Abstract
Acute liver failure (ALF) is a potentially life-threatening disorder without any effective treatment strategies. d-Galactosamine (GalN)/lipopolysaccharide (LPS)–induced ALF is a widely used animal model to identify novel hepato-protective agents. In the present study, we investigated the potential of a cannabinoid receptor 2 (CB2) agonist, JWH-133 [(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran], in the amelioration of GalN/LPS-induced ALF. JWH-133 treatment protected the mice from ALF-associated mortality, mitigated alanine transaminase and proinflammatory cytokines, suppressed histopathological and apoptotic liver damage, and reduced liver infiltration of mononuclear cells (MNCs). Furthermore, JWH-133 pretreatment of M1/M2-polarized macrophages significantly increased the secretion of anti-inflammatory cytokine interleukin-10 (IL-10) in M1 macrophages and potentiated the expression of M2 markers in M2-polarized macrophages. In vivo, JWH-133 treatment also suppressed ALF-triggered expression of M1 markers in liver MNCs, while increasing the expression of M2 markers such as Arg1 and IL-10. microRNA (miR) microarray analysis revealed that JWH-133 treatment altered the expression of only a few miRs in the liver MNCs. Gene ontology analysis of the targets of miRs suggested that Toll-like receptor (TLR) signaling was among the most significantly targeted cellular pathways. Among the altered miRs, miR-145 was found to be the most significantly decreased. This finding correlated with concurrent upregulated expression of its predicted target gene, interleukin-1 receptor–associated kinase 3, a negative regulator of TLR4 signaling. Together, these data are the first to demonstrate that CB2 activation attenuates GalN/LPS-induced ALF by inducing an M1 to M2 shift in macrophages and by regulating the expression of unique miRs that target key molecules involved in the TLR4 pathway.
Introduction
Acute liver failure (ALF) is a highly devastating and potentially fatal syndrome caused by a sudden destruction of hepatocytes (Lee, 2012). Etiologies associated with ALF include drug overdose, viral hepatitis, and bacterial toxins (Zhan et al., 2014). The prognosis for ALF is very poor, and the syndrome is usually associated with a high mortality of up to 80% (Bernal et al., 2010). There is a definite need for the development of new and effective therapeutic strategies for the treatment of this syndrome (Mas and Rodes, 1997; Neuberger, 2005).
Injecting a combination of d-galactosamine (GalN) and lipopolysaccharide (LPS) causes acute liver failure in animals, which closely resembles the immunologic and metabolic dysfunctions seen in the clinical syndrome (Rahman and Hodgson, 2000). Thus, GalN/LPS-induced ALF has been widely used as an animal model to study the pathogenesis of ALF and to develop new therapeutic strategies against it (Tunon et al., 2009). The model triggers ALF through the binding of LPS to Toll-like receptor 4 (TLR4) on liver resident macrophages, the Kupffer cells (KCs), leading to their activation and secretion of a number of proinflammatory cytokines (Zhan et al., 2014). These cytokines provide a signal for the ensuing massive infiltration of immune cells in the liver and initiate apoptosis in the liver parenchyma, ultimately leading to ALF (Liang et al., 2014).
The cannabinoid (CB) system comprises the cannabinoid receptors, their ligands, and the proteins that mediate their synthesis and degradation (Mallat and Lotersztajn, 2008). The ligands include both endogenous lipidic signaling molecules and exogenous cannabinoids derived from Cannabis sativa or synthetic agonists and antagonists for the cannabinoid receptors (Adhikary et al., 2012). The CB receptors include CB1 and CB2, both of which are G protein–coupled receptors. CB1, the psychoactive receptor, is expressed in a number of tissues with a particularly high expression in the brain and peripheral neurons (Adhikary et al., 2012), whereas CB2 is mainly expressed in hematopoietic cells and immune cells, including the macrophages (Louvet et al., 2011), and is not associated with any psychotropic effects (Miller and Stella, 2008).
Accumulating evidence suggests that cannabinoids and their receptors act as key players in a number of liver pathologies (Mallat and Lotersztajn, 2008; Teixeira-Clerc et al., 2010). Multiple studies have implicated CB1 in the pathogenesis of liver fibrosis (Teixeira-Clerc et al., 2006), alcoholic liver disease (Jeong et al., 2008), and nonalcoholic fatty liver disease (Kunos and Osei-Hyiaman, 2008). CB2, on the other hand, has largely been demonstrated to possess anti-inflammatory properties. For example, endogenous CB2 activity has been shown to suppress CCl4-induced hepatic fibrosis (Julien et al., 2005), whereas exogenous activation by different ligands has been demonstrated to ameliorate hepatic ischemia/reperfusion injury (Batkai et al., 2007), concanavalin-A–induced hepatitis (Hegde et al., 2008), and alcoholic liver disease (Louvet et al., 2011). In addition, a recent study showed that in vitro CB2 activation causes a suppression in LPS-induced inflammatory responses and suppresses the proinflammatory M1 macrophage phenotype, favoring a switch to the anti-inflammatory M2 phenotype (Louvet et al., 2011). Furthermore, in vitro CB2 activation has been shown to increase production of the anti-inflammatory cytokine interleukin-10 (IL-10) in LPS/interferon-γ (IFN-γ)–activated macrophages (Correa et al., 2005). Together, these findings have led to the generally accepted idea that CB1 antagonists and/or CB2 agonists could have therapeutic potential in the context of liver pathologies (Lotersztajn et al., 2008; Tam et al., 2011).
MicroRNAs (miRs) are endogenously encoded, small noncoding regulatory RNA molecules, about 21–22 nt in length. Increasing evidence from functional studies has shown that miRs are involved in the regulation of almost all biologic processes, including development and function of the immune system (O'Connell et al., 2010). miRs achieve this regulatory function by binding directly to the 3′-untranslated region of the target mRNA to cause a translational inhibition or mRNA degradation of the target gene (O'Connell et al., 2010). Results from a number of studies also suggest that miRs play a critical role in regulating different aspects of innate immune system and macrophage activation in response to TLR ligands (O'Neill et al., 2011). In this study, we investigated the efficacy of a select CB2 agonist, JWH-133 [(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran], against ALF induced by GalN/LPS. We found that the CB2 agonist protected the mice from ALF-associated lethality primarily by inducing significant alterations in the miRs that regulated the TLR4 pathway, and by promoting a switch in macrophage phenotype from M1 to M2.
Materials and Methods
Mice.
