies, there were no significant differences between any ASD treatment group and VEH (CBZ versus VEH, t = 1.59, P = 0.13; VPA versus VEH, t = 0.77, P = 0.45). Thus, the present results suggest that neither CBZ nor VPA treatment was associated with significant long-term protective effects on astrogliosis or neurodegeneration relative to VEH-treated mice.
Discussion
The potential benefit of 8-day treatment with VPA or CBZ to modify short- and long-term outcomes associated with central administration of TMEV was evaluated in C57BL/6J mice. These results demonstrate the utility of the TMEV model for drug-testing purposes in an etiologically relevant, syndrome-specific animal model of encephalitis-induced seizures. Interestingly, long-term evaluation of anxiety-like behavior and histopathology suggests that whereas acute reductions in seizure burden can be achieved with ASDs, long-term measures of disease outcome are not similarly improved, indicating that this model may identify novel therapies.
Treatment with therapeutic doses of VPA and CBZ during the acute seizure period conferred different effects on behavioral seizures. VPA reduced the acute seizure burden. Conversely, CBZ exacerbated the consequences associated with TMEV infection. This is supported by the observation that more mice in the CBZ-treatment group presented with seizures than VEH-treated, TMEV-infected mice. Additionally, the OR to have a stage 5 seizure was greatest in CBZ-treated mice, suggesting an increased susceptibility to disease in the CBZ-treated mice because more animals presented with seizures associated with the viral infection. Furthermore, the latency to first seizure presentation in the CBZ-treatment group was significantly accelerated relative to VEH- and VPA-treated mice. These observations are an important clinical consideration for the management of encephalitis-induced seizures. CBZ can be immunosuppressive and induce hypogammaglobulinaemia in humans (Sorrell and Forbes, 1975; Spickett et al., 1996), and has even been attributed to recurrent seizures in Herpes simplex encephalitis (Rice et al., 2007). Thus, the effect of CBZ on immunocompetence may have contributed to the presently observed increase in the numbers of TMEV-infected mice with acute seizure.
The observed effects of drug administration on acute histopathology suggest that VPA treatment may have potentiated astrogliosis at the early time point (Fig. 6B). Such results are intriguing given that VPA treatment significantly improved short-term (15 DPI) measures of recovery in rotarod performance relative to VEH-treated controls. This may suggest that acutely elevated astrogliosis may promote disease recovery in this model. As astrocytes can be both neuroprotective and neurodegenerative in the context of neurologic insult (Sofroniew, 2005), further investigations into the temporal contributions of astrocyte reactivity and disease outcomes in this model are necessary.
Approximately 65% of mice that present with acute seizures during the viral infection subsequently develop epilepsy (Stewart et al., 2010a), and the population of TMEV-infected mice that exhibit acute seizures can go on to develop behavioral impairments weeks later (Umpierre et al., 2014). Thus, we attempted to evaluate the effect of ASD treatment on measures of disease progression 36 DPI, when viral clearance is complete (Libbey et al., 2008) and behavioral deficits are apparent (Umpierre et al., 2014). CBZ, but not VPA, treatment increased the proportion of mice with handling-induced seizures. Not surprisingly, CBZ-treated mice demonstrated significantly worse anxiety-like behavior 36 DPI; for example, decreased distance traveled in the OF center (Fig. 5C). Additionally, CBZ-treated mice had significantly greater horizontal activity throughout the OF, indicative of greater spatial memory deficits. Hippocampal lesions tend to enhance measures of hyperactivity due to the disruption of memory formation, thereby causing animals to ambulate to a greater extent throughout an OF (Praag et al., 1994; Walker et al., 2011). That acute CBZ treatment exacerbated long-term reductions in center distance traveled and increased horizontal activity may suggest greater hippocampal damage in CBZ-treated mice relative to VEH-treated mice. CBZ-treated mice also presented with astrogliosis 36 DPI (Fig. 6, A and C), despite no difference in astrogliosis 8 DPI relative to VEH-treated mice. As TMEV infection is associated with both hippocampal sclerosis and deficits in OF activity (Stewart et al., 2010a,b; Umpierre et al., 2014), the observations that CBZ potentiated behavioral impairments and astrogliosis further suggest that CBZ may not be the preferred treatment in instances of viral encephalitis–induced seizures. Therefore, the TMEV model not only has the potential to identify promising treatments for encephalitis-induced epilepsy, but it may also be useful for identifying treatments to be avoided.
