Abstract
Context
Some cancer patients experience pain and fatigue whereas some others experience only one of the two. Yet, it is not clear who experiences these unique patterns and why.
Objectives
This study aimed to identify subgroups of cancer patients with unique pain and fatigue experiences in two different chemotherapy cycles, to examine how selected factors influenced subgroup membership, and identify how subgroups differed in concurrently measured functional-limitation outcome.
Methods
The sample included 276 patients with diverse cancer types from four U.S. sites. To investigate subgroups, latent profile analyses were performed. Multinomial logistic regression and one-way analyses of variance type analyses were conducted to examine the influencing variables of subgroup membership and to examine differences among subgroups in patient outcome.
Results
At both time points, the high-pain/high-fatigue and low-pain/low-fatigue subgroups were found. The low-pain/high-fatigue subgroup was present only in the first chemotherapy cycle. Pain and fatigue levels significantly differentiated subgroups at each time point (all p < .05). Across two time points, experiencing higher depressed mood increased the risk to be in the high-pain and high-fatigue subgroup (all p <.01). The high-pain and high-fatigue subgroup had the most serious limitations in activities (all p < .01).
Conclusion
This study confirmed the existence of a unique symptom experience of pain and fatigue. This pattern should be acknowledged for symptom assessment and management.
Keywords: pain, fatigue, depressed mood, symptom cluster, symptom assessment, symptom management, cancer
Introduction
Managing and assessing pain and fatigue, both prevalent in cancer patients, are major priorities of oncology nursing research and practice.1 Of cancer patients, 70% suffer severe pain, requiring immediate intervention.2 Fatigue, prevalent in 60 to 90% of patients,3 can occur before diagnosis, during treatment, or years after treatment completion.4 Pain and fatigue are key symptoms that lower the quality of life for individuals with cancer.2,5
Symptom-cluster researchers examine the co-occurring tendency of symptoms6 and often report that pain and fatigue tend to form a symptom cluster either alone or with other symptoms.7–9 However, several studies reported that pain and fatigue separately formed clusters with other symptoms,10,11 suggesting that pain and fatigue occur together only in a subgroup of cancer patients.
Recent studies examining subgroups with respect to unique symptom experience also suggest that some cancer patients experience both pain and fatigue while some others experience only one of the two.12–15 For instance, a cross-sectional study examined subgroups with different symptom experience of pain, fatigue, depression, and sleep disturbance in a mixed sample of cancer patients.13 This study found that 15% of the sample experienced high intensity of all four symptoms; 15% experienced high intensity of pain and low intensity of fatigue; 35% experienced high fatigue and low pain; and 35% experienced low levels of all four symptoms. In another study, about 20% had only fatigue at four different time points after diagnosis while 23–45% experienced fatigue with pain and/or insomnia across all time points.15
It is unclear who experiences both or only one symptom and what leads to their unique experience. This information helps delineate symptom management tailored to patients with a particular symptom experience. Previous studies included other symptoms such as insomnia, depressed mood, and cognitive disturbance, in addition to pain and fatigue, to create subgroups.12–14 The inclusion of multiple symptoms in analyses could have hampered the examination of unique experiences concerning pain and fatigue as well as the influence of factors on the two symptoms. For example, the subgroups may have been created as a function of other symptoms, not of pain and fatigue only. Previous studies employed cluster analysis of subjects.12,13 The weakness of cluster analysis includes subjectivity in determining the number of subgroups; technical barriers in establishing replicability (i.e., reliability) of the final solution in the sample; and difficulty handling missing data.
