Abstract
The relation between posttraumatic stress symptoms and smoking is well documented but poorly understood. The present investigation sought to evaluate the impact of posttraumatic stress symptoms on subjective and behavioral tobacco abstinence effects both directly and indirectly through negative affect reduction smoking outcome expectancies. Participants included 275 (68.7% male; Mage=43.9, 10+ cig/day) adult non-treatment seeking smokers, who attended two counterbalanced laboratory sessions (16 h of smoking deprivation vs ad libitum smoking), during which they completed self-report measures of withdrawal symptoms and mood followed by a smoking lapse task in which they could earn money for delaying smoking and purchase cigarettes to smoke. Results supported a mediational pathway whereby higher baseline symptoms of posttraumatic stress predicted greater endorsement of expectancies that smoking will effectively reduce negative affect, which in turn predicted greater abstinence-provoked exacerbations in nicotine withdrawal symptoms and negative affect. Posttraumatic stress symptoms also predicted number of cigarettes purchased independent of negative affect reduction expectancies, but did not predict delaying smoking for money. Findings highlight tobacco abstinence effects as a putative mechanism underlying posttraumatic stress disorder (PTSD)-smoking comorbidity, indicate an important mediating role of beliefs for smoking-induced negative affect reduction, and shed light on integrated treatment approaches for these two conditions.
Keywords: Posttraumatic stress, cigarette smoking, nicotine withdrawal, negative affect, abstinence
Cigarette smoking and posttraumatic stress symptoms and syndromes commonly co-occur (Feldner et al., 2007a; Fu et al., 2007). Indeed, relative to the general population, individuals who have been exposed to trauma or diagnosed with posttraumatic stress disorder (PTSD) endorse significantly higher rates of current smoking, lifetime smoking, and nicotine dependence (Feldner et al., 2007a; Fu et al., 2007; Hapke et al., 2005; McClernon et al., 2013). Further, individuals with posttraumatic stress symptoms evidence lower overall quit rates (Hapke et al., 2005; Lasser et al., 2000) as well as a higher risk of early smoking lapse in comparison to non-psychiatric controls (Beckham et al., 2013; Zvolensky et al., 2008). Such findings have stimulated scholarly interest in elucidating the nature of, and mechanisms underlying, PTSD-smoking relations (Rasmusson et al., 2006).
One promising avenue for better understanding the association between PTSD and smoking has been to explore the impact of posttraumatic stress symptoms on the expression of distressing nicotine withdrawal symptoms upon acute tobacco abstinence. Nicotine withdrawal has been posited to play a central role underlying addiction motivation, given that the desire to suppress negative affect and other aversive nicotine withdrawal symptoms (Baker et al., 2004), coupled with the heightened reward value placed on smoking, may produce a strong motivation to resume smoking following abstinence. Thus, explicating the factors that may influence the expression of nicotine withdrawal symptoms upon acute abstinence is vital from both a scientific and clinical perspective as it could provide further insight into the maintenance of smoking behavior.
Several retrospective and prospective studies have documented that smokers with posttraumatic stress symptoms report more severe nicotine withdrawal symptoms during tobacco abstinence (Breslau et al., 1992; Dedert et al., 2012; Feldner et al., 2008; Weinberger et al., 2009). However, this research has been limited to primarily examining subjective nicotine withdrawal symptoms, which span negative affect (e.g. anxiety irritability) and somatic features (e.g. hunger). It is likely that other changes that occur upon tobacco abstinence may be central factors to consider in the maintenance of smoking among those with posttraumatic stress symptoms. Moving beyond subjective nicotine withdrawal symptoms, inflations in the reward value of initiating and continuing smoking behavior following abstinence may reflect key markers of the nicotine dependence phenotype and ability to quit smoking (Baker et al., 2007; McKee, 2009). To this end, McKee (2009) developed an objective behavioral task which assesses these aspects of smoking reward valuations by measuring smokers’ ability to delay smoking for proportionally increasing monetary reward and willingness to purchase individual cigarettes once the opportunity to smoke arises. To our knowledge, no study has directly evaluated the role of posttraumatic stress symptoms on these aspects of smoking reward valuation.
Furthermore, little is known about the factors that underlie (or mediate) the relations between posttraumatic stress symptoms and tobacco abstinence effects and smoking behavior more generally. Outcome expectancies, which reflect anticipated consequences of smoking and include beliefs about positive reinforcement, negative reinforcement/negative affect reduction, negative consequences, and appetite control, are believed to be core cognitive mechanisms underlying smoking motivation (Brandon and Baker, 1991). Increasing evidence suggests that individuals with posttraumatic stress symptoms and syndromes have higher expectations that smoking will effectively reduce negative affect, to a greater extent than those without the disorder (Calhoun et al., 2011; Carmody et al., 2012; Feldner et al., 2007b; Marshall et al., 2008). For example, in a cross-sectional study, Carmody and colleagues (2012) found that negative affect reduction smoking expectancies significantly mediated relations between posttraumatic stress symptoms and tobacco dependence as well as abstinence self-efficacy among military veterans diagnosed with PTSD.