VPA possesses known effects on transcription factors (Rosenberg, 2007; Yasuda et al., 2009; Chiu et al., 2013), which may underlie disease modification (Liu et al., 2013). However, VPA (200 mg/kg b.i.d.) did not improve long-term behavioral measures relative to VEH-treated mice, suggesting that this dose may have been insufficient to target pathways necessary for long-term disease modification (Ravizza et al., 2011; Vezzani et al., 2011; Liu et al., 2013). VPA is neuroprotective in models of Alzheimer’s disease (Kilgore et al., 2010), TBI (Dash et al., 2010), and septic encephalopathy (Wu et al., 2013), but our present results failed to demonstrate any neuroprotection. Relative to VEH-treated mice, VPA-treated mice were no different in OF activity (Fig. 5; Table 1), showed no short- or long-term differences in NeuN immunoreactivity, and displayed hippocampal sclerosis 36 DPI (Fig. 6A, right). Our present results thus demonstrate that, whereas seizure burden was improved during VPA treatment, there was no long-term disease modification as a consequence of VPA treatment at this dose and time point tested.
Our present observations are reminiscent of clinical trials designed to evaluate the prophylactic efficacy of ASDs for the treatment and prevention of seizures following TBI or febrile seizures (Temkin, 2001) and support the utility of the TMEV model as a preclinical drug screening platform. These clinical studies suggested that, whereas ASDs may block acute provoked seizures following a neurologic insult, most treatments failed to prevent subsequent epileptogenesis (Temkin, 2001). In our present study, VPA, but not CBZ, conferred notable beneficial effects on seizure burden, but there were few positive effects on long-term associated comorbidities. In fact, CBZ potentiated much of the effects of TMEV infection alone, making long-term behavioral deficits more severe for CBZ-treated mice. Future studies using vEEG monitoring may confirm a correlation between the behavioral comorbidities examined in this work and TMEV infection-induced epileptogenesis (Stewart et al., 2010a); such work would clarify whether our present results align with clinical ASD prophylaxis efforts (Temkin, 2001). However, the present results suggest that the TMEV model has the potential to identify compounds possessing both acute seizure-suppressive effects and long-term disease-modifying potential. It is difficult to evaluate an antiepileptogenic effect of ASD treatment in post-traumatic or infection-induced epilepsy in clinical trials due to the low incidence of acquired epilepsy (Annegers et al., 1988, 1998; Barker-Haliski et al., 2014a), most likely a cause of many clinical failures of antiepileptogenesis trials (Mani et al., 2011). Moreover, prior attempts at antiepileptogenesis with ASDs have come with considerable adverse effects on cognition and general health outcomes that have limited the complete evaluation of any antiepileptic effect (Dikmen et al., 1991). For these reasons, the TMEV mouse model offers a novel platform for investigator-controlled studies on preclinical ASD efficacy for disease modification.
