Abstract
OBJECTIVE
Cardiorespiratory fitness (VO2max) is associated with glycemic control, yet the relationship between VO2max and the underlying determinants of glycemic control is less clear. Our aim was to determine whether VO2max is associated with insulin sensitivity, insulin secretion, and the disposition index, a measure of compensatory pancreatic β-cell insulin secretion relative to insulin sensitivity, in subjects representing the entire range of the glucose tolerance continuum.
RESEARCH DESIGN AND METHODS
A cohort of subjects (N = 313) with heterogeneous age, sex, BMI, and glycemic control underwent measurements of body composition, HbA1c, fasting glucose, oral glucose tolerance (OGTT), and VO2max. OGTT-derived insulin sensitivity (SiOGTT), glucose-stimulated insulin secretion (GSISOGTT), and the disposition index (DIOGTT) (the product of SiOGTT and GSISOGTT) were measured, and associations between VO2max and these determinants of glycemic control were examined.
RESULTS
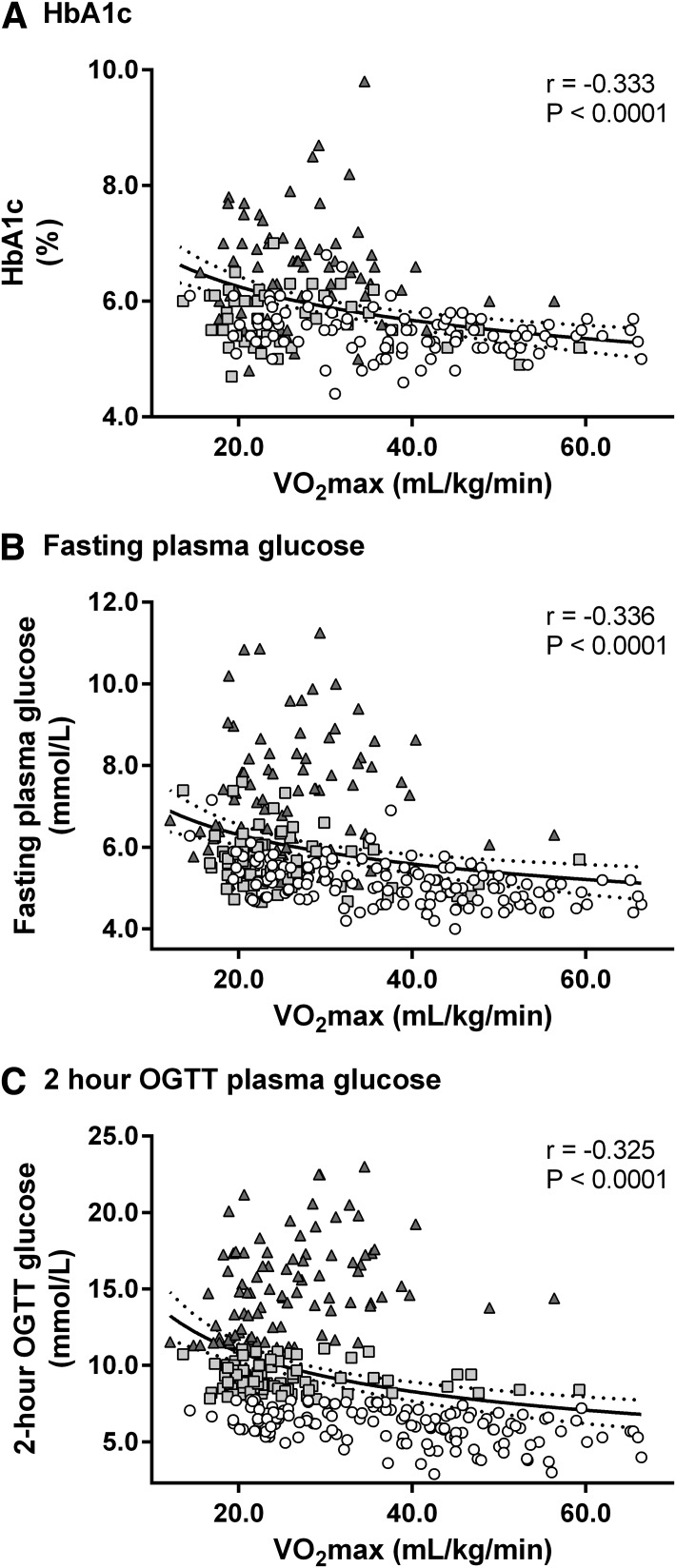
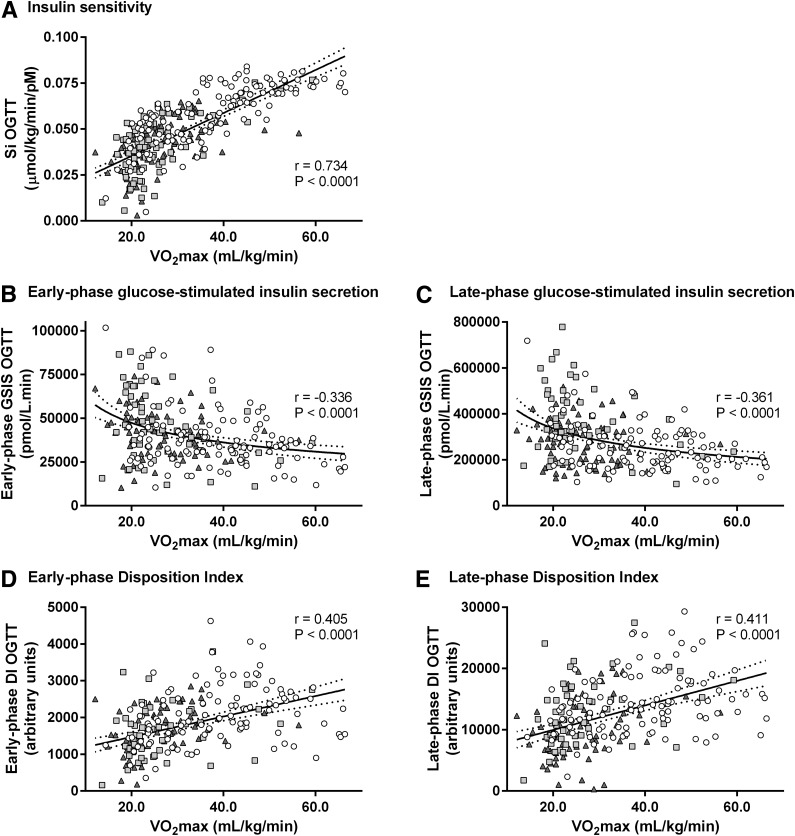
A low VO2max was associated with high HbA1c (r = −0.33), high fasting glucose (r = −0.34), high 2-h OGTT glucose (r = −0.33), low SiOGTT (r = 0.73), and high early-phase (r = −0.34) and late-phase (r = −0.36) GSISOGTT. Furthermore, a low VO2max was associated with low early- and late-phase DIOGTT (both r = 0.41). Interestingly, relationships between VO2max and either glycemic control or late-phase GSISOGTT deteriorated across the glucose tolerance continuum.