Female C57BL/6 mice (8–10 weeks old) were purchased from the National Cancer Institute (Frederick, MD). Mice were housed and maintained under specific pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facility of the School of Medicine at the University of South Carolina. All animal procedures were performed according to National Institutes of Health guidelines, and the protocols were preapproved by the Institutional Animal Care and Use Committee of the University of South Carolina.
Induction of Acute Liver Failure and Treatment with JWH-133 Compound.
GalN and LPS (Sigma-Aldrich, St. Louis, MO) were dissolved in phosphate-buffered saline (PBS). JWH-133 was obtained from the National Institute on Drug Abuse (Rockville, MD). To induce ALF, mice were injected intraperitoneally with a combination of GalN and LPS at doses of 400 mg/kg and 5 µg/kg body weight, respectively (n = 10–12 in each group). PBS was used as vehicle (veh) for these experiments. JWH-133, obtained at an initial concentration of 5 mg/ml in 100% ethanol, was concentrated to 40 mg/ml using a speed vacuum system and diluted further in PBS for treatments. For treatment with JWH-133, mice were treated with two doses of 20 mg/kg each: the first dose was administered 24 hours before the GalN/LPS injection, and the second, 2 hours before the GalN/LPS injection. Blood samples were collected at 12 hours after GalN/LPS injection by retro-orbital bleeding, and sera were separated and stored at −20°C until further use. The survival of mice was observed for 12 hours after GalN/LPS injection. At 12 hours, mice were sacrificed and liver tissues were harvested for histology and isolation of mononuclear cells. The liver tissues were frozen and stored at −80°C until further use. All mice were carefully monitored, and any moribund mice were immediately euthanized.
Serum Cytokine Analysis and Alanine Transaminase Activity Assay.
Sera samples collected 12 hours after GalN/LPS injections were analyzed for levels of cytokines [tumor necrosis factor α (TNF-α), IL-6, and monocyte chemotactic protein-1 (MCP-1) (BioLegend, San Diego, CA)] and to measure liver enzyme alanine transaminase (ALT) levels (Pointe Scientific, Canton, MI) as described earlier (Hegde et al., 2011).
Liver Histology.
Liver tissues isolated at 12 hours after GalN/LPS injection were rinsed gently with PBS and fixed in 10% neutral buffered formalin for 24 hours, paraffin embedded, and sectioned using microtome to obtain 5-µm-thick sections. The sections were stained with H&E and examined under light microscopy to evaluate tissues for histopathological damage.
Isolation of Liver Mononuclear Cells.
The liver-infiltrating cells were isolated using a modification of a protocol described earlier (Dong et al., 2004). In brief, livers were perfused with liver perfusion medium, cut into small pieces, and digested using liver digestion medium (Gibco/Life Technologies, Grand Island, NY). The digestion medium was washed and hepatocytes were removed using low-speed centrifugation at 50g at 4°C for 5 minutes. Mononuclear cells (MNCs) were then isolated from supernatant using Percoll-based (GE Healthcare Life Sciences, Pittsburgh, PA) density gradient centrifugation. MNCs were then filtered using a 70-µm nylon mesh filter and counted.
RNA Isolation, miRNA Microarray, Pathway Analysis, and Real-Time Polymerase Chain Reaction.
RNA was isolated from liver MNCs using an miRNeasy kit (Qiagen, Valencia, CA). Total RNA isolated from two biologic duplicate samples of liver MNCs from both the LPS+vehicle group and LPS+JWH-133 group was analyzed using the Affymetrix Gene Chip miRNA 3.0 array platform, according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA). Using Ingenuity Pathway Analysis (IPA) software (Qiagen, Redwood City, CA), the results from the miRNA microarray were analyzed to identify the gene ontologies associated with molecular pathways potentially altered by single or multiple miRNA target genes. The set of miRNAs that had greater than an average 1.5-fold linear fold difference between treatment versus vehicle groups were analyzed within immunologically relevant pathways in the database to identify target genes for each of these miRNAs. The list of targets was then uploaded to Cytoscape using the ClueGo plugin for gene ontology analysis (Bindea et al., 2009). Gene ontology analysis of immunologically relevant pathways was then performed for the pathways having P values <0.05 with right-sided hypergenomic enrichment and Benjamini-Hochberg false discovery rate testing. The TLR pathway, as regulated by identified miRNAs, was generated using the IPA software. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to quantify the levels of miRNAs using miScript SYBR Green PCR kits and the following primer assays (Qiagen, Valencia, CA): mmu-miR-145 and Snord61. Snord61 was used as the reference gene for qRT-PCR.
Immunoblotting.
Total protein was isolated from the liver tissues and MNCs collected 12 hours after GalN/LPS injection using radioimmunoprecipitation assay buffer supplemented with protease inhibitors. The protein samples were quantified using a BCA protein estimation kit (Fisher Scientific, Pittsburgh, PA). Twenty micrograms of protein was loaded for each sample. The proteins were transferred on the nitrocellulose membrane using the wet transfer method. The membranes were blocked in 5% milk in Tris-buffered saline/Tween 20 for 1 hour and probed with the following antibodies (Cell Signaling Technologies, Danvers, MA): β-actin (1:1000) and caspase-3 (1:2000).
Cell Culture.
Thioglycollate-induced macrophages were isolated from the peritonea of mice, washed in PBS, plated for 90 minutes at 37°C in a CO2 incubator, harvested, and allowed to adhere for 24 hours. Macrophages were then treated with vehicle (0.05% dimethylsulfoxide) or JWH-133 (25 or 5 µM). One hour later, cells were treated with LPS (100 ng/ml). For macrophage polarization studies, cells were pretreated with 5 µM JWH-133 for 1 hour and then stimulated with either IFN-γ (150 U/ml) for 6 hours and then with LPS (10 ng/ml) for M1 polarization or with IL-4 (20 U/ml) for M2 polarization. RNA samples were isolated from macrophages 6 hours after LPS or IL-4 stimulation. Cell culture supernatants were isolated 24 hours later for enzyme-linked immunosorbent assay to quantify TNF-α, IL-12, and IL-10 secretion by macrophages.
Statistics.
For in vivo experiments, 10–12 mice were used per experimental group. For in vitro assays and qRT-PCRs, all experiments were performed in triplicate. For statistical differences, one-way analysis of variance was used to analyze significance for each experiment, and Tukey’s post-hoc test was performed to analyze differences between the groups. Results are shown as the average ± S.E.M. wherever applicable. A P value ≤0.05 was used to determine statistical significance. Single and double asterisks represent significant differences with P values <0.05 and <0.01, respectively. The graphs were plotted using GraphPad Prism (GraphPad Software, La Jolla, CA), and the densitometric analysis was performed using ImageJ software (NIH, Bethesda, MD).