Notably, much of the work described above has conceptualized PTSD as a categorical construct, which is classified by the presence or absence of the disorder based upon specific diagnostic criteria. However, converging evidence suggests that posttraumatic stress symptoms are phenomenologically heterogeneous and may be better understood from a dimensional approach (Broman-Fulks et al., 2006; Greenberg et al., 2012; Simms et al., 2002). In terms of PTSD-smoking comorbidity, it is important to study such relations among a community sample as well as include individuals who may experience posttraumatic stress symptoms below the diagnostic threshold, given prior work illustrating that the degree of symptoms (regardless of PTSD diagnosis status) is associated with smoking and nicotine dependence in the general population (Greenberg et al., 2012). Further, research suggests that subthreshold posttraumatic stress symptoms are associated with significant psychological burden, and warrant greater attention (Mylle and Maes, 2004; Jakupcak et al., 2007; Pietrzak et al., 2012). Consistent with this perspective, examination of smokers who may not receive a formal PTSD diagnosis, yet are still symptomatic, may yield valuable information about the interplay between posttraumatic stress symptoms and smoking behavior.
Therefore, the present investigation sought to extend the post-traumatic stress and smoking literature by: (a) examining posttraumatic stress symptoms in relation to other established factors that potentially maintain smoking behavior, namely, abstinence-induced changes in nicotine withdrawal symptoms, negative affect, and objective smoking reward; (b) employing a well-controlled counterbalanced within-participant experimental design, which assessed the relative reward value of smoking, and ability to resist smoking when delaying and abstaining are monetarily rewarded; and (c) including community-recruited participants experiencing varying degrees of posttraumatic stress symptoms. It was hypothesized that greater symptoms of posttraumatic stress would be significantly related to greater abstinence-provoked exacerbations in nicotine withdrawal symptoms and negative affect as well as inflations in smoking reward valuation (i.e. diminished ability to delay smoking for monetary compensation and increased cigarette purchases). We further hypothesized that these relations would be mediated by negative affect reduction smoking expectancies, such that posttraumatic stress symptoms would be indirectly related to abstinence-induced increases in nicotine withdrawal and negative affect as well as speed of initiating smoking and number of cigarettes smoked during the laboratory smoking lapse task, through negative affect reduction smoking expectancies.
Methods
Participants
Participants included 275 (68.7% male; Mage=43.9, standard deviation (SD)=10.6) adult non-treatment seeking smokers, recruited from the Los Angeles, California, area of the USA, who responded to advertisements to take part in a study examining individual differences in tobacco withdrawal effects. In terms of race/ethnicity, 52% identified as Black, 35% identified as Caucasian, and 5% identified as other, with 8% self-identifying as Hispanic. Participants reported smoking an average of 19.6 cigarettes/day (SD=5.3), smoking their first cigarette at 16.7 years of age (SD=4.8), and smoking regularly at 19.5 years of age (SD=5.6). The average score on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was 5.3 (SD=1.9), indicating moderate overall levels of nicotine dependence. Regarding post-traumatic stress symptoms, participants endorsed an average score of 25.8 (SD=10.6) on the Posttraumatic Stress Disorder Checklist-Civilian Version (PCL-C; Weathers et al., 1994), with approximately 8% of the sample scoring ≥35, suggesting probable PTSD, based upon recommended cut-points in civilian or general population samples (National Center for PTSD, 2014).
Inclusion criteria required participants to be 18 years or older, regular cigarette smoking for a minimum of two years (≥10 cig/day), and fluent in English. Exclusion criteria included: (a) current DSM-IV non-nicotine substance dependence; (b) acute DSM-IV mood disorder or psychotic symptoms or use of psychiatric or psychoactive medications to prevent modulation of tobacco abstinence effects by acute distress or pharmacological mechanisms; (c) breath carbon monoxide (CO) levels<10 ppm at baseline; (d) use of non-cigarette forms of tobacco or nicotine products; (e) currently pregnant. Participants were compensated $200 USD for completing the study. The procedures followed were in accordance with the ethical standards of the University of Southern California Internal Review Board.
Procedure
Following a preliminary phone screening, participants were invited to attend a baseline assessment session to determine final eligibility, consistent with the inclusion/exclusion criteria identified above. The baseline session involved informed consent, breath CO analysis, psychiatric interview, and baseline questionnaire measures. Participants deemed eligible at the baseline session subsequently attended two counterbalanced experimental sessions beginning at 12:00; abstinent (16 h of smoking abstinence beginning at 20:00 the evening prior to the visit) and non-abstinent (smoking ad libitum). The experimental sessions were scheduled a minimum of 24 h apart and generally no longer than 14 d apart. Identical procedures were employed across the two conditions with one exception – at the commencement of the non-abstinent session participants were instructed to smoke one cigarette of their preferred brand in the laboratory in order to standardize recency of smoking. In previous studies employing this methodology the length of time in between experimental sessions has varied, and the time between sessions has not been significantly related to the outcomes of interest (McKee et al., 2011). At the outset of both experimental sessions, breath alcohol analyses and CO assessments were performed. Participants screening positive for alcohol use (i.e. breath alcohol concentration >0.00 g/dL) were prohibited from completing the session and rescheduled to return to the laboratory at a later time. In the abstinent condition, a breath CO analysis of 10 ppm or higher was considered indicative of recent smoking (Leventhal et al., 2010; Society for Research on Nicotine and Tobacco (SRNT) Subcommittee on Biochemical Verification, 2002). As such, participants with a breath CO analysis greater than 9 ppm were considered non-abstinent, and permitted to return to the laboratory a week later to make a second attempt to complete their abstinent session. Those with a breath CO analysis greater than 9 ppm on their second attempt were excluded from further participation.
Participants arrived at the laboratory at 12:00 to begin the experimental sessions. In the non-abstinent session, participants started the session by smoking a cigarette followed by breath analysis for alcohol and CO levels, whereas the abstinent session started with breath analysis for alcohol and CO levels. Following breath analysis procedures, participants completed measures of nicotine withdrawal and affect. Participants were then instructed to engage in a smoking lapse analogue task (described below), which consisted of a delay period, self-administration period, and rest period.