CONCLUSIONS
The association between poor cardiorespiratory fitness and compromised pancreatic β-cell compensation across the entire glucose tolerance continuum provides additional evidence highlighting the importance of fitness in protection against the onset of a fundamental pathophysiological event that leads to type 2 diabetes.
Introduction
Type 2 diabetes (T2D) is characterized by chronic hyperglycemia that develops when pancreatic β-cell insulin secretion fails to compensate for the deterioration in insulin sensitivity (1). Physical activity aimed at improving cardiorespiratory fitness is prescribed as part of standard-of-care treatment for T2D (2), primarily because randomized controlled clinical trials show that exercise training reduces hyperglycemia in patients with T2D (3–5) and delays the onset of T2D in at-risk individuals (6). Interestingly, in a longitudinal study of 8,633 nondiabetic men, Blair and colleagues showed that high cardiorespiratory fitness (as determined by maximal oxygen consumption [VO2max], measured during exhaustive incremental workload exercise) confers protection against developing T2D-related hyperglycemia (7). A further longitudinal study by Church et al. (8), examining 2,316 men with T2D, reported that high cardiorespiratory fitness reduced cardiovascular disease mortality. Consequently, poor fitness is considered a key determinant of the pathophysiological progression of glucose intolerance. However, because poor glucose disposition, driven by inadequate β-cell insulin secretory function in the presence of poor insulin sensitivity, is the fundamental cause of hyperglycemia in T2D, it is prudent to determine whether cardiorespiratory fitness is related to these pathophysiological factors. Indeed, we and others have shown that aerobic exercise training that improves cardiorespiratory fitness also increases insulin sensitivity (9–14) and improves β-cell insulin secretory function (10,14,15) in patients with T2D. Nonetheless, whether the predictive value of cardiorespiratory fitness for determining longitudinal glycemic control is explained by an association between fitness and the underlying determinants of glycemic control (insulin sensitivity and/or insulin secretory function) is not clear.
With the a priori knowledge (9–16) that exercise training improves VO2max and β-cell insulin secretory compensation for changing insulin sensitivity (the glucose disposition index) and the evidence that both variables are reduced in normoglycemic first-degree relatives of T2D patients (17), we hypothesized that low cardiorespiratory fitness would be associated with low disposition index, the underlying pathophysiological determinant of glucose intolerance. Therefore, our aim was to examine this relationship in a large cohort representing the entire glucose tolerance continuum from normal glucose tolerance (NGT) to T2D.
Research Design and Methods
Subjects
Potential participants underwent medical screening to determine their eligibility for the study. This included a medical history assessment, an electrocardiogram, and blood chemistry screening. Evidence of prior or current chronic pulmonary, hepatic, renal, gastrointestinal, or hematological disease; weight loss (>2 kg in the last 6 months); smoking; pregnancy; and contraindication to an exercise test were used as exclusion criteria. Subjects were recruited by newspaper/radio advertisement from the local municipal areas in Copenhagen, Denmark, and Cleveland, OH. All subjects provided oral and written informed consent prior to participation, and the methods were approved by ethics committees at both locations (Institutional Review Board, Cleveland Clinic, and Scientific Ethics Committee of the Capital Region of Denmark). Data from some subjects participating in this work have previously been published (3,9,10,18,19). Subjects included in the study were stratified by glucose tolerance status (i.e., NGT, impaired glucose tolerance, or T2D) according to their 2-h glucose on an oral glucose tolerance test (OGTT) (2). The final study cohort included 313 subjects.
Pretest Control Period
Tests took place at the Clinical Research Unit at the Cleveland Clinic and at the clinical research laboratory of the Centre of Inflammation and Metabolism, Rigshospitalet. Subjects being treated with antidiabetic drugs (metformin, N = 38; sulfonylureas, N = 14; GLP-1 analogs, N = 12; and dipeptidyl peptidase-4 inhibitors, N = 2) withheld their medications for 5 days prior to metabolic testing, during which time diet and physical activity records were used to ensure there were no pretesting variations in dietary or activity habits. Subjects also abstained from structured exercise for at least 24 h prior to metabolic testing.