Results
Attenuation of ALF in JWH-133–Treated Mice.
We first investigated if treatment of mice with the CB2 agonist JWH-133 can protect mice from GalN/LPS-induced ALF. To this end, we treated the mice with JWH-133 at a dose of 20 mg/kg body weight. This dose was established on the basis of our previous findings in which this dose was found to be anti-inflammatory and protective in an acute model of dextran sulfate sodium–induced colitis (Singh et al., 2012). Our results showed that around 50% of the mice treated with GalN/LPS+vehicle died within 12 hours, whereas remarkably, 100% of the mice treated with GalN/LPS+JWH-133 survived (Fig. 1A). Also, all mice treated with vehicle alone and JWH-133 alone survived for the duration of studies and did not show any signs of morbidity (data not shown). Injection of GalN/LPS+vehicle also caused a significant increase in serum ALT levels indicative of liver failure (Fig. 1B). On the other hand, mice treated with GalN/LPS+JWH-133 showed significantly lower ALT levels. The control mice that received only vehicle or JWH-133 alone did not show any elevation in serum ALT levels. Next, we investigated the histopathological damage in the liver tissues of mice in the four groups. Mice injected with GalN/LPS+vehicle showed severe tissue damage and massive infiltration of inflammatory cells in the liver (Fig. 1C). In contrast, mice that received the GalN/LPS+JWH-133 treatment showed a significant reduction in liver tissue damage and immune cell infiltration.
Fig. 1.
JWH-133 treatment attenuates LPS-induced ALF. (A) Percent survival of mice at different time (hours) intervals after coadministration of GalN/LPS+vehicle (depicted as LPS+Veh) or GalN/LPS+JWH-133 (LPS+JWH-133), as described in Materials and Methods. (B) Sera collected at 12 hours post-LPS treatment were analyzed for ALT levels. (C) Histologic examination of livers from mice following H&E staining. *P < 0.05; **P < 0.01.
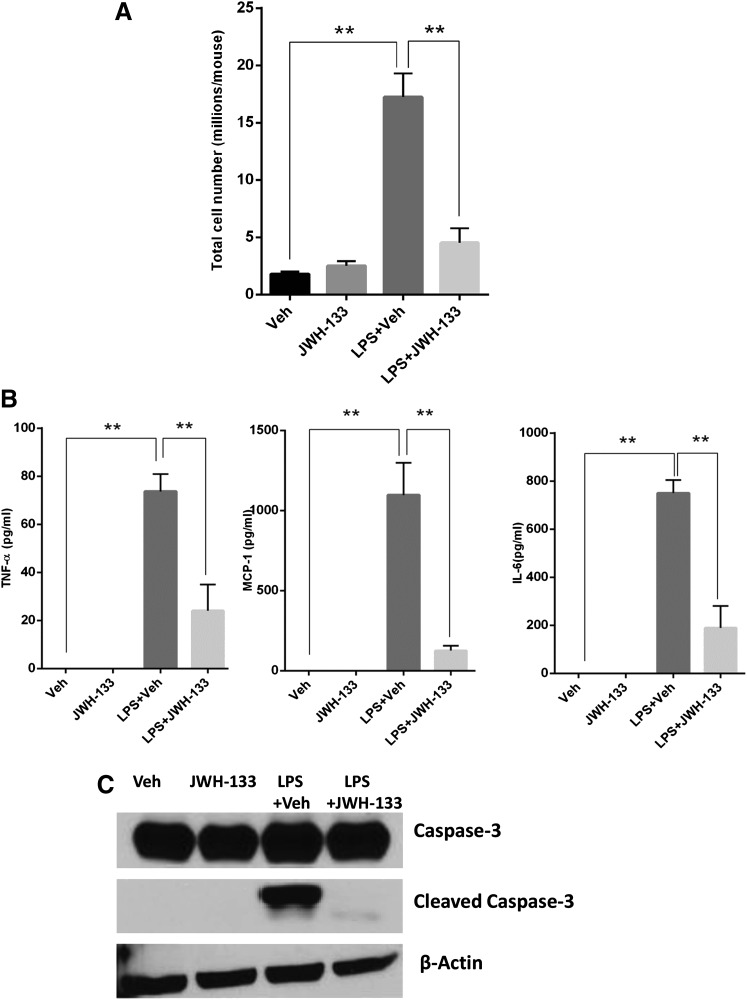
JWH-133 Treatment Decreases Liver Infiltration of MNCs, Production of Proinflammatory Cytokines, and Liver Apoptosis upon GalN/LPS Injection.
Based on the marked reduction of infiltration of immune cells in the liver tissues, we next quantified the differences in liver infiltration of immune cells. To this end, we isolated the liver mononuclear cells from the livers of the different groups of mice. Mice with GalN/LPS+vehicle-induced ALF showed a significant increase in numbers of MNCs that infiltrated the liver, whereas mice that received treatment with GalN/LPS+JWH-133 had a significantly lower number of MNCs in the liver tissues (Fig. 2A). We next investigated the production of proinflammatory cytokines in different groups of mice by quantifying the serum levels of TNF-α, MCP-1, and IL-6 12 hours after injecting mice with GalN/LPS. We observed a significant increase in the levels of all of these proinflammatory cytokines in mice that had ALF post-GalN/LPS+vehicle injection. We also noted a significant decrease in the serum levels of these proinflammatory cytokines in the mice that received GalN/LPS+JWH-133 treatment (Fig. 2B). Because hepatocyte apoptosis is the major event that leads to ALF (Liang et al., 2014), and caspase-3 is a major mediator of apoptosis, we next studied the effect of JWH-133 upon the GalN/LPS+vehicle-triggered apoptosis in liver tissues by immunoblotting. Liver tissues isolated from mice 12 hours after GalN/LPS+vehicle injection showed a very significant increase in caspase-3 cleavage indicative of liver tissue damage by apoptosis (Fig. 2C). In contrast, GalN/LPS+JWH-133 treatment completely suppressed caspase-3 cleavage in the liver tissues, suggesting protection of liver from tissue apoptosis.
Fig. 2.
JWH-133 treatment decreases liver infiltration, apoptosis, and production of systemic proinflammatory cytokines. (A) Liver-infiltrating MNCs were isolated as described in Materials and Methods. The cells were counted to get absolute numbers of infiltrating cells in liver. Blood was collected from mice at 12 hours after GalN/LPS+vehicle (veh) injection and (B) sera were analyzed for cytokines using sandwich enzyme-linked immunosorbent assay for TNF-α, MCP-1, and IL-6. (C) Protein samples were isolated from liver tissues harvested 12 hours after GalN/LPS+vehicle injection and run on SDS-PAGE for immunoblotting to probe for caspase-3 (both uncleaved and cleaved) and β-actin as a control. **P < 0.01.