Measures
Baseline assessments
Screening, descriptive, and covariate measures
The Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-NP; First et al., 2002) was used to assess mood disorders, psychosis, and substance use disorders in the context of exclusionary criteria. The SCID-NP was administered by trained bachelors, masters, and doctoral-level research staff, who were supervised by a licensed clinical psychologist (second author: AML). The Center for Epidemiologic Studies Depression Scale (CES-D; Shafer, 2006) assessed baseline depressive symptomatology. The Mood and Anxiety Symptom Questionnaire – Short Form – Anxious Arousal subscale (MASQ-AA; Clark and Watson, 1991) measured baseline anxiety symptoms of somatic tension and arousal. An author-constructed questionnaire assessed demographic variables of interest, including, participant sex and smoking characteristics (e.g. cigs/day, age of smoking onset, etc.). Finally, the FTND (Heatherton et al., 1991) was administered as a measure of nicotine dependence severity.
PCL-C
Posttraumatic stress symptomatology was assessed with the PCL-C (Weathers et al., 1994). The PCL-C is a 17-item self-report instrument that reflects the DSM-IV diagnostic criteria for PTSD (American Psychiatric Association (APA), 2000), and assesses each relevant domain of posttraumatic stress symptoms, including: re-experiencing (e.g. “Repeated, disturbing dreams of a stressful experience from the past?”), avoidance (e.g. “Avoid activities or situations because they remind you of a stressful experience from the past?”), emotional numbing (e.g. “Feeling emotionally numb or being unable to have loving feelings for those close to you?”), and hyperarousal (e.g. “Feeling jumpy or easily startled?”). Given that this measure is employed in community samples (as opposed to military samples), the instructions on the civilian version are worded to assess symptoms in response to “stressful life experiences” more broadly, and not limited to “stressful military experiences.” Respondents are asked to indicate the degree to which they have been “bothered” by each PTSD-related symptom over the prior month, on a five-point scale (1=not at all to 5=extremely). The PCL-C has demonstrated strong internal consistency and good test-retest reliability in a community sample, as well as good convergent validity with other well-established measures of PTSD (Ruggierio et al., 2003). Notably, in non-clinical samples, the PCL-C was more highly correlated with alternative measures of trauma symptoms than with other measures of psychological distress (e.g. social anxiety, worry, depression), therefore, evidencing good discriminant validity (Coneybeare et al., 2012). The total score, which was devised from the sum of 17 items, was utilized as our primary predictor variable.
Smoking Consequences Questionnaire (SCQ)
The SCQ (Brandon and Baker, 1991) is a 50-item self-report measure that assesses smoking expectancies on a Likert-type scale, ranging from 0 (“completely unlikely”) to 9 (“completely likely”). The measure and its constituent factors have excellent psychometric properties (Brandon and Baker, 1991; Buckley et al., 2005; Downey and Kilbey, 1995). The SCQ includes the following sub-scales: positive reinforcement (e.g. “I enjoy the taste sensations while smoking”), negative reinforcement/negative affect reduction (e.g. “Smoking helps me calm down when I feel nervous”), negative consequences (e.g. “The more I smoke, the more I risk my health”), and appetite control (e.g. “Smoking helps me control my weight”). The four subscales of the SCQ were employed as mediators in the present analyses.
Experimental assessments
Minnesota Nicotine Withdrawal Scale (MNWS)
In the present study, nicotine withdrawal symptom severity was assessed using an 11-item variant of the MNWS (Hughes and Hatsukami, 1986) that has shown good psychometric properties in acute abstinence research (Leventhal et al., 2010). Participants were asked to rate to what extent they experienced each symptom associated with nicotine withdrawal (e.g. craving, irritability) “so far today” using a six-point scale (0=“none” to 5=“severe”). A mean composite scale was derived from each of the items.
The Profile of Mood States (POMS)
The POMS (McNair et al., 1971) is a multidimensional affect scale commonly used in prior tobacco withdrawal research (Gilbert et al., 1999, 2002). The present study employed a 72-item version of the POMS, in which participants rated affect adjectives on a five-point scales based on how they were feeling “right now” (0=“not at all” to 4=“extremely”). Five negative affect subscales (Anger, Anxiety, Confusion, Depression, and Fatigue) and three positive affective subscales (Elation, Friendliness, and Vigor) were derived from the POMS. Each subscale score is computed as a mean score per item. For the present analyses, we utilized the composite scores for negative affect (NA, mean of all negative affect subscales) only.
Laboratory Analogue Smoking Lapse Task (McKee et al., 2006)
This task assesses the relative reward value of smoking (vs money), and ability to resist smoking when delaying and abstaining is advantageous (i.e. monetarily rewarded). During this task, participants received a tray containing eight cigarettes of their preferred brand, a lighter, and an ashtray. At the outset of the delay period, participants were instructed they could commence smoking at any point over the next 50 min, but they would earn $0.20 for each 5 min they delay smoking. Thus, participants could earn a maximum of $2 for delaying smoking. The delay period ended when the participant indicated they wanted to smoke or at the end of 50 min for participants choosing not to smoke. Following the delay period, participants began the self-administration period. During this period, participants could smoke as much or little as they wanted for 60 min. They were accurately instructed they would not have another opportunity to smoke again until 16:00. Participants were told they had a $1.60 credit and for each cigarette lit would cost $0.20. Following the self-administration period, participants experienced a rest period of no smoking until 16:00. To avoid disrupting natural smoking processes or draw attention to craving, we did not collect self-report measures throughout the delay and self-administration periods. Two variables were computed from this task: time delay (i.e. the total amount of time participant resisted smoking; range 0–50 min) and cigarettes smoked (i.e. total number of cigarettes smoked during the self-administration period; range 0–8 cigarettes). The laboratory analogue smoking lapse task has been validated in numerous studies using a variety of relapse precipitants, including, but not limited to: nicotine deprivation (Leeman et al., 2010; Pang and Leventhal, 2013), alcohol (McKee et al., 2006), and stress (Ashare et al., 2012; McKee et al., 2011). These studies support the utility of this methodology to effectively model lapse behavior in the laboratory setting. The monetary value used in this study was based on prior piloting from smokers sampled from the same local population in order to generate the greatest dispersion of values and sensitivity to deprivation effects.