Clinical Procedures
Height and weight were measured using standard techniques. Whole-body adiposity was estimated using DEXA (Lunar iDXA; GE Healthcare, Madison, WI). Subjects performed an exercise test to determine their VO2max during exhaustive incremental workload exercise to volitional exhaustion, which provides a measure of cardiorespiratory fitness. Heart rate was measured using a chest-worn monitor (Polar Electro, Kempele, Finland) and VO2 was measured online using an automated system (Oxycon Pro, Erich Jaeger, Hoechberg, Germany; CPET, Cosmed, Rome, Italy). VO2max was determined when at least two of the following criteria were met: respiratory exchange ratios >1.1; no further increase in VO2 despite increasing workloads, with heart rate greater than age-predicted maximum; or volitional fatigue. The VO2max test was conducted 48–72 h prior to subsequent metabolic assessments to avoid the acute effects of physical exercise on glycemic control. On a separate day, after an 8- to 10-h overnight fast, subjects came to the laboratory and an antecubital venous cannula was placed. Baseline blood samples were collected and subjects ingested 75 g anhydrous glucose dissolved in 300 mL water (standard OGTT). After glucose ingestion, venous blood samples were collected every 15 min for 2 h. Venous blood was collected into tubes containing EDTA and 500 kiU/mL aprotinin (serine protease inhibitor; Sigma) or into plain tubes containing no anticoagulant. Plain tubes were allowed to clot for 30 min at room temperature, while EDTA tubes were kept on ice. Blood was centrifuged at 2,000g for 15 min at room temperature, and respective serum/plasma was stored at −80°C until analysis.
Blood Chemistry Analysis
Glucose concentrations were measured using a bedside analyzer (YSI Stat, Yellow Springs; ABL, Radiometer Medical, Brønshøj, Denmark); insulin and C-peptide concentrations were determined by electrochemiluminescence immunoassay (E-modular; Roche, Basel, Switzerland); total cholesterol and triglycerides were determined by colorimetric assays (P-modular; Roche). A further venous blood sample was collected into an EDTA tube so that hemoglobin A1c (HbA1c) levels could be determined by high-performance liquid chromatography (HPLC) (Tosoh G7 analyzer; San Francisco, CA).
Calculations
Insulin sensitivity during OGTT (SiOGTT) was calculated using a model previously validated against the gold-standard hyperinsulinemic-euglycemic clamp method (19): SiOGTT (µmol/kg/min/pmol ⋅ L) = 0.138 − (0.00231 × BMI) − (0.00118 × G120) − (0.0000135 × I30) − (0.00000678 × I90), where BMI is measured as weight in kilograms divided by the square of height in meters, G120 is glucose at 120 min, and I30 and I90 are insulin at 30 and 90 min during OGTT. GSISOGTT was determined from the OGTT plasma C-peptide response rather than the insulin response to overcome the confounding effects of hepatic insulin extraction, which removes up to 80% of insulin at first pass prior to measurement in the peripheral venous blood (20). Early- and late-phase oral glucose–stimulated insulin secretion (GSISOGTT) was calculated as the area under the C-peptide curve from 0 to 30 and 30 to 120 min during OGTT, respectively. We previously showed that the log-log relationship between these measures of GSISOGTT and SiOGTT is described by an inverse linear model (19); therefore, the oral disposition index (DIOGTT), which is a measure of glucose disposal and estimates pancreatic β-cell insulin secretory compensation for changing insulin sensitivity during OGTT, was calculated as the product of GSISOGTT and SiOGTT.
Statistics
Relationships between VO2max and measures of glycemic control (HbA1c, fasting glucose, and 2-h OGTT glucose) and GSISOGTT were best fit by log-log curvilinear regression. Relationships between VO2max and measures of SiOGTT and DIOGTT were best fit by linear regression. For testing of the difference between correlation coefficients, r values were converted to z scores using Fisher r-to-z transformation and were then compared using the Cohen test (21). Forced-entry multiple regression was used to determine the relative contributions of VO2max and other independent variables (age, sex, body weight, BMI, and whole-body fat percentage) to the variance in glycemic control (HbA1c, fasting glucose, 2-h OGTT glucose), SiOGTT, GSISOGTT, and DIOGTT. One-way ANOVA and Bonferroni post hoc tests were used to assess differences between glucose tolerance groups. Statistical significance was set at P < 0.05 and determined using Prism v6 (GraphPad, La Jolla, CA) and SPSS v20 (IBM, New York, NY).
Results
Glycemic Control, Insulin Sensitivity, Insulin Secretion, and Disposition Index
Table 1 shows subject characteristics stratified by glucose tolerance status. Glycemic control (HbA1c, fasting glucose, and 2-h glucose) was progressively reduced across the glucose tolerance continuum. SiOGTT was markedly lower in subjects with impaired glucose tolerance and T2D compared with those with NGT. Early- and late-phase GSISOGTT were greatest in impaired glucose tolerant subjects, and lowest in the T2D group. Early- and late-phase DIOGTT showed progressive deterioration across the glucose tolerance groups.
Table 1.