JWH-133 Treatment Suppresses the LPS-Induced Activation Macrophages and Triggers an M1 to M2 Phenotype Shift in Macrophage Polarization In Vitro.
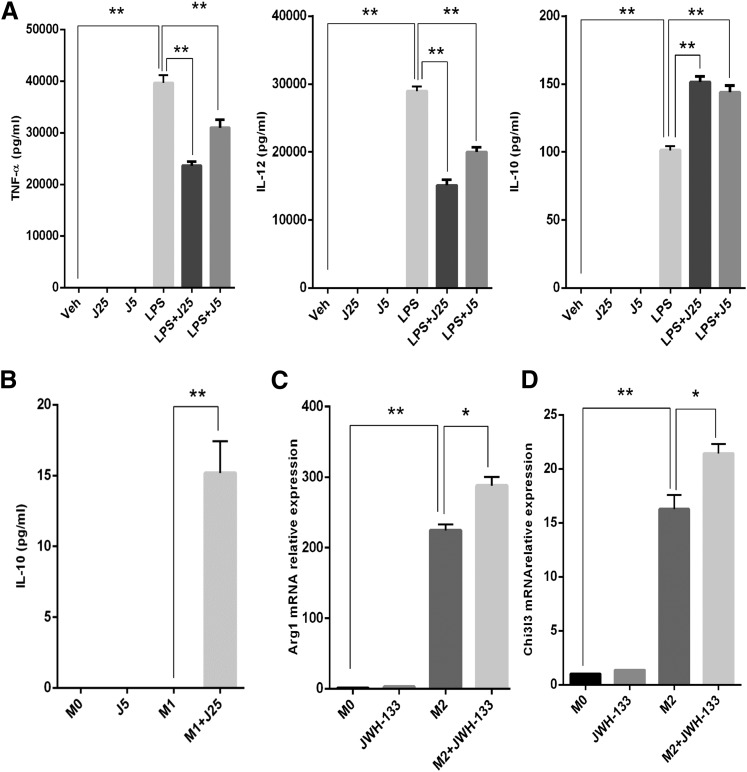
Because macrophages play an important role in the pathogenesis of GalN/LPS-induced ALF, we investigated the effects of JWH-133 on macrophages in vitro, specifically to produce proinflammatory cytokines upon LPS activation. Based on our pilot studies and other published studies, we determined that doses of 25 and 5 µM suppressed LPS-induced activation and were noncytotoxic to macrophages over 24-hour cultures. Therefore, we used these doses of JWH-133 for pretreatment of peritoneal macrophages 1 hour prior to LPS stimulation. We observed that JWH-133 treatment caused a significant suppression in the production of proinflammatory cytokines, including TNF-α and IL-12, whereas it increased the production of the anti-inflammatory cytokine IL-10 (Fig. 3A).
Fig. 3.
CB2 stimulation by JWH-133 decreases M1 while increasing M2 phenotype activation of macrophages in vitro. (A) Thioglycollate-induced peritoneal macrophages were treated with JWH-133 (25 and 5 µM, shown as J25 and J5), and 1 hour later, cells were stimulated with LPS (100 ng/ml). Conditioned media from macrophage cultures were analyzed for proinflammatory cytokines (TNF-α, IL-12, and anti-inflammatory cytokine IL-10) by enzyme-linked immunosorbent assay. Peritoneal macrophages were treated with JWH-133 (5 µM) for 1 hour and then stimulated toward M1 or M2 phenotypes. (B) IL-10 secretion was quantified in conditioned medium from M1-polarized macrophages using enzyme-linked immunosorbent assay. RNA isolated from the macrophage cultures was used for quantitative PCR to quantify relative expression of genes: Arg-1 (C) and Chi3l3 (D). *P < 0.05; ** P < 0.01. Veh, vehicle.
Because a switch from M1 to M2 macrophage phenotype leads to suppression of inflammation, we next determined if JWH-133 used this mechanism to suppress ALF. To this end, we pretreated peritoneal macrophages with JWH-133 and then stimulated the cells, polarizing them toward M1 or M2 phenotype, and the culture supernatants were analyzed for TNF-α, IL-12 (indicative of M1), and IL-10 (indicative of M2). Interestingly, our data showed that JWH-133 increased the secretion of IL-10 in the M1-stimulated cultures (Fig. 3B). We could not detect any IL-10 in the M2 macrophage cultures; therefore, we investigated the expression of typical M2 phenotype markers such as arginase-1 (Arg-1) and chitinase3 like 3 (Chi3l3) using qRT-PCR. Interestingly, we observed a significant increase in the expression of these M2 phenotype–associated markers. More importantly, JWH-133 caused a significant additional increase in the expression of both Arg-1 and Chi3l3 (Fig. 3, C and D). These results suggested that JWH-133 suppresses M1 activation while potentiating M2 phenotype.
JWH-133 Treatment of GalN/LPS-Injected Mice Increases M2 Macrophage Phenotype Polarization in Liver.
To corroborate if the M1 to M2 switch can be seen in vivo, we investigated the effect of JWH-133 treatment on the expression of M1 and M2 macrophage phenotypic markers in liver MNCs isolated 12 hours after GalN/LPS injections by qRT-PCR. We observed that expression of the typical M1 phenotype markers, TNF-α and nitric oxide synthase 2 (Nos2), was significantly increased in mice with GalN/LPS+vehicle, whereas GalN/LPS+JWH-133 treatment significantly suppressed this expression (Fig. 4A). On the other hand, expression of the typical M2 markers, Arg-1 and IL-10, was increased upon JWH-133 treatment (Fig. 4B). Together, these results suggested that JWH-133 may ameliorate LPS-induced ALF, at least in part, by promoting M1 to M2 switch in macrophage phenotype in the liver.
Fig. 4.
JWH-133 treatment induces in vivo M2 polarization of macrophages in mice with LPS-induced ALF. Liver MNCs were isolated for the different groups of animals treated with vehicle (Veh) alone, JWH-133 alone, LPS+Veh, or LPS+JWH-133 at 12 hours after GalN/LPS injection. Relative expression of typical M1 markers, TNF-α and Nos2 (A), and typical M2 markers, Arg-1 and IL-10 (B), in liver MNCs as quantified by qRT-PCR is shown. *P < 0.05; **P < 0.01.