TMEV infection in mice elevates inflammatory mediators known to contribute to seizure induction and maintenance (Wilcox and Vezzani, 2014). Moreover, this cytokine response promotes the development of acute seizures in TMEV-infected C57BL/6J mice (Libbey et al., 2011a,b; Cusick et al., 2013). There is thus potential for combined acute seizure control with traditional ASDs and subsequent disease modification with compounds possessing alternative mechanisms of action that may modify or prevent the disease, for example, anti-inflammatory compounds (Schmidt, 2012; Loscher et al., 2013). As Temkin (2001) and others (White and Loscher, 2014) have suggested, it is feasible that entirely different classes of compounds will be needed to prevent the development of epilepsy than those that effectively suppress the seizures once the process has progressed into epilepsy. In fact, other models of TLE after insult have suggested that anti-inflammatory agents may be disease-modifying (Maroso et al., 2010, 2011; Vezzani et al., 2013). Furthermore, these agents represent a clinically unexploited mechanism to prevent or modify epilepsy. As postencephalitic epilepsy is often pharmacoresistant (Cruzado et al., 2002), the TMEV model provides a novel and valuable drug development platform. Moreover, the development of acute seizures in TMEV-infected mice can be prevented with the repurposed anti-inflammatory agent, minocycline (Libbey et al., 2011a,b; Cusick et al., 2013), highlighting the potential to evaluate mechanistically novel agents in this model. However, long-term follow-up (>15 DPI) to evaluate treatment effects on behavioral comorbidities was not included in these studies (Libbey et al., 2011a,b; Cusick et al., 2013). Future efforts to characterize any subsequent effects of therapeutic intervention with other agents on disease course, or biomarkers thereof, are thus warranted in this model.
In this study, we demonstrate the utility of the TMEV model of infection-induced seizures and subsequent behavioral comorbidities as a platform to evaluate potentially novel therapeutic approaches for acute seizure control and disease modification. Treatment with VPA during the infection significantly improved acute seizure burden. However, neither ASD treatment prevented long-term behavioral comorbidities associated with TMEV infection. Nevertheless, these results should not detract from the obvious utility of the TMEV model of infection-induced encephalitis and subsequent behavioral comorbidities as an etiologically relevant drug-testing platform. This model may have the potential to identify innovative and mechanistically novel seizure-suppressive, and possibly disease-modifying, therapies for epilepsy.
Acknowledgments
The authors thank Dr. Robert Fujinami for purified TMEV titers.
Abbreviations
- ASD
antiseizure drug
- CBZ
carbamazepine
- DPI
days postinfection
- GFAP
glial fibrillary acidic protein
- OF
open field
- OR
odds ratio
- TBI
traumatic brain injury
- TLE
temporal lobe epilepsy
- TMEV
Theiler’s murine encephalomyelitis virus
- vEEG
video electroencephalogram
- VEH
vehicle (0.5% methylcellulose)
- VPA
valproic acid
Authorship Contributions
Participated in research design: Barker-Haliski, Wilcox, White.
Conducted experiments: Barker-Haliski, Dahle, Pruess, Heck, Vanegas.
Performed data analysis: Barker-Haliski, White.
Wrote or contributed to the writing of the manuscript: Barker-Haliski, Wilcox, White.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS065434 (to K.S.W. and H.S.W.) and Contract HHSN271201100029C (to H.S.W.)].
Part of this work was presented as follows: Barker-Haliski ML, Heck TD, Dahle E, Pruess TH, Wilcox KS, and White H (2014) Treatment of acute behavioral seizures in the Theiler's murine encephalomyelitis virus model of acquired epilepsy disrupts long-term, but not acute, histopathology. Society for Neuroscience Meeting; 2014 Nov 16; Washington, D.C. Abstract 201.01.