Characteristics of subjects representing the glucose tolerance continuum
| NGT | IGT | T2D | PNGT vs. IGT | PNGT vs. T2D | PIGT vs. T2D | |
|---|---|---|---|---|---|---|
| N (male/female) | 137 (84/53) | 85 (44/41) | 91 (44/47) | — | — | — |
| Age (years) | 51 ± 1 | 61 ± 1 | 59 ± 1 | <0.001 | <0.001 | ns |
| Weight (kg) | 82.7 ± 1.3 | 93.9 ± 1.7 | 87.9 ± 1.6 | <0.0001 | <0.05 | <0.05 |
| BMI (kg/m2) | 26.8 ± 0.4 | 32.5 ± 0.3 | 30.6 ± 0.6 | <0.001 | <0.001 | <0.05 |
| Adiposity (%) | 28.6 ± 1.0 | 38.2 ± 1.2 | 37.1 ± 1.1 | <0.001 | <0.001 | ns |
| VO2max (mL/kg/min) | 37.0 ± 1.1 | 25.1 ± 0.9 | 26.2 ± 0.8 | <0.0001 | <0.0001 | ns |
| VO2max (L/min) | 3.02 ± 0.08 | 2.32 ± 0.07 | 2.28 ± 0.07 | <0.0001 | <0.0001 | ns |
| Fasting plasma total cholesterol (mmol/L) | 5.00 ± 0.08 | 5.15 ± 0.11 | 5.17 ± 0.10 | ns | ns | ns |
| Fasting plasma triglycerides (mmol/L) | 1.15 ± 0.06 | 1.60 ± 0.09 | 1.89 ± 0.23 | <0.05 | <0.001 | ns |
| Systolic blood pressure (mmHg) | 131 ± 2 | 133 ± 2 | 141 ± 2 | ns | <0.001 | <0.05 |
| Diastolic blood pressure (mmHg) | 78 ± 1 | 80 ± 2 | 85 ± 1 | ns | <0.0001 | <0.05 |
| Glycemic control | ||||||
| HbA1c (%) | 5.54 ± 0.08 | 5.69 ± 0.07 | 6.59 ± 0.11 | ns | <0.0001 | <0.0001 |
| HbA1c (mmol/mol) | 37.3 ± 0.8 | 39.0 ± 0.7 | 48.9 ± 1.2 | ns | <0.0001 | <0.0001 |
| Fasting plasma glucose (mmol/L) | 5.16 ± 0.04 | 5.63 ± 0.07 | 7.43 ± 0.22 | <0.01 | <0.0001 | <0.0001 |
| 2-h OGTT plasma glucose (mmol/L) | 6.05 ± 0.09 | 9.11 ± 0.09 | 15.08 ± 0.32 | <0.0001 | <0.0001 | <0.0001 |
| SiOGTT (µmol/kg/min/pmol ⋅ L) | 0.0606 ± 0.0012 | 0.0415 ± 0.0018 | 0.0409 ± 0.0015 | <0.0001 | <0.0001 | ns |
| GSISOGTT (AUC C-peptide [pmol/L ⋅ min]) | ||||||
| Early phase | 36,083 ± 1,331 | 45,802 ± 2,661 | 41,153 ± 1,520 | <0.001 | ns | ns |
| Late phase | 249,414 ± 8,325 | 351,893 ± 19,681 | 255,493 ± 11,131 | <0.0001 | ns | <0.001 |
| DIOGTT (arbitrary units) | ||||||
| Early phase | 2,085 ± 66 | 1,677 ± 104 | 1,665 ± 69 | <0.001 | <0.001 | ns |
| Late phase | 14,470 ± 430 | 12,840 ± 724 | 10,202 ± 560 | ns | <0.0001 | <0.01 |
Data are means ± SEM. Differences between group means were compared by one-way ANOVA adjusted for multiple comparisons. AUC, area under the curve; early phase, measures made during 0–30 min of OGTT; late phase, measures made during 30–120 min of OGTT.
Relationships With Cardiorespiratory Fitness (VO2max)
Table 2 and Figs. 1 and 2 show the correlation coefficients for the associations between VO2max and glycemic control, SiOGTT, GSISOGTT, and DIOGTT. VO2max was inversely associated with clinical markers of glycemic control: HbA1c (Fig. 1A), fasting glucose (Fig. 1B), and 2-h glucose during OGTT (Fig. 1C); however, these relationships were strongest in NGT subjects, while significant correlations were not evident in the subgroup of subjects with T2D (Table 2). Furthermore, these relationships were significantly weaker in subjects with T2D compared with those with NGT (NGT vs. T2D z scores for HbA1c, fasting glucose, and 2-h glucose were −2.51 [P < 0.05], −2.48 [P < 0.05], and −2.67 [P < 0.01], respectively). Fig. 2A demonstrates that VO2max was directly correlated with SiOGTT. This significant relationship was evident across the entire glucose tolerance continuum (Table 2), although with decreasing r values across the continuum where the relationship in T2D subjects was significantly weaker than in NGT subjects (NGT vs. T2D z score = 2.52, P < 0.05). Fig. 2B and C indicate that VO2max was inversely related to early- and late-phase GSISOGTT; nevertheless, these relationships were weaker in subjects with T2D, particularly during late phase (NGT vs. T2DM z score = −1.73, P = 0.08), where the relationship was not significant (Table 2). Fig. 2D and E show that VO2max was directly related to early- and late-phase DIOGTT. This finding was also consistent across the whole glucose tolerance continuum (Table 2). These significant relationships persisted when analyzing absolute VO2max (liters per minute) vs. HbA1c (r = −0.37, P < 0.0001), fasting glucose (r = −0.22, P < 0.001), 2-h OGTT glucose (r = −0.34, P < 0.0001), SiOGTT (r = 0.44, P < 0.0001), GSISOGTT (r = −0.26, P < 0.05; r = −0.21, P < 0.05 [early and late phase, respectively), and DIOGTT (r = 0.25, P < 0.0001; r = 0.26, P < 0.0001). However, r values were lower compared with the analyses made against relative VO2max (milliliters per kilogram per minute) indicating that differing body mass accounts for some of the variance in these relationships. Multiple regression analysis (Table 3) where VO2max was entered simultaneously with the other independent variables (age, sex, weight, BMI, and % body fat) showed that although VO2max was significantly associated with glycemic control (HbA1c, fasting glucose, and 2-h OGTT glucose), SiOGTT, GSISOGTT, and DIOGTT, the other independent variables also explained some of the variance. By comparing significant β-values in Table 3, these analyses also indicate that the majority of the variance in glycemic control was related to VO2max, while the majority of variance in GSISOGTT, SiOGTT, and DIOGTT was related to body weight and BMI, respectively.