JWH-133 Treatment of GalN/LPS-Injected Mice Significantly Alters the miRNA Profile of Liver MNCs.
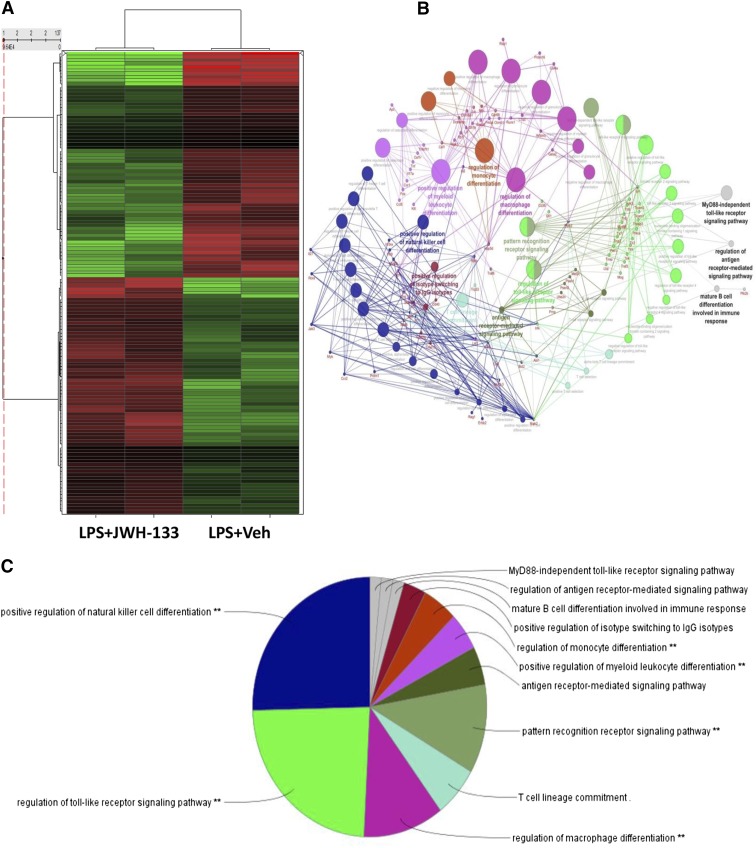
Because GalN/LPS-induced ALF results from acute inflammation, which is also regulated by microRNAs, we next investigated their role in the current model. For this purpose, we investigated the change in expression of miRNAs in response to JWH-133 treatment of mice exposed to GalN/LPS. To this end, we performed microarray analysis for the expression of 2023 miRNAs in the liver MNCs of GalN/LPS+JWH-133–treated mice versus those that received GalN/LPS+vehicle. JWH-133 treatment caused a significant change in the miRNA expression profile of liver MNCs, as seen in the heat map (Fig. 5A).
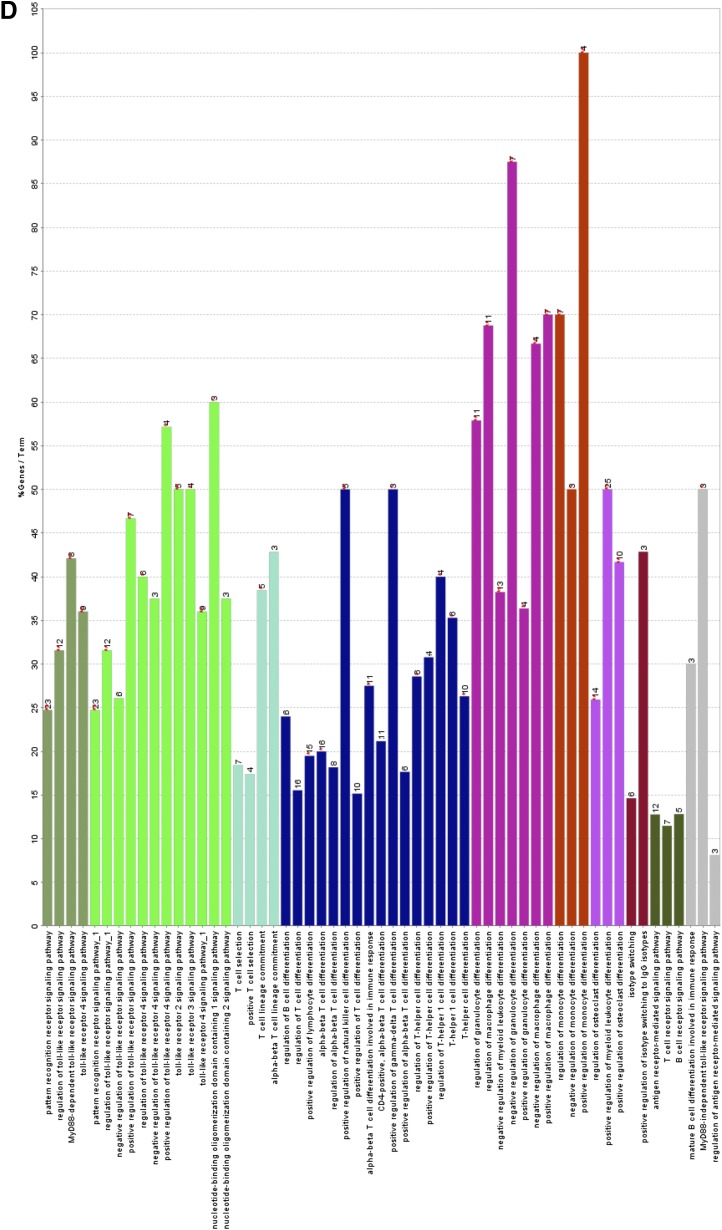
Fig. 5.
JWH-133 treatment causes a differential expression of a number of miRNAs in liver MNCs that can potentially regulate TLR signaling pathway. Mice were treated with LPS+vehicle or LPS+JWH-133 as described in Fig. 1, and liver MNCs were isolated for each group as described in Materials and Methods. (A) Heat map for the expression of 2023 miRNAs, with the first two lanes showing LPS+JWH-133 treatment and the latter two lanes showing LPS+vehicle treatment. (B) Gene ontology analysis of the predicted target genes of miRNAs identifies the major cellular pathways altered in the JWH-133+LPS group as compared with the LPS+vehicle group. A pie chart (C) and bar graph analysis (D) of the major cellular pathways altered in the JWH-133+LPS group when compared with the LPS+vehicle based on miRNAs altered by more than 1.5-fold.