The present study focused on only pain and fatigue in patients undergoing chemotherapy (CTX) in two selected time points. Our previous analysis12 found the subgroups had opposite directions of pain and fatigue, as discussed above, and the present study further examined those subgroups with a different data set using a different approach. The treatment modality of CTX and radiation treatment both influence pain and fatigue experience.12,15,16 The present study focused on symptom experiences during CTX cycles. The present study used latent profile analysis (LPA). LPA is a latent variable method and an extension of latent class analysis for continuous indicators. Using LPA, psychologists and sociologists examined subgroups with unique characteristics, such as substance-use behaviors17 or phenotypes of eating disorders.18 In LPA, subgroups are determined by a latent nominal variable and each subgroup has a unique profile of means and variances corresponding to specified indicator variables (i.e., fatigue and pain intensity in this study). The goals of LPA are often similar to those of cluster analysis.19 Yet, LPA solutions can be replicated and are statistically robust in selecting numbers of subgroups, especially in situations with non-normal distributions of indicators.20
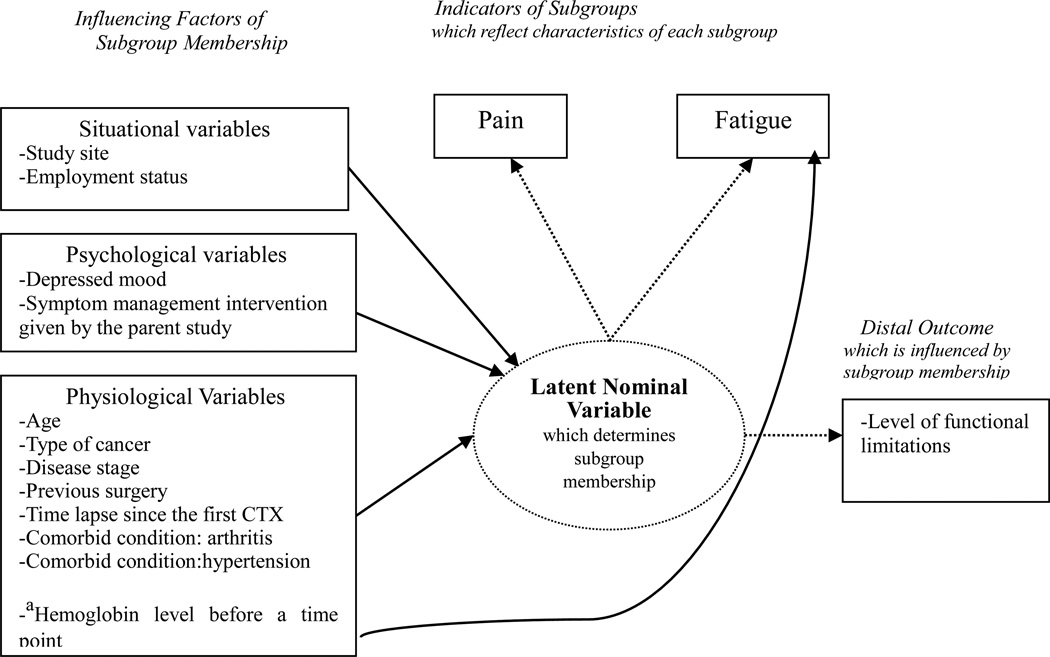
Age, disease stage, cancer type, and comorbid conditions may be associated with experiencing pain or fatigue.12,13,15,16 These variables were selected as influencing factors in the present study. Figure 1 shows variables categorized by the theory of unpleasant symptoms,21 describing physiological, psychological, and situational factors. These factors influence symptom experiences that can lower performance. Depressed mood has been associated with fatigue and/or pain experience in many studies.12,13,22 In the present study, depressed mood is categorized as a psychological factor rather than symptom per se, because the present study planned to examine its influence on the pain and fatigue experience.
Figure 1.
Statistical model of the study
Note. athe direct influence of the hemoglobin level on fatigue was allowed in this statistical model but its effect was not tested.
The purposes of this study were (a) to identify the subgroups of cancer patients with unique experience of pain and fatigue at two CTX cycles, (b) to examine how selected factors influenced subgroup membership, and (c) to examine how subgroups differed in concurrently measured functional limitations.
Methods
Design
This study employed a secondary analysis. The data were originally collected as a randomized clinical trial testing the effects of a cognitive behavioral intervention on fatigue and sleep in cancer patients undergoing CTX.23 The experimental group received education on energy-conservation strategies for fatigue and on sleep-enhancement strategies. The control group received general information on nutrition and diet for the equivalent time. A specially trained oncology nurse provided the intervention over the course of three interactive telephone sessions during the second, third, and fourth week after the first CTX treatment.
Baseline data were collected at two time points: on Day 1 of the first CTX cycle before receiving treatment (Time 0) and on Day 4 after the first CTX treatment (Time 1). The follow-up time point (Time 2) was on Days 43–46 for patients on a weekly or 21-day regimen, or on Days 57–60 for those on a 14-day or 28-day regimen. The follow-up time point fell within 3 days of the most recent CTX treatment (after the 7th CTX for weekly cycles, the 3rd CTX for 21-day cycles, the 5th CTX for 14-day cycles, and the 3rd CTX for 28-day cycles). Thus, Times 1 and 2 fell within 4 days of the most recent CTX. For the parent study, the criteria for the post-CTX time points was based on the time points found to have maximal levels of symptoms after CTX (i.e., within 3 days of CTX).24,25 The present study only used symptom data collected at Time 1 and Time 2, since this study focused on post-CTX symptom experience.
Settings and Sample
A convenience sample of oncology patients from four U.S. clinical sites were collected from 2004 to 2007: University of Utah, University of Cincinnati, Christiana Medical Center, and Fox Chase Cancer Center. Patients were at least 18 years of age; initiated a new CTX regimen with at least one drug administered intravenously in a cyclic manner; were diagnosed with lymphoma or breast, lung, colorectal, prostate, gynecologic, bladder, or testicular cancer; had no treatment other than surgery for at least one month before enrollment (concurrent radiation treatment allowed); and could read and understand spoken English. Patients were excluded with marrow or stem-cell transplantation, interleukins, interferon, or tumor-necrosis factor; chronic-fatigue disorder; sleep disorder; psychiatric disorder; anemia or depression during the prior 3 weeks; communication impairment; or enrollment in another psychoeducational intervention study.
A total of 276 patients composed the final sample for analyses in both the parent and present study