Table 2.
Relationships between VO2max and OGTT-derived variables
| NGT | IGT | T2D | All subjects | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Glycemic control | ||||||||
| HbA1c (%) | −0.392 | <0.0001 | −0.217 | ns | −0.015 | ns | −0.333 | <0.0001 |
| Fasting plasma glucose (mmol/L) | −0.475 | <0.0001 | −0.288 | <0.05 | −0.175 | ns | −0.336 | <0.0001 |
| 2-h OGTT plasma glucose (mmol/L) | −0.436 | <0.0001 | −0.343 | <0.001 | −0.097 | ns | −0.325 | <0.0001 |
| SiOGTT (µmol/kg/min/pmol ⋅ L) | 0.733 | <0.0001 | 0.609 | <0.0001 | 0.525 | <0.0001 | 0.734 | <0.0001 |
| GSISOGTT (AUC C-peptide [pmol/L ⋅ min]) | ||||||||
| Early phase | −0.325 | <0.001 | −0.309 | <0.05 | −0.233 | <0.05 | −0.336 | <0.0001 |
| Late phase | −0.406 | <0.0001 | −0.312 | <0.05 | −0.166 | ns | −0.361 | <0.0001 |
| DIOGTT (arbitrary units) | ||||||||
| Early phase | 0.399 | <0.01 | 0.361 | <0.01 | 0.390 | <0.01 | 0.405 | <0.0001 |
| Late phase | 0.404 | <0.01 | 0.360 | <0.01 | 0.354 | <0.01 | 0.411 | <0.0001 |
In a cohort representative of the whole glucose tolerance continuum, we determined whether statistically significant associations existed between cardiorespiratory fitness (VO2max) (mL/kg/min) and HbA1c (total N = 218; n = 102 NGT, n = 47 IGT, and n = 69 T2D), fasting glucose and 2-h OGTT (total N = 313; n = 137 NGT, n = 85 IGT, and n = 91 T2D), SiOGTT (total N = 304; n = 134 NGT, n = 83 IGT, and n = 87 T2D), GSISOGTT (total N = 240; n = 111 NGT, n = 54 IGT, and n = 75 T2D), and DIOGTT (total N = 235; n = 110 NGT, n = 53 IGT, and n = 72 T2D). Data indicate correlation coefficients (r) for comparisons between variables. AUC, area under the curve.
Figure 1.
Cardiorespiratory fitness is associated with markers of glycemic control. VO2max was measured during incremental workload and exhaustive aerobic exercise in subjects representative of a heterogeneous population with respect to age, BMI, adiposity, and glucose tolerance status (white circles, NGT; light-gray squares, IGT; and dark-gray triangles, T2D). Regression analysis demonstrated inverse curvilinear log-log relationships between VO2max and HbA1c (log10y = −0.14 log10x + 0.98) (A), fasting glucose (log10y = −0.17 log10x + 1.03) (B), and 2-h glucose during OGTT (log10y = −0.39 log10x + 1.55) (C). Solid and dotted lines represent the regression curves and 95% CI, respectively, and show unadjusted data.
Figure 2.
Cardiorespiratory fitness is associated with SiOGTT, GSISOGTT, and DIOGTT. VO2max was measured during incremental workload and exhaustive aerobic exercise in subjects representative of a heterogeneous population with respect to age, BMI, adiposity, and glucose tolerance status (white circles, NGT; light-gray squares, IGT; and dark-gray triangles, T2D). VO2max was directly associated with SiOGTT (y = 0.00117x + 0.0119) (A) but had an inverse curvilinear log-log relationship with early-phase (log10y = −0.39 log10x + 5.18) (B) and late-phase (log10y = −0.42 log10x + 6.07) (C) GSISOGTT. Finally, there were direct linear relationships between VO2max and early-phase (y = 27.8x + 916) (D) and late-phase (y = 200x + 5,966) (E) DIOGTT, a measure of pancreatic β-cell insulin secretory compensation for changing insulin sensitivity. Solid and dotted lines represent the regression curves and 95% CIs, respectively, and show unadjusted data.
Table 3.