We also investigated the gene ontologies associated with the cellular pathways potentially changed by using IPA software, and determined the miRNAs with more than 1.5-fold change in the GalN/LPS+JWH-133 group when compared with the GalN/LPS+vehicle group. Next, immunologically relevant target genes for such miRNAs were identified using IPA. Gene ontology analysis of these target genes revealed a number of pathways most affected by the target genes altered in the GalN/LPS+JWH-133–treated mice when compared with the GalN/LPS+vehicle-treated mice. Here, we observed that TLR signaling was one of the most affected ontologies pertinent to macrophage function as well as regulation of macrophage differentiation (Fig. 5, B–D). These pathways were strongly predicted to suppress the expression of various negative regulators of the TLR4 signaling pathway, as shown in the pathway analysis.
JWH-133 Treatment Decreases Expression of miR-145 to Regulate Interleukin-1 Receptor–Associated Kinase 3 and Modulate TLR4 Signaling.
Our analysis led us to identify a relatively small number of miRNAs that were significantly altered in GalN/LPS+JWH-133–treated mice when compared with GalN/LPS+vehicle-treated mice (Fig. 6A). Among these, miR-145 emerged as the most significantly altered miRNA, with a 5-fold decrease in the mice treated with JWH-133. Next, we used the miRWalk database (Dweep et al., 2011), a powerful tool that can predict miRNA–target gene interactions to identify predicted targets most relevant to TLR4 signaling. One such strongly predicted miRNA-gene interaction that caught our attention was miR-145-5p to the 3′-untranslated region of the interleukin-1 receptor–associated kinase 3 (IRAK3) mRNA (Fig. 6B). In addition to miRWalk-based predicted binding between miR-145 and IRAK3, we also used the miRanda algorithm to verify the predicted interaction between the two sequences. This analysis also suggested a strong interaction between miR-145 and its predicted target, IRAK3, based on the mirSVR score (Fig. 6C).
Fig. 6.
JWH-133 treatment suppresses TLR signaling in liver MNCs by decreasing the expression of miR-145 to regulate IRAK3. (A) Table of miRNAs significantly increased or decreased by more than 1.5-fold change in LPS+JWH-133–treated mice when compared with LPS+vehicle (Veh)-treated mice. (B) Toll-like receptor pathway showing the predicted interaction between miR-145, the most significantly altered miRNAs in the LPS+JWH-133group with its predicted target IRAK3 (shown in blue), and how they relate to the rest of the members of the pathway. (C) miRanda algorithm generated miR-145 and mRNA alignment at the 3′-untranslated region of IRAK3 mRNA. mirSVR and PhastCons conservation scores are also shown. miR-145 (D) and its predicted target IRAK3 in liver MNCs (E), as quantified by qRT-PCR at 12 hours after GalN/LPS injection. *P < 0.05; **P < 0.01. CYLD, cylindromatosis (turban tumor syndrome); IKK, inhibitor of κ light polypeptide gene enhancer in B cells, kinase β; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; PPARA, peroxisome proliferator-activated receptor α; TIRAP, Toll-interleukin 1 receptor (TIR) domain containing adaptor protein.
IRAK3 is a member of the IRAK family and, unlike other IRAK family members, has been shown to negatively regulate the downstream signaling from the stimulated TLRs. Thus, next we validated the expression of miR-145 in liver MNCs isolated at 12 hours post-GalN/LPS+vehicle treatment using qRT-PCR. Whereas the expression of miR-145 was increased by about 3-fold in mice with GalN/LPS+vehicle-induced ALF, mice with GalN/LPS+JWH-133 treatment indeed showed decreased expression of miR-145 when compared with the mice with LPS-induced ALF (Fig. 6D). Concurrently, the expression of IRAK3, the predicted target gene of miR-145, on the other hand, was found to be increased in mice treated with GalN/LPS+JWH-133 when compared with mice with GalN/LPS-induced ALF (Fig. 6E). These results taken together demonstrate that JWH-133 can suppress GalN/LPS-induced ALF through changes in macrophage polarization and miRNA expression modulation to tame the TLR4 signaling in response to LPS-induced inflammatory response.
Discussion
Acute liver failure represents the most destructive form of liver damage (Lee, 2012). Considering the substantial evidence underscoring the roles of systemic inflammatory response syndrome, multiple organ dysfunction syndrome, and sepsis in the progression of ALF (Antoniades et al., 2008), a therapeutic agent for ALF could also represent good promise against these similar types of inflammatory disorders. Several studies have underlined the significance of LPS in pathogenesis of both chronic and acute liver pathologies, including ALF (Nolan, 2010; Zhan et al., 2014), which further strengthens the clinical relevance of the GalN/LPS-induced ALF animal model.
CB2 receptor activation has been previously shown to mediate anti-inflammatory effects in liver injury (Munoz-Luque et al., 2008; Louvet et al., 2011). However, the role of microRNAs in such models of protection has not been previously investigated. In the current study, we demonstrated that treatment with a select CB2 agonist, JWH-133, protected the mice from GalN/LPS-induced ALF, based on increased survival, decreased serum ALT levels, decreased number of liver-infiltrating MNCs, and reduced histologic damage to the liver. The effectiveness of JWH-133 was remarkable in this acute liver injury model inasmuch as 100% of such mice survived the GalN/LPS challenge. JWH-133 treatment significantly upregulated the expression of markers for anti-inflammatory macrophage phenotype M2 in both polarized macrophage cultures and liver MNCs of mice with GalN/LPS-induced ALF. Additionally, in liver MNCs, JWH-133 significantly decreased the expression of miR-145 relative to the mice with LPS-induced ALF, with a concomitant increased expression of its predicted target gene: IRAK3. Thus, our results suggested that JWH-133 may cause a switch in macrophage phenotype to the anti-inflammatory M2. Also, these changes may be mediated through induction of miRNAs, such as miR-145, and its target genes in the liver MNCs, to repress the LPS-induced TLR4 pathway.
In the GalN/LPS-induced ALF model, LPS-induced TLR4 stimulation of KCs causes an increase in the release of proinflammatory cytokines and chemokines, such as TNF-α, MCP-1, and IL-6 (Masaki et al., 2004), which play a pivotal role in the pathogenesis of ALF (Antoniades et al., 2008). In our study, we observed that JWH-133 treatment decreased the serum levels of these proinflammatory cytokines and chemokines that were induced by LPS. In addition, JWH-133 treatment also decreased the infiltration of MNCs in the liver and significantly reduced the apoptosis mediated by caspase-3 activation in liver tissues, suggesting CB2-mediated protection of mice from LPS-induced ALF.