Data analytic strategy
We conducted repeated measures analyses of variance (ANOVAs) to determine whether the outcomes of interest varied as a function of experimental condition.
To evaluate the effects of posttraumatic stress symptoms on tobacco abstinence effects (i.e. nicotine withdrawal, negative affect, time delay, and cigarettes smoked), both directly and indirectly through negative affect reduction smoking expectancies on tobacco abstinence effects, the nonparametric bootstrapping method recommended by Preacher and Hayes (2008) was employed. Bootstrapping offers the advantage of generating an empirical approximation of the sampling distribution through resampling of the full data set without assuming indirect effects are normally distributed. Direct and indirect effects were tested based on bootstrapped standard errors (SEs), with 5000 bootstrap samples. For each value, we used abstinence-induced change scores (abstinent – non-abstinent score). Given that we sought to test the relative specificity of negative affect reduction smoking expectancies over and above the other smoking expectancies of positive reinforcement, negative consequences, and weight concerns, multiple mediation models were constructed. Multiple mediation analyses include all hypothesized mediators in the model simultaneously, each serving as a control for the other. Within these analyses, the strength of each individual indirect effect was compared against the other by conducting pairwise contrasts between the four indirect effects (Preacher and Hayes, 2008). Given that posttraumatic stress may covary with sex, other psychiatric factors that co-occur with smoking, and more severe dependence, the following variables were also included in our model as statistical controls to elucidate the specificity of findings to posttraumatic stress symptoms per se: participant sex (dummy coded: 0=female, 1=male); nicotine dependence (FTND assessed at baseline); anxiety symptoms (MASQ-AA assessed at baseline); depressive symptomatology (CES-D assessed at baseline); and corresponding non-abstinent outcome measure. Point estimates of indirect effects were considered significant if 95% confidence intervals (CIs) did not contain zero (Preacher and Hayes, 2008)1.
Results
Preliminary analyses
Across the sample, nicotine withdrawal (F(1,301)=5.31, p<0.0001), negative affect (F(1,301)=4.35, p<0.0001), time delay (F(1,300)=4.20, p<0.0001), and cigarettes smoked (F(1,299)=5.25, p<0.0001) significantly differed between the two experimental conditions (16 h of smoking deprivation vs ad libitum smoking). In general, participants reported more severe symptoms of nicotine withdrawal and negative affect during the abstinence condition, compared to the smoking ad libitum condition. Further, participants evidenced shorter speed to smoking initiation, and smoked a greater number of cigarettes during the abstinence condition, compared to the smoking ad libitum condition (abstinence condition: time delay (M=23.93, SD=22.92); cigarettes smoked (M=1.52, SD=0.94); smoking ad libitum condition: time delay (M=39.15, SD=17.84); cigarettes smoked (M=1.22, SD=0.94)).
Primary analyses
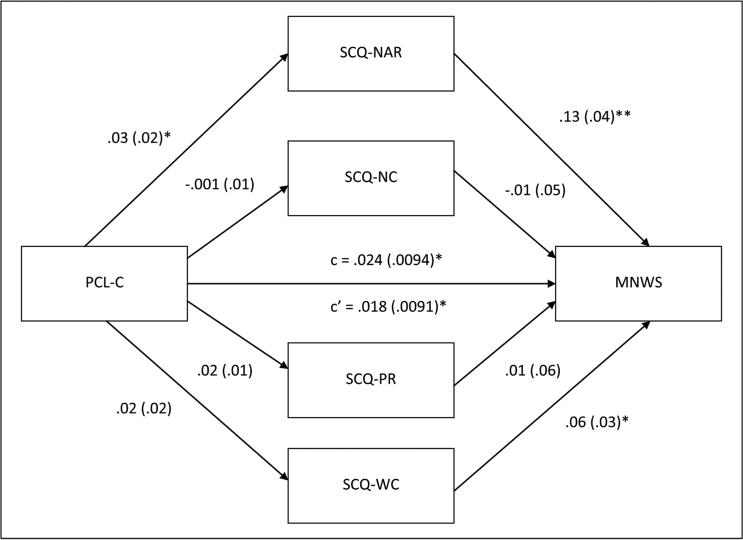
Figure 1 displays the results of multiple mediation analyses simultaneously examining negative consequences, negative affect reduction, positive reinforcement, and weight control smoking expectancies as mediators of the association between posttraumatic stress symptoms and abstinence-induced nicotine withdrawal symptoms. Posttraumatic stress symptoms evidenced a total effect on abstinence-induced nicotine withdrawal symptoms (β=0.02, SE=0.01, p<0.05), with greater symptoms of posttraumatic stress being associated with more severe abstinence-induced increases in nicotine withdrawal. The total indirect effect of post-traumatic stress symptoms on abstinence-induced withdrawal symptoms through all four smoking outcome expectancy variables was also significant (point estimate=0.006; CI: 0.001–0.01). Regarding specific indirect effects of each smoking outcome expectancy variable in the multiple mediation model, negative affect reduction was the only significant mediator of the effect of posttraumatic stress symptoms on abstinence-induced withdrawal (point estimate=0.004; CI: 0.001–0.01; see Table 1).