References
- Annegers JF, Hauser WA, Beghi E, Nicolosi A, Kurland LT. (1988) The risk of unprovoked seizures after encephalitis and meningitis. Neurology 38:1407–1410. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA. (1998) A population-based study of seizures after traumatic brain injuries. N Engl J Med 338:20–24. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski M, Friedman D, White HS, French JA. (2014a) How clinical development can, and should, inform translational science. Neuron 84:582–593. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski M, Sills GJ, White HS. (2014b) What are the arguments for and against rational therapy for epilepsy? Adv Exp Med Biol 813:295–308. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski ML, Pastuzyn ED, Keefe KA. (2012) Expression of the core exon-junction complex factor eukaryotic initiation factor 4A3 is increased during spatial exploration and striatally-mediated learning. Neuroscience 226:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Cherif W, Dridi I, Aouam K, Ben-Attia M, Reinberg A, Boughattas NA. (2013) Circadian variation of valproic acid pharmacokinetics in mice. Eur J Pharm Sci 49:468–473. [DOI] [PubMed] [Google Scholar]
- Bialer M, Twyman RE, White HS. (2004) Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav 5:866–872. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, Kanner AM, O’Brien TJ, Whittemore VH, Winawer MR, et al. (2013) Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia 54 (Suppl 4):44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley MD, Haynes DR. (2013) Epigenetic regulation of inflammation: progressing from broad acting histone deacetylase (HDAC) inhibitors to targeting specific HDACs. Inflammopharmacology 21:301–307. [DOI] [PubMed] [Google Scholar]
- Caserta MT, Hall CB, Schnabel K, Long CE, D’Heron N. (1998) Primary human herpesvirus 7 infection: a comparison of human herpesvirus 7 and human herpesvirus 6 infections in children. J Pediatr 133:386–389. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Wang Z, Hunsberger JG, Chuang DM. (2013) Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzado D, Masserey-Spicher V, Roux L, Delavelle J, Picard F, Haenggeli CA. (2002) Early onset and rapidly progressive subacute sclerosing panencephalitis after congenital measles infection. Eur J Pediatr 161:438–441. [DOI] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. (2013) Infiltrating macrophages are key to the development of seizures following virus infection. J Virol 87:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, Moore AN. (2010) Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 5:e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Temkin NR, Miller B, Machamer J, Winn HR. (1991) Neurobehavioral effects of phenytoin prophylaxis of posttraumatic seizures. JAMA 265:1271–1277. [PubMed] [Google Scholar]
- Dunham NW, Miya TS. (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc 46:208–209. [DOI] [PubMed] [Google Scholar]
- Friend DM, Keefe KA. (2013) Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J Neurochem 125:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JJ, Li S, Shu HF, Yu SX, Liu SY, Yin Q, Yang H. (2013) The interleukin 17 system in cortical lesions in focal cortical dysplasias. J Neuropathol Exp Neurol 72:152–163. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Czapp M, Löscher W. (2008) Increase in antiepileptic efficacy during prolonged treatment with valproic acid: role of inhibition of histone deacetylases? Epilepsy Res 81:107–113. [DOI] [PubMed] [Google Scholar]
- Hu MH, Huang GS, Wu CT, Lin JJ, Hsia SH, Wang HS, and Lin KL; CHEESE Study Group (2014) Analysis of plasma multiplex cytokines for children with febrile seizures and severe acute encephalitis. J Child Neurol 29:182–186. [DOI] [PubMed] [Google Scholar]
- Kan AA, de Jager W, de Wit M, Heijnen C, van Zuiden M, Ferrier C, van Rijen P, Gosselaar P, Hessel E, van Nieuwenhuizen O, et al. (2012) Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. J Neuroinflammation 9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. (2010) Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 35:870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. (2011a) Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol 85:6913–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. (2011b) Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J Neurovirol 17:496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, Fujinami RS. (2008) Seizures following picornavirus infection. Epilepsia 49:1066–1074. [DOI] [PubMed] [Google Scholar]
- Liu G, Gu B, He XP, Joshi RB, Wackerle HD, Rodriguiz RM, Wetsel WC, McNamara JO. (2013) Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron 79:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Klitgaard H, Twyman RE, Schmidt D. (2013) New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 12:757–776. [DOI] [PubMed] [Google Scholar]