One of the possible mechanisms of JWH-133–mediated attenuation of ALF could be the suppression of the LPS-induced activation of liver resident macrophages. Indeed, recent studies have shown that in vitro CB2 activation suppresses LPS-induced stimulation of macrophages by decreasing the secretion of proinflammatory cytokines (Louvet et al., 2011). In the present study, we also observed that JWH-133 treatment of macrophages decreased the secretion of proinflammatory cytokines, such as TNF-α and IL-12 in response to LPS stimulation, and increased the secretion of the anti-inflammatory cytokine IL-10.
Insights from recent studies have led us to identify different functional subsets of macrophages, two main phenotypes being M1 and M2. M1 macrophages are associated with cytotoxic and proinflammatory functions, whereas M2 macrophages have anti-inflammatory and tissue injury–resolving functions (Sica and Mantovani, 2012). In addition, macrophages are believed to be plastic, able to reversibly transition from one subset to another in response to distinct microenvironmental cues (Sica and Mantovani, 2012). In our current study, we observed that JWH-133 treatment increased the secretion of IL-10, a typical M2 phenotype marker in macrophages that had been stimulated toward M1 phenotype by using IFN-γ+LPS. Interestingly, the CB2 agonist also enhanced the expression of other typical M2 phenotype markers, Arg-1 and Chi3l3, suggesting that CB2 activation not only causes an M1 to M2 shift in M1-stimulated macrophages but also potentiates stimulation to the anti-inflammatory M2 macrophage phenotype. IL-10 is an anti-inflammatory cytokine that has been shown to be hepato-protective in the GalN/LPS-induced ALF model (Santucci et al., 1996).
For our in vitro study, we chose to use doses of 5 and 25 µM JWH-133 1 hour prior to LPS activation of macrophages. These doses of JWH-133 to activate CB2 receptors on the macrophages are similar to what have been published earlier in the in vitro studies, showing its protective effects against different liver pathologies (Teixeira-Clerc et al., 2010; Louvet et al., 2011). One can also question if, at these concentrations of JWH-133, the compound is acting through CB1 receptors due to some cross-reactivity. We believe that this is unlikely because the reported expression of the CB1 receptor on the inflammatory macrophages/monocytes is up to 100 times lower than that of the CB2 receptor (Pacher et al., 2006; Han et al., 2009), which makes it much more likely that the protective effects of JWH-133 are mediated through a specific activation of the CB2 receptor. Nevertheless, a partial CB1 activation cannot be definitively ruled out at this point.
Although we focused our current study on macrophages, one can reason that JWH-133 could be acting on other cells in the liver as well. Although this possibility cannot be ruled out entirely, we reason that this is less likely based on the previous findings that the CB receptors, particularly CB2, are expressed at low levels or even absent in healthy liver tissues (Pacher et al., 2006; Floreani et al., 2010). Furthermore, to our knowledge, there are no reports to suggest an increase in liver tissue levels of CB receptors during the course of acute liver pathologies in humans or animals.
A switch in KCs to the M2 phenotype has been shown to be protective against liver pathologies, such as alcoholic liver disease (Louvet et al., 2011) and obesity-induced insulin resistance (Odegaard et al., 2008). Thus, based on our in vitro findings, we investigated the effect of JWH-133 treatment on the expression of typical M1 and M2 markers in mice with LPS-induced ALF. We noticed an increase in the expression of M1 markers, TNF-α and Nos2, in the liver MNCs of ALF-induced mice, whereas the CB2 agonist decreased the expression of these markers. It is worthy to note here that TNF-α is the most important mediator that triggers hepatocyte apoptosis in LPS-induced ALF (Leist et al., 1995; Nowak et al., 2000), and JWH-133 treatment of mice was able to decrease both systemic serum levels and liver-specific expression of TNF-α. Nos2 has also been identified as one of the major mediators of inflammation, both in clinical ALF (Leifeld et al., 2002) and in GalN/LPS-induced ALF (Sass et al., 2001). Thus, the ability of JWH-133 to suppress liver-specific expression of Nos2 in mice with LPS-induced ALF is compelling. Even more interestingly, we observed that JWH-133 treatment increased the expression of typical M2 markers, Arg-1 and IL-10, within the liver MNCs. These results together suggested that CB2 activation induces an M1 to M2 shift within the liver resident macrophages in mice with LPS-induced ALF.
TLR4 activation by LPS initiates a signal transduction pathway involving MyD88 recruitment to TLR4, leading to nuclear translocation of nuclear factor κB, which promotes the transcription of various proinflammatory genes (Lu et al., 2008). Recent studies have suggested that miRNAs play an important role in the regulation of TLR responses (O'Neill et al., 2011). Thus, we next investigated if JWH-133–induced miRNA changes were a potential mechanism by which the CB2 agonist was able to suppress LPS-induced ALF. In our present study, we found that JWH-133 treatment significantly decreased the expression of miR-145 in liver MNCs. Furthermore, we identified a number of immunologically relevant gene ontologies that were predicted to be targeted by the changes in miR expression among the JWH-133–treated mice. In particular, we showed that the changes in miRs could potentially regulate the expression of a number of genes involved in macrophage function and differentiation, including regulation of TLR4 signaling in response to TLR ligands, such as LPS. Our in silico analysis showed that IRAK3, previously shown to inhibit LPS-induced TLR4 activation (Kobayashi et al., 2002), was a highly predicted target gene that could be suppressed by miR-145. This suggests that induction of IRAK3 expression could be a useful strategy to dampen the LPS-induced TLR4 responses and hence attenuate LPS-induced ALF pathogenesis. Therefore, we validated our findings from in silico analyses and found an increase in IRAK3 expression in liver MNCs from GalN/LPS+JWH-133–treated mice relative to GalN/LPS+vehicle-treated mice, along with a concurrent decrease in the expression of miR-145, thus suggesting that miR-145–led increase in IRAK3 may be a potential mechanism of action by which JWH-133 suppresses LPS-induced ALF.
In summary, we demonstrate that JWH-133, a CB2 agonist, can protect mice from liver injury triggered by inflammation. Our findings are significant inasmuch as this represents the first finding of attenuation of LPS-triggered TLR4 signaling by JWH-133 through a negative feedback involving changes in expression of distinct miRNAs to regulate critical regulators of TLR4 signaling, such as IRAK3.
Acknowledgments
The authors thank the National Institute on Drug Abuse (Bethesda, MD) for providing the JWH-133 used in these studies. The authors also thank the Instrumentation Resource Facility at School of Medicine of the University of South Carolina for processing liver specimens for histology.