Figure 1.
Multiple mediation model for posttraumatic stress symptoms and abstinence-induced nicotine withdrawal symptoms via smoking outcome expectancies. Unstandardized path coefficients and standard error are listed below. The coefficient appearing above the line connecting Posttraumatic Stress Disorder Checklist-Civilian Version (PCL-C) to abstinence-induced nicotine withdrawal symptoms represents the original path before the mediators were added to the model. SCQ-NAR: Smoking Consequences Questionnaire, Negative Affect Reduction subscale; SCQ-NC: Smoking Consequences Questionnaire, Negative Consequences subscale; SCQ-PR: Smoking Consequences Questionnaire, Positive Reinforcement subscale; SCQ-WC: Smoking Consequences Questionnaire, Weight Concern subscale. MNWS: Minnesota Nicotine Withdrawal Scale. *p<0.05, **p<0.01.
Table 1.
Summary of bootstrap results for indirect effects.
| Mediating variable | Point estimates β SE | Lower limit | Upper limit |
|---|---|---|---|
| MNWS | |||
| SCQ-NARa | 0.004, 0.003 | 0.0005 | 0.0108 |
| SCQ-NC | −0.0001, 0.001 | −0.0010 | 0.0015 |
| SCQ-PR | 0.0002, 0.001 | −0.0019 | 0.0035 |
| SCQ-WC | 0.001, 0.002 | −0.0007 | 0.0056 |
| POMS-NA | |||
| SCQ-NARa | 0.003, 0.001 | 0.0005 | 0.0064 |
| SCQ-NC | 0.0001, 0.001 | −0.0012 | 0.0019 |
| SCQ-PR | 0.0000, 0.001 | −0.0020 | 0.0016 |
| SCQ-WC | 0.001, 0.001 | −0.0003 | 0.0040 |
| Time delay | |||
| SCQ-NAR | −0.01, 0.05 | −0.1177 | 0.0674 |
| SCQ-NC | 0.0009, 0.02 | −0.0319 | 0.0322 |
| SCQ-PR | 0.03, 0.04 | −0.0215 | 0.1269 |
| SCQ-WC | −0.009, 0.02 | −0.0821 | 0.0168 |
| Cigs/smoked | |||
| SCQ-NAR | 0.001, 0.002 | −0.0018 | 0.0047 |
| SCQ-NC | 0.0000, 0.001 | −0.0011 | 0.0014 |
| SCQ-PR | 0.001, 0.001 | −0.0012 | 0.0047 |
| SCQ-WC | 0.001, 0.001 | −0.0004 | 0.0036 |
MNWS: Minnesota Nicotine Withdrawal Scale; POMS-NA: Profiles of Mood Scale, Negative Affect subscale; SCQ-NAR: Smoking Consequences Questionnaire, Negative Affect Reduction subscale; SCQ-NC: Smoking Consequences Questionnaire, Negative Consequences subscale; SCQ-PR: Smoking Consequences Questionnaire, Positive Reinforcement subscale; PCL-C: Posttraumatic Stress Disorder Checklist-Civilian Version; SCQ-WC: Smoking Consequences Questionnaire, Weight Concern subscale; SE: standard error.
Significant point estimates as determined by the absence of zero within the confidence interval. IV: Independent Variable; PCL-C. Mediators: SCQ-NAR; SCQ-NC; SCQ-PR; SCQ-WC. DVs Dependent Variable; MNWS; POMS-NA; Time delay (range 0-50 min); Cigs/smoked (range 0-8). β: unstandardized path coefficient.
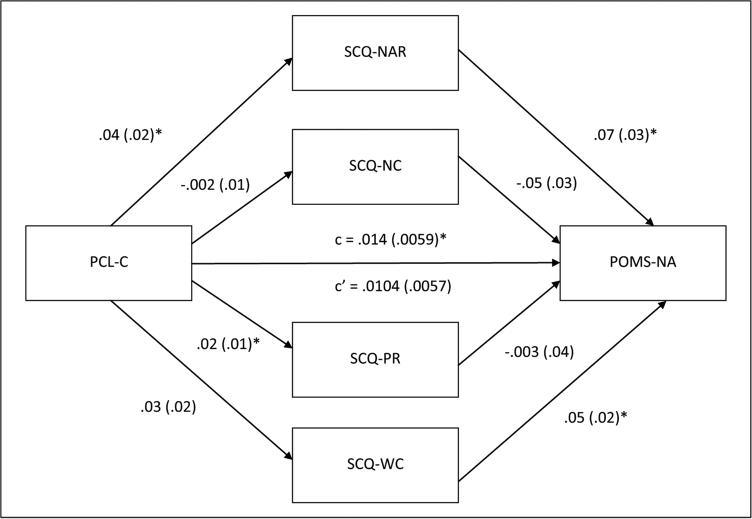
Figure 2 displays the results of multiple mediation analyses simultaneously examining negative consequences, negative affect reduction, positive reinforcement, and weight control smoking expectancies as mediators of the association between posttraumatic stress symptoms and abstinence-induced negative affect. Posttraumatic stress symptoms evidenced a total effect on abstinence-induced negative affect (β=0.01, SE=0.01, p<0.05), with greater symptoms of posttraumatic stress being associated with more severe nicotine withdrawal. Again, the total indirect effect of posttraumatic stress symptoms on abstinence-induced negative affect through all four smoking outcome expectancy variables was also significant (point estimate=0.004; CI: 0.001– 0.01). Regarding specific indirect effects of each smoking outcome expectancy variable in the multiple mediation model, negative affect reduction was the only significant mediator of the effect of posttraumatic stress symptoms on abstinence-induced negative affect (point estimate=0.003; CI: 0.001–0.01; Table 1).