Multiple regression analyses
| Independent variables | HbA1c (R2 = 0.20) |
FPG (R2 = 0.15) |
2-h OGTT glucose (R2 = 0.22) |
SiOGTT (R2 = 0.86) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | P | B | SE B | β | P | B | SE B | β | P | B | SE B | β | P | |
| Constant | 11.29 | 1.04 | 5.8 × 10−22 | 14.06 | 1.66 | 1.3 × 10−15 | 37.02 | 4.20 | 1.1 × 10−16 | 0.08 | 0.007 | 2.4 × 10−24 | ||||
| VO2max | −0.06 | 0.01 | −0.83 | 4.8 × 10−9 | −0.10 | 0.02 | −0.68 | 3.7 × 10−8 | −0.32 | 0.04 | −0.87 | 7.3 × 10−13 | 0.0005 | 0.00007 | 0.34 | 1.1 × 10−10 |
| Age | −0.02 | 0.01 | −0.29 | 0.001 | −0.02 | 0.01 | −0.17 | 0.04 | −0.08 | 0.03 | −0.21 | 0.006 | 0.0002 | 0.00005 | 0.13 | 7.6 × 10−5 |
| Sex | −0.30 | 0.21 | −0.16 | 0.15 | −1.03 | 0.32 | −0.32 | 0.002 | −2.84 | 0.82 | −0.34 | 0.0006 | 0.007 | 0.001 | 0.20 | 4.0 × 10−6 |
| Weight | −0.03 | 0.01 | −0.43 | 0.006 | −0.04 | 0.01 | −0.41 | 0.003 | −0.12 | 0.03 | −0.48 | 0.0003 | 0.0001 | 0.00006 | 0.13 | 0.02 |
| BMI | 0.04 | 0.03 | 0.25 | 0.14 | 0.09 | 0.04 | 0.30 | 0.05 | 0.18 | 0.11 | 0.23 | 0.10 | −0.003 | 0.0002 | −0.81 | 1.5 × 10−31 |
| Fat % |
−0.02 |
0.01 |
−0.29 |
0.03 |
−0.04 |
0.02 |
−0.29 |
0.01 |
−0.10 |
0.04 |
−0.26 |
0.02 |
−0.0001 |
0.00007 |
−0.09 |
0.05 |
| Early-phase GSISOGTT (R2 = 0.18) |
Late-phase GSISOGTT (R2 = 0.18) |
Early-phase DIOGTT (R2 = 0.33) |
Late-phase DIOGTT (R2 = 0.32) |
|||||||||||||
| Independent variables |
B |
SE B |
β |
P |
B |
SE B |
β |
P |
B |
SE B |
β |
P |
B |
SE B |
β |
P |
| Constant | 29,034 | 14,130 | 0.04 | 195,511 | 100,528 | 0.05 | 3,130 | 770 | 0.0001 | 18,623 | 5,346 | 0.0006 | ||||
| VO2max | −6,057 | 2,278 | −0.32 | 0.008 | −31,946 | 16,306 | −0.24 | 0.03 | 16.8 | 8.0 | 0.22 | 0.04 | 96.7 | 55.5 | 0.23 | 0.04 |
| Age | −240 | 126 | −0.16 | 0.06 | −1,339 | 899 | −0.13 | 0.14 | −1.1 | 5.2 | −0.02 | 0.84 | 33.6 | 36.3 | 0.08 | 0.36 |
| Sex | 1,644 | 4,057 | 0.05 | 0.69 | −4,922 | 28,840 | −0.02 | 0.87 | 172 | 163 | 0.11 | 0.29 | 513 | 1133 | 0.05 | 0.65 |
| Weight | 538 | 166 | 0.51 | 0.001 | 2,574 | 1178 | 0.35 | 0.03 | 17.3 | 6.4 | 0.38 | 0.007 | 83.4 | 44.4 | 0.26 | 0.06 |
| BMI | −223 | 502 | −0.07 | 0.66 | −649 | 3,571 | −0.03 | 0.86 | −121 | 21 | −0.90 | 1.4 × 10−8 | −785 | 142 | −0.85 | 9.4 × 10−8 |
| Fat % | −23 | 220 | −0.02 | 0.92 | 1,640 | 1,579 | 0.16 | 0.30 | 10.9 | 9.4 | 0.17 | 0.25 | 132 | 66 | 0.30 | 0.05 |
In a cohort representative of the whole glucose tolerance continuum, we determined the relative contributions of VO2max and other independent variables such as age, sex, body weight, BMI, and whole-body fat percentage to the variance of glycemic control (HbA1c, fasting glucose [FPG], 2-h OGTT glucose), SiOGTT, GSISOGTT, and DIOGTT using a forced-entry multiple regression method. Dependent variables are the actual values and not log transformed. The statistical significance of β values is indicated by the individual P values. B, the unstandardized β, which represents the change in the dependent variable resulting from 1 unit change in the independent variable; SE B, the SE of β; β, standardized β, which represents the change in the dependent variable resulting from 1 SD change in the independent variable.
Conclusions
In the current study, we demonstrate that cardiorespiratory fitness is inversely related with clinical markers of glycemic control, such that high HbA1c, high fasting glucose, and impaired oral glucose tolerance are associated with low VO2max. We further found that cardiorespiratory fitness is positively related to insulin sensitivity and inversely related to GSISOGTT. However, the major novel finding of this work is that cardiorespiratory fitness is associated with pancreatic β-cell insulin secretory compensation for changing insulin sensitivity (DIOGTT) in a cohort representing the entire glucose tolerance continuum.
The Italian Diabetes and Exercise Study (IDES [22]) demonstrated that changes in fitness after aerobic training predicted improvements in HbA1c (and other cardiovascular disease risk factors). In this study, we show that VO2max is inversely associated with HbA1c (and other markers of glycemic control such as fasting and 2-h OGTT glucose) in a large cohort representing the entire glucose tolerance continuum, supporting the findings from Blair’s group, who showed that high VO2max confers protection against the development of T2D over a 6-year period even when adjusted for age and parental diabetes (7). These data provide strength to the notion that maintaining cardiorespiratory fitness will help prevent T2D. That said, it should be noted that when the T2D subgroup was analyzed in isolation, the relationship between VO2max and glycemic control (HbA1c and fasting and OGTT glucose) was not evident (Table 2), possibly suggesting that once T2D is present cardiorespiratory fitness is a lesser determinant of the state of glycemia.