- Mani R, Pollard J, Dichter MA. (2011) Human clinical trails in antiepileptogenesis. Neurosci Lett 497:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A. (2011) Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–419. [DOI] [PubMed] [Google Scholar]
- Misra UK, Tan CT, Kalita J. (2008) Viral encephalitis and epilepsy. Epilepsia 49 (Suppl 6):13–18. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Honda N, Sugioka N, Takada K, Shibata N. (2008) Evaluation of carbamazepine pharmacokinetic profiles in mice with kainic acid-induced acute seizures. Biol Pharm Bull 31:2302–2308. [DOI] [PubMed] [Google Scholar]
- Pernot F, Heinrich C, Barbier L, Peinnequin A, Carpentier P, Dhote F, Baille V, Beaup C, Depaulis A, Dorandeu F. (2011) Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia 52:2315–2325. [DOI] [PubMed] [Google Scholar]
- Praag Hv, Dreyfus CF, Black IB. (1994) Dissociation of motor hyperactivity and spatial memory deficits by selective hippocampal lesions in the neonatal rat. J Cogn Neurosci 6:321–331. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. [DOI] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Rauch HC, Montgomery IN, Hinman CL, Harb W, Benjamins JA. (1987) Chronic Theiler’s virus infection in mice: appearance of myelin basic protein in the cerebrospinal fluid and serum antibody directed against MBP. J Neuroimmunol 14:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Balosso S, Vezzani A. (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497:223–230. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29:142–160. [DOI] [PubMed] [Google Scholar]
- Rice CM, Johnston SL, Unsworth DJ, Glover SC, Donati M, Renowden SA, Holloway J, Lhatoo SD. (2007) Recurrent herpes simplex virus encephalitis secondary to carbamazepine induced hypogammaglobulinaemia. J Neurol Neurosurg Psychiatry 78:1011–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. (2007) The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci 64:2090–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley NM, White HS. (2010) Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: correlation with other seizure and epilepsy models. Epilepsy Res 92:163–169. [DOI] [PubMed] [Google Scholar]
- Schmidt D. (2012) Is antiepileptogenesis a realistic goal in clinical trials? Concerns and new horizons. Epileptic Disord 14:105–113. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407. [DOI] [PubMed] [Google Scholar]
- Solomon T, Hart IJ, Beeching NJ. (2007) Viral encephalitis: a clinician’s guide. Pract Neurol 7:288–305. [DOI] [PubMed] [Google Scholar]
- Sorrell TC, Forbes IJ. (1975) Depression of immune competence by phenytoin and carbamazepine: studies in vivo and in vitro. Clin Exp Immunol 20:273–285. [PMC free article] [PubMed] [Google Scholar]
- Spickett GP, Gompels MM, Saunders PW. (1996) Hypogammaglobulinaemia with absent B lymphocytes and agranulocytosis after carbamazepine treatment. J Neurol Neurosurg Psychiatry 60:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. (2010a) Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol 69:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. (2010b) Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia 51:1418–1428. [DOI] [PubMed] [Google Scholar]
- Temkin NR. (2001) Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42:515–524. [DOI] [PubMed] [Google Scholar]
- Tolley ND, Tsunoda I, Fujinami RS. (1999) DNA vaccination against Theiler’s murine encephalomyelitis virus leads to alterations in demyelinating disease. J Virol 73:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. (2001) Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol 75:7494–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, West PJ, White HS, Wilcox KS. (2014) Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol Dis 64:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A. (2011) Brain inflammation as a biomarker in epilepsy. Biomarkers Med 5:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J. (2004) Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis 17:385–402. [DOI] [PubMed] [Google Scholar]
- Walker JM, Fowler SW, Miller DK, Sun AY, Weisman GA, Wood WG, Sun GY, Simonyi A, Schachtman TR. (2011) Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav Brain Res 222:169–175. [DOI] [PubMed] [Google Scholar]
- White HS, Löscher W. (2014) Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies. Neurotherapeutics 11:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Smith MD, Wilcox KS. (2007) Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol 81:85–110. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Vezzani A. (2014) Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol 813:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dong L, Zhang M, Jia M, Zhang G, Qiu L, Ji M, Yang J. (2013) Class I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathy. Neurochem Res 38:2440–2449. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. (2009) The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14:51–59. [DOI] [PubMed] [Google Scholar]