Figure 2.
Multiple mediation model for posttraumatic stress symptoms and abstinence-induced negative affect via smoking outcome expectancies. Unstandardized path coefficients and standard error are listed below. The coefficient appearing above the line connecting Posttraumatic Stress Disorder Checklist-Civilian Version (PCL-C) to abstinence-induced negative affect represents the original path before the mediators were added to the model. POMS-NA: Profiles of Mood Scale, Negative Affect subscale; SCQ-NAR: Smoking Consequences Questionnaire, Negative Affect Reduction subscale; SCQ-NC: Smoking Consequences Questionnaire, Negative Consequences subscale; SCQ-PR: Smoking Consequences Questionnaire, Positive Reinforcement subscale; SCQ-WC: Smoking Consequences Questionnaire, Weight Concern subscale. *p<0.05.
For the smoking task outcomes, posttraumatic stress symptoms did not evidence total (β=0.09, SE=0.21, p=ns), direct (β=0.08, SE=0.21, p=ns) or indirect effects (β=0.01, SE=0.05, p=ns) on latency to first cigarette. By contrast, significant total (β=0.03, SE=0.01, p<0.01) and direct (β=0.02, SE=0.01, p<0.05) effects of posttraumatic stress symptoms on number of cigarettes smoked during the self-administration period was observed; although, none of the smoking outcome expectancies mediated this relation (see Table 1). Here, higher levels of posttraumatic stress symptoms were associated with smoking a greater number of cigarettes during the observation period.
Discussion
The present investigation sheds light on a putative mechanism through which posttraumatic stress symptoms maintain smoking behavior. That is, we demonstrated that smokers with elevated posttraumatic stress symptoms were more sensitive to the exacerbating effects of tobacco abstinence on nicotine withdrawal symptoms, negative affect, and reward value of smoking when provided with the opportunity. Further, the present study provides initial support for the potential mediating role of negative affect reduction smoking outcome expectancies in terms of the relations between posttraumatic stress symptoms and tobacco abstinence effects in this community sample of adult non-treatment seeking smokers. Specifically, higher baseline symptoms of posttraumatic stress were associated with greater endorsement of expectancies that smoking will effectively reduce negative affect, which in turn predicted greater abstinence-provoked exacerbations in nicotine withdrawal symptoms and negative affect. Although this study cannot determine the temporal order of posttraumatic stress symptoms and negative affect reduction expectancies given that they were assessed cross-sectionally, it nonetheless, suggests that negative affect reduction expectancies may be a possible intervening variable. These results are broadly consistent with previous research documenting that smokers with posttraumatic stress symptoms are likely to report: (a) more severe nicotine withdrawal symptoms and negative affect during tobacco abstinence (Breslau et al., 1992; Dedert et al., 2012; Feldner et al., 2008; Weinberger et al., 2009); (b) greater motivation to smoke in an effort to manage negative affective states (Marshall et al., 2008); and (c) stronger expectations that smoking will attenuate negative affect (Calhoun et al., 2011; Carmody et al., 2012). This study uniquely extends past extant work by: (a) examining the explanatory value of negative affect reduction smoking expectancies, relative to other smoking outcome expectancies, in relation to abstinence-induced nicotine withdrawal and negative affect; (b) utilizing a dimensional, as opposed to categorical, approach to examining posttraumatic stress symptoms in relation to smoking- and affect- relevant outcomes; and (c) employing a novel and objective laboratory smoking lapse task to assess impact of smoking behavior.
As previously described, the current study also extended prior work by incorporating a behavioral economic task to objectively measure the ability to delay smoking for monetary compensation as well as willingness to purchase cigarettes when permitted to smoke. This type of paradigm empirically assesses the relative reward value of smoking, which has been conceptualized as an index of nicotine dependence, self-control, and the ability to quit smoking (Baker et al., 2007; McKee, 2009). Results regarding this outcome were partially consistent with prediction. Specifically, neither posttraumatic stress symptoms nor any of the smoking outcome expectancies were significantly related to the latency to first cigarette. By contrast, posttraumatic stress symptoms (yet not smoking outcome expectancies) were significantly associated with a greater number of cigarettes purchased when permitted to smoke. Together, these findings suggest that symptoms of posttraumatic stress may be less relevant to the ability to delay smoking for monetary reward and yet provide further support for the premise that smokers experiencing symptoms of posttraumatic stress may smoke in such a way as to deliver higher nicotine levels when given the opportunity (Beckham et al., 1997; McClernon et al., 2005). Consistent with this perspective, several reports have documented that emotional numbing symptoms of PTSD, which encompass restricted positive affect, disinterest in activities, and emotional detachment from others, are highly related to increased smoking, above and beyond other symptom clusters (e.g. effortful avoidance; Cook et al., 2009; Greenberg et al., 2012). Further, other work has demonstrated that emotional numbing is associated with diminished appetitive functioning (i.e. low levels of positive affect; anhedonic symptoms) among those receiving treatment for PTSD (Kashdan et al., 2006). Thus, during periods of abstinence, the incentive value of smoking and corresponding self-control abilities to resist smoking may dramatically increase as persons experiencing symptoms of posttraumatic stress may be motivated to engage in smoking to overcome emotional blunting (Cook et al., 2007).