Hyperglycemia in individuals with T2D develops when insulin secretion no longer compensates for poor insulin sensitivity, indicating a state of β-cell dysfunction. Indeed, exercise training improves glycemic control (3–6,23), and it is traditionally thought that this benefit is driven entirely by increases in muscle glucose uptake and/or increased insulin sensitivity (rev. in 24,25). Furthermore, VO2max is associated with insulin sensitivity, as first shown in 1983 by Rosenthal et al. (26), and we now confirm this finding across the entire continuum of glucose tolerance. We also found VO2max to be inversely associated with GSISOGTT; however, as for variables of glycemic control (HbA1c and fasting and 2-h glucose), such associations were less evident in subjects with T2D. Given that VO2max is well correlated with insulin sensitivity even in subjects with T2D, the poor relationship between VO2max and variables of glycemic control in subjects with diabetes is potentially explained by the fact that the solid inverse relationship between insulin sensitivity and insulin secretion is also diminished in subjects with T2D, as evidenced by the deterioration in glucose disposition. Thus, again, in T2D once the disease is established, cardiorespiratory fitness is a lesser determinant of insulin secretion. However, it is important to note that glycemic control is not entirely governed by insulin sensitivity. The ability to restore β-cell function is particularly relevant for the treatment of T2D, a disease in which β-cell insulin secretory function is no longer able to overcome the poor underlying degree of insulin sensitivity. Thus, it is of importance to examine insulin secretory compensation (i.e., the disposition index). Interestingly, aerobic exercise training increases the disposition index in subjects without (16,27) and in subjects with (10) T2D, and Chen et al. (28) have demonstrated that self-reported weekly minutes of physical activity are directly associated with disposition index. In support of such findings, we now demonstrate that high VO2max is also predictive of high β-cell insulin secretory compensation for poor insulin sensitivity (DIOGTT) across the entire glucose tolerance continuum. While these relationships do not indicate causality, our findings do highlight an important association between cardiorespiratory fitness and the key determinant of T2D-related hyperglycemia. However, it should also be noted that the genetic component of VO2max (29) has not been addressed in this study and should be examined in future work.
The underlying mechanisms as to why VO2max (or aerobic training) may be positively associated with β-cell compensation are not well defined. Physical activity per se, which is a major determinant of VO2max, decreases blood glucose and lipids and may thereby relieve glucolipotoxicity in β-cells, allowing restoration of appropriate insulin secretory function (30). Further to this, increased physical activity lowers circulating levels of proinflammatory cytokines (tumor necrosis factor-α, leptin) and increases levels of various growth factors, hormones, and cytokines (growth hormone, insulin-like growth factor-1, GLP-1, interleukin-6, and interleukin-1 receptor antagonist) that have direct beneficial effects on β-cells (30). Irrespective of the speculated mechanisms, our findings support the need for future work examining the role of exercise in β-cell health.
There are some limitations to our study. Firstly, it is important to note that pausing antidiabetic drugs for 5 days prior to metabolic testing may induce acute increases in glycemia, potentially inducing glucotoxicity. On the contrary, this time period may not be sufficient to wash out all drugs. With these issues in mind, care should be taken when interpreting, for example, the relationship between VO2max and HbA1c and whether it would be altered when pausing the antidiabetic drugs for 5 days. Therefore, future studies should carefully consider the rationale for withholding drugs in the context of the primary outcomes of the study. Particular attention should also be drawn to the strength of the correlations. Despite finding significant relationships in the whole cohort, VO2max explains just ∼11% of the variance in measures of glycemic control (HbA1c and fasting and OGTT glucose), which indicates that other factors, such as the other independent variables included in the multiple regression analyses (Table 3), are also involved. Furthermore, for all variables, the strength of the relationships with VO2max progressively diminishes with advancing glucose intolerance. This is particularly interesting because it highlights the complexity of the pathophysiology of T2D. Indeed, prior work in subjects with T2D has found a relationship between aerobic exercise training–induced changes in fitness and glycemic control (31). Furthermore, we previously found that patients randomized to a training intervention that does not increase VO2max fail to improve glycemic control compared with patients randomized to training that increases VO2max (3). However, we (32) and others (33) have also shown that exercise training–induced improvements in VO2max do not always extrapolate to improved glycemia. Accordingly, while VO2max explains ∼11% of the variance in measures of glycemic control, it accounted for ∼54% of the variance in insulin sensitivity. Thus, although cardiorespiratory fitness is predictive of insulin sensitivity, this does not extrapolate to the same magnitude of prediction of glycemic control. On the other hand, ∼17% of the variance in DIOGTT was accounted for by VO2max. As such, the underlying mechanisms linking cardiorespiratory fitness to improvements in glycemic control require more detailed investigation. Lastly, it is important to note that body weight and BMI explain a large part of the variance of GSISOGTT and of SiOGTT and DIOGTT, respectively (Table 3). Consequently, it is likely that weight loss combined with exercise training–induced increases in cardiorespiratory fitness is required to mediate the most optimal beneficial adaptation in glycemic control.
In conclusion, our study has advanced the prior knowledge that high cardiorespiratory fitness is associated with good glycemic control by showing that VO2max is also positively associated with the disposition index in a cohort of adults representing the entire glucose tolerance continuum. While the large contribution of body weight and BMI must not be ignored, these findings are clinically significant because they also highlight the importance of an individual’s cardiorespiratory fitness level in relation to the fundamental pathophysiological cause of hyperglycemia-related chronic disease.