Abbreviations
- ALF
acute liver failure
- ALT
alanine transaminase
- Arg-1
arginase-1
- CB
cannabinoid
- Chi3l3
chitinase3 like 3
- GalN
d-galactosamine
- IFN-γ
interferon-γ
- IL
interleukin
- IPA
Ingenuity Pathway Analysis
- IRAK3
interleukin-1 receptor–associated kinase 3
- JWH-133
(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran
- KC
Kupffer cell
- LPS
lipopolysaccharide
- MCP-1
monocyte chemotactic protein-1
- miR
microRNA
- MNC
mononuclear cell
- Nos2
nitric oxide synthase 2
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time polymerase chain reaction
- TLR4
Toll-like receptor 4
- TNF-α
tumor necrosis factor α
Authorship Contributions
Participated in research design: Tomar, Zumbrun, M. Nagarkatti, P. S. Nagarkatti.
Conducted experiments: Tomar.
Contributed new reagents or analytic tools: M. Nagarkatti, P. S. Nagarkatti.
Performed data analysis: Tomar, Zumbrun, M. Nagarkatti, P. S. Nagarkatti.
Wrote or contributed to the writing of the manuscript: Tomar, Zumbrun, M. Nagarkatti, P. S. Nagarkatti.
Footnotes
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grant R01-MH094755]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant R01-ES019313]; the National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM103641]; the National Institutes of Health National Center for Complementary and Integrative Health [Grants P01-AT003961 and R01-AT006888]; and the U.S. Department of Veterans Affairs [Grant BX001357].
References
- Adhikary S, Kocieda VP, Yen JH, Tuma RF, Ganea D. (2012) Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood 120:3741–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades CG, Berry PA, Wendon JA, Vergani D. (2008) The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol 49:845–861. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G, et al. (2007) Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21:1788–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal W, Auzinger G, Dhawan A, Wendon J. (2010) Acute liver failure. Lancet 376:190–201. [DOI] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25:1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Mestre L, Docagne F, Guaza C. (2005) Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: role of IL-10 and ERK1/2 kinase signaling. Br J Pharmacol 145:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZJ, Wei HM, Sun R, Tian ZG, Gao B. (2004) Isolation of murine hepatic lymphocytes using mechanical dissection for phenotypic and functional analysis of NK1.1+ cells. World J Gastroenterol 10:1928–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweep H, Sticht C, Pandey P, Gretz N. (2011) miRWalk—database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 44:839–847. [DOI] [PubMed] [Google Scholar]
- Floreani A, Lazzari R, Macchi V, Porzionato A, Variola A, Colavito D, Leon A, Guido M, Baldo V, De Caro R, et al. (2010) Hepatic expression of endocannabinoid receptors and their novel polymorphisms in primary biliary cirrhosis. J Gastroenterol 45:68–76. [DOI] [PubMed] [Google Scholar]
- Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ. (2009) CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res 84:378–386. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. (2008) Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol 74:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti PS, Nagarkatti M. (2011) Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS ONE 6:e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WI, Osei-Hyiaman D, Park O, Liu J, Bátkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B, et al. (2008) Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 7:227–235. [DOI] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. (2005) Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128:742–755. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D. (2008) Endocannabinoids and liver disease. IV. Endocannabinoid involvement in obesity and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 294:G1101–G1104. [DOI] [PubMed] [Google Scholar]
- Lee WM. (2012) Acute liver failure. Semin Respir Crit Care Med 33:36–45. [DOI] [PubMed] [Google Scholar]
- Leifeld L, Fielenbach M, Dumoulin FL, Speidel N, Sauerbruch T, Spengler U. (2002) Inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) expression in fulminant hepatic failure. J Hepatol 37:613–619. [DOI] [PubMed] [Google Scholar]
- Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. (1995) Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Liu LM, Ye CG, Zhao L, Yu FP, Gao DY, Wang YY, Yang ZW, Wang YY. (2014) Inhibition of UII/UTR system relieves acute inflammation of liver through preventing activation of NF-κB pathway in ALF mice. PLoS ONE 8:e64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, Tran-Van-Nhieu J, Karsak M, Zimmer A, Mallat A. (2008) CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol 153:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, Pecker F, Mallat A, Lotersztajn S. (2011) Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 54:1217–1226. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. [DOI] [PubMed] [Google Scholar]
- Mallat A, Lotersztajn S. (2008) Endocannabinoids and liver disease. I. Endocannabinoids and their receptors in the liver. Am J Physiol Gastrointest Liver Physiol 294:G9–G12. [DOI] [PubMed] [Google Scholar]
- Mas A, Rodés J. (1997) Fulminant hepatic failure. Lancet 349:1081–1085. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. (2004) Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 40:177–184. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. (2008) CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol 153:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Luque J, Ros J, Fernández-Varo G, Tugues S, Morales-Ruiz M, Alvarez CE, Friedman SL, Arroyo V, Jiménez W. (2008) Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther 324:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger J. (2005) Prediction of survival for patients with fulminant hepatic failure. Hepatology 41:19–22. [DOI] [PubMed] [Google Scholar]
- Nolan JP. (2010) The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology 52:1829–1835. [DOI] [PubMed] [Google Scholar]
- Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK, 3rd, Moldawer LL. (2000) LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol 278:R1202–R1209. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. (2008) Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10:111–122. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Sheedy FJ, McCoy CE. (2011) MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 11:163–175. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman TM, Hodgson HJ. (2000) Animal models of acute hepatic failure. Int J Exp Pathol 81:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci L, Fiorucci S, Chiorean M, Brunori PM, Di Matteo FM, Sidoni A, Migliorati G, Morelli A. (1996) Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology 111:736–744. [DOI] [PubMed] [Google Scholar]
- Sass G, Koerber K, Bang R, Guehring H, Tiegs G. (2001) Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest 107:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. (2012) Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol 258:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. (2011) Endocannabinoids in liver disease. Hepatology 53:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, Louvet A, Zimmer A, Tordjmann T, Mallat A, et al. (2010) Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology 52:1046–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. (2006) CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12:671–676. [DOI] [PubMed] [Google Scholar]
- Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. (2009) An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol 15:3086–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Wang Z, Yang P, Wang T, Xia L, Zhou M, Wang Y, Wang S, Hua Z, Zhang J. (2014) Adenosine 5′-monophosphate ameliorates D-galactosamine/lipopolysaccharide-induced liver injury through an adenosine receptor-independent mechanism in mice. Cell Death Dis 5:e985. [DOI] [PMC free article] [PubMed] [Google Scholar]