The present investigation has several limitations and related future directions that warrant further discussion. First, one should consider the timing of assessments when interpreting study findings. Specifically, symptoms of posttraumatic stress as well as smoking outcome expectancies were assessed at the baseline session, and thus, preclude definitive conclusions regarding temporal order or causal relations. Furthermore, self-report measures were not collected during the delay or self-administration period. Although this type of approach was intentionally selected to minimize the disruption of natural smoking processes, it nonetheless limits our knowledge about factors precipitating the decision to smoke. Past reports employing the analogue smoking lapse task have demonstrated that during the ad libitum period emotion ratings begin to change once the decision to start smoking has been made, even in the absence of actual smoking (McKee et al., 2011). Thus, future research may benefit from incorporating self-report measures during the delay and/or self-administration period, which may provide a more nuanced understanding of the studied variables.
Second, several of the constructs examined within this investigation relied on self-report methodologies (e.g. smoking outcome expectancies). To address this concern, future research could use alternative assessment methodologies that incorporate multi-method approaches. For instance, to increase validity, future studies may choose to use experimental cognitive methodologies that tap both strategic and automatic aspects of psychological processes (e.g. outcome expectancies predicting attentional biases for emotional-relevant stimuli). Related to this limitation, the present study employed self-reported symptoms of posttraumatic stress; however, the content of an index trauma and/or the diagnostic criteria for PTSD were not specifically assessed. Although studies have examined the severity of post-traumatic stress symptoms in non-clinical samples that may not have specifically experienced a Criterion A traumatic event (Cameron et al., 2010; Coneybeare et al., 2012), failure to empirically categorize a traumatic event precludes the ability to directly relate such symptoms to PTSD. That is, the non-specificity of several of the symptoms (e.g. irritability, sleep disturbance), suggests that this measure could be tapping into general mood or anxiety symptoms, rather than posttraumatic stress symptoms. Therefore, replication of these findings in clinical populations formally diagnosed with PTSD via clinician-administered structured interviews may be an important next step.
Third, we controlled for a wide range of potential variables that could influence tobacco abstinence effects. However, it is possible that other factors not assessed or experimentally controlled for (e.g. caffeine use) may impact these processes, which should be addressed in future work. Finally, the sample was comprised of non-treatment seeking smokers. While the goal of the present study was to examine the impact of posttraumatic stress symptoms on abstinence-induced exacerbations in nicotine withdrawal and negative affect as well as lapse behavior, it is unclear whether these findings would generalize to those undergoing a cessation attempt. To offset this concern, the present study utilized monetary compensation to provide an incentive for not smoking and to increase the likelihood of detecting effects of posttraumatic stress symptoms on the relative reinforcing value of nicotine. However, to rule out potential self-selection bias and increase the generalizability of these findings, it will be important for researchers to extend these findings to populations with expressed interest in quitting smoking.
Despite these limitations, the present findings offer insight into possible mechanisms potentiating smoking among persons experiencing symptoms of posttraumatic stress. Namely, holding the belief that smoking is an effective strategy for managing negative emotional states, particularly in the context of acute tobacco deprivation, may amplify the effects of nicotine withdrawal and negative affect. This study also adds to the growing literature suggesting that smokers with posttraumatic stress symptoms may smoke in such a way as to maximize the delivery of nicotine (Beckham et al., 1997; McClernon et al., 2005). From a clinical perspective, these results highlight the application of integrated PTSD-smoking cessation treatment which addresses common mechanisms underlying this comorbidity (Feldner et al., 2013).
Acknowledgments
Funding
This project was supported by a National Research Service Award (NRSA) F31DA026634 awarded to KJ Langdon by the National Institute on Drug Abuse. This work was also supported by grants R01DA026831 and K08DA025041 awarded to AM Leventhal by the National Institute of Health.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
The present study also tested for moderated mediation to identify whether the mediation pathway from posttraumatic stress to abstinence-induced nicotine withdrawal and negative affect, through negative affect reduction smoking expectancies, differed as a function of participant sex. However, results of these analyses revealed a similar pattern of findings for male and female participants, suggesting that sex does not moderate these relations. Please contact KJ Langdon for the full results of these analyses.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Ashare RL, Sinha R, Lampert R, et al. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology. 2012;220:256–268. doi: 10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, et al. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of the ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9:555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, et al. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res. 2013;15:1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, et al. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict Behav. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The subjective expected utility of smoking in college students. Psych Assess. 1991;3:484–491. [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemio-logic study of young adults. Am J Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Broman-Fulks JJ, Ruggiero KJ, Green BA, et al. Taxometric investigation of PTSD: Data from two nationally representative samples. Behav Ther. 2006;37:364–380. doi: 10.1016/j.beth.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Kamholz BW, Mozley SL, et al. A psychometric evaluation of the Smoking Consequences Questionnaire-Adult in smokers with psychiatric conditions. Nicotine Tob Res. 2005;7:739–745. doi: 10.1080/14622200500259788. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Levin HF, Dedert EA, et al. VA MIRECC Registry Workgroup The relationship between posttraumatic stress disorder and smoking outcome expectancies among U.S. military veterans who served since September 11, 2001. J Trauma Stress. 2011;24:303–308. doi: 10.1002/jts.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Palm K, Follette V. Reaction to stressful life events: What predicts symptom severity? J Anxiety Disord. 2010;24:645–649. doi: 10.1016/j.janxdis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Carmody TP, McFall M, Saxon AJ, et al. Smoking outcome expectancies in military veteran smokers with posttraumatic stress disorder. Nicotine Tob Res. 2012;14:919–926. doi: 10.1093/ntr/ntr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cook J, Jakupcak M, Rosenheck R, et al. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11:1189–1195. doi: 10.1093/ntr/ntp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, McFall MM, Calhoun PS, et al. Posttraumatic Stress Disorder and smoking relapse: A theoretical model. J Trauma Stress. 2007;20:989–998. doi: 10.1002/jts.20275. [DOI] [PubMed] [Google Scholar]