Article Information
Acknowledgments. The authors thank Julianne Filion, RN (Department of Pathobiology, Cleveland Clinic), for her clinical nursing support and Lisbeth Andreasen, MSc (Department of Clinical Biochemistry, Rigshospitalet), for her assistance with biochemical assays.
Funding. This work was funded by a Paul Langerhans Program grant from the European Foundation for the Study of Diabetes/Amylin Pharmaceuticals, Inc. (to T.P.J.S.). Additional support for this study came from the Danish Center for Strategic Research in Type 2 Diabetes (Danish Council for Strategic Research, grant nos. 09-067009 and 09-075724), and the National Institutes of Health (NIH) (RO1 AG12834) (to J.P.K.). S.K.M. was supported by an NIH T32 grant (DK007319 [training grant] to J.P.K.). The Centre of Inflammation and Metabolism is supported by the Danish National Research Foundation (grant no. 02-512-55).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.P.J.S. designed the study and drafted the manuscript. T.P.J.S., S.K.M., K.K., S.H.K., J.M.H., M.J.L., and J.P.K. implemented the study and edited the manuscript. T.P.J.S., S.K.M., J.M.H., M.J.L., and J.P.K. had access to and analyzed data. T.P.J.S., S.K.M., K.K., J.M.H., M.J.L., and J.P.K. discussed the data. T.P.J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Jallut D, Golay A, Munger R, et al. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism 1990;39:1068–1075 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association . Standards of medical care in diabetes--2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karstoft K, Winding K, Knudsen SH, et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2013;36:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balducci S, Zanuso S, Nicolucci A, et al.; Italian Diabetes Exercise Study (IDES) Investigators . Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med 2010;170:1794–1803 [DOI] [PubMed] [Google Scholar]
- 5.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 7.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 1999;130:89–96 [DOI] [PubMed] [Google Scholar]
- 8.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med 2005;165:2114–2120 [DOI] [PubMed] [Google Scholar]
- 9.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2009;297:E151–E156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010;33:1561–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010;59:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Gorman DJ, Karlsson HK, McQuaid S, et al. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 2006;49:2983–2992 [DOI] [PubMed] [Google Scholar]
- 13.Winnick JJ, Sherman WM, Habash DL, et al. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 2008;93:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krotkiewski M, Lönnroth P, Mandroukas K, et al. The effects of physical training on insulin secretion and effectiveness and on glucose metabolism in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1985;28:881–890 [DOI] [PubMed] [Google Scholar]
- 15.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E1024–E1031 [DOI] [PubMed] [Google Scholar]
- 16.Slentz CA, Tanner CJ, Bateman LA, et al. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009;32:1807–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thamer C, Stumvoll M, Niess A, et al. Reduced skeletal muscle oxygen uptake and reduced beta-cell function: two early abnormalities in normal glucose-tolerant offspring of patients with type 2 diabetes. Diabetes Care 2003;26:2126–2132 [DOI] [PubMed] [Google Scholar]
- 18.Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol (1985) 2008;104:1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon TP, Malin SK, Karstoft K, et al. Determining pancreatic β-cell compensation for changing insulin sensitivity using an oral glucose tolerance test. Am J Physiol Endocrinol Metab 2014;307:E822–E829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 21.Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Internet], 2002. Available from http://quantpsy.org. Accessed 15 January 2015 [Google Scholar]
- 22.Balducci S, Zanuso S, Cardelli P, et al.; Italian Diabetes Exercise Study (IDES) Investigators . Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care 2012;35:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulé NG, Weisnagel SJ, Lakka TA, et al.; HERITAGE Family Study . Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 2005;28:108–114 [DOI] [PubMed] [Google Scholar]
- 24.Maarbjerg SJ, Sylow L, Richter EA. Current understanding of increased insulin sensitivity after exercise - emerging candidates. Acta Physiol (Oxf) 2011;202:323–335 [DOI] [PubMed] [Google Scholar]
- 25.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 2003;284:E453–E467 [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal M, Haskell WL, Solomon R, Widstrom A, Reaven GM. Demonstration of a relationship between level of physical training and insulin-stimulated glucose utilization in normal humans. Diabetes 1983;32:408–411 [DOI] [PubMed] [Google Scholar]
- 27.Malin SK, Solomon TP, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic β-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab 2013;305:E1248–E1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Black MH, Watanabe RM, et al. Self-reported physical activity is associated with β-cell function in Mexican American adults. Diabetes Care 2013;36:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 1999;87:1003–1008 [DOI] [PubMed] [Google Scholar]
- 30.Narendran P, Solomon TP, Kennedy A, Chimen M, Andrews RC. The time has come to test the beta cell preserving effects of exercise in patients with new onset type 1 diabetes. Diabetologia 2015;58:10–18 [DOI] [PubMed] [Google Scholar]
- 31.Larose J, Sigal RJ, Khandwala F, Prud’homme D, Boulé NG, Kenny GP; Diabetes Aerobic and Resistance Exercise (DARE) trial investigators . Associations between physical fitness and HbA₁(c) in type 2 diabetes mellitus. Diabetologia 2011;54:93–102 [DOI] [PubMed] [Google Scholar]
- 32.Solomon TP, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic β-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab 2013;98:4176–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksen L, Dahl-Petersen I, Haugaard SB, Dela F. Comparison of the effect of multiple short-duration with single long-duration exercise sessions on glucose homeostasis in type 2 diabetes mellitus. Diabetologia 2007;50:2245–2253 [DOI] [PubMed] [Google Scholar]