- Coneybeare D, Behar E, Solomon A, et al. The PTSD Checklist-Civilian Version: Reliability, validity and factor structure in a non-clinical sample. J Clin Psychol. 2012;68:699–713. doi: 10.1002/jclp.21845. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Harper LE, et al. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine Tob Res. 2012;14:372–376. doi: 10.1093/ntr/ntr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey KK, Kilbey MM. Relationship between nicotine and alcohol expectancies and substance dependence. Exp Clin Psycho-pharmacol. 1995;3:174–182. [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clin Psychol Rev. 2007a;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ, et al. Posttraumatic stress symptoms and smoking to reduce negative affect: An investigation of trauma-exposed daily smokers. Addict Behav. 2007b;32:214–227. doi: 10.1016/j.addbeh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Smith RC, Monson CM, et al. Initial evaluation of an integrated treatment for comorbid PTSD and smoking using a nonconcurrent, multiple-baseline design. Behav Ther. 2013;44:514–528. doi: 10.1016/j.beth.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Vujanovic AA, Gibson LE, et al. Posttraumatic stress disorder and anxious and fearful reactivity to bodily arousal: A test of the mediating role of nicotine withdrawal among daily smokers in 12-hour nicotine deprivation. Exp Clin Psychopharmacol. 2008;16:144–155. doi: 10.1037/1064-1297.16.2.144. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fu S, McFall M, Saxon A, et al. Post-traumatic stress disorder and smoking: A systematic review. Nicotine Tob Res. 2007;9:1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Exp Clin Psychopharmacol. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J Consult Clin Psychol. 2002;70:142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Greenberg JB, Ameringer KJ, Trujillo MA, et al. Associations between posttraumatic stress disorder symptom clusters and cigarette smoking. Psychol Addict Behav. 2012;26:89–98. doi: 10.1037/a0024328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapke U, Schumann A, Rumpf H, et al. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis. 2005;193:843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Conybeare D, Phelps L, et al. Anger, hostility, and aggression among Iraq and Afghanistan war veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007;20:945–954. doi: 10.1002/jts.20258. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behav Res Ther. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, et al. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leeman RF, O'Malley SS, White MA, et al. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology. 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, et al. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35:1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Beckham JC, Mozley SL, et al. The effects of trauma recall on smoking topography in posttraumatic stress disorder and non-posttraumatic stress disorder trauma survivors. Addict Behav. 2005;30:247–257. doi: 10.1016/j.addbeh.2004.05.013. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Calhoun PS, Hertzberg JS, et al. VA MIRECC Registry Workgroup Associations between smoking and psychiatric comorbidity in U.S. Iraq and Afghanistan-Era Veterans. Psychol Addict Behav. 2013;27:1182–1188. doi: 10.1037/a0032014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin K, Shi J, et al. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman DL. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, et al. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. J Anxiety Disord. 2008;22:1214–1226. doi: 10.1016/j.janxdis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylle J, Maes M. Partial posttraumatic stress disorder revisited. J Affect Disord. 2004;78:37–48. doi: 10.1016/s0165-0327(02)00218-5. [DOI] [PubMed] [Google Scholar]
- National Center for PTSD [1 August 2014];Using the PTSD Checklist for DSMIV (PCL) 2014 Available at: http://www.ptsd.va.gov/professional/pages/assessments/assessment-pdf/PCL-handout.pdf.
- Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: A laboratory study. Exp Clin Psychopharmacol. 2013;21:269–276. doi: 10.1037/a0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Schechter CB, Bromet EJ, et al. The burden of full and subsyndromal posttraumatic stress disorder among police involved in the World Trade Center rescue and recovery effort. J Psychiatr Res. 2012;46:835–842. doi: 10.1016/j.jpsychires.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Picciotto MR, Krishnan-Sarin S. Smoking as a complex but critical covariate in neurobiological studies of posttraumatic stress disorders: A review. J Psychopharmacol. 2006;20:693–707. doi: 10.1177/0269881106060193. [DOI] [PubMed] [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, et al. Psychometric properties of the PTSD Checklist-Civilian version. J Trauma Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Simms LJ, Watson D, Doebbeling BN. Confirmatory factor analyses of posttraumatic stress symptoms in deployed and nondeployed veterans of the Gulf War. J Abnorm Psychol. 2002;111:637–647. doi: 10.1037//0021-843x.111.4.637. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco (SRNT) Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Huska JA, et al. PTSD Checklist-Civilian Version. National Center for PTSD, Behavioral Science Division; Boston: 1994. [Google Scholar]
- Weinberger AH, Maciejewski PK, McKee SA, et al. Gender differences in associations between lifetime alcohol, depression, panic disorder, and posttraumatic stress disorder and tobacco withdrawal. Am J Addict. 2009;18:140–147. doi: 10.1080/10550490802544888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, et al. Impact of post-traumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine Tob Res. 2008;10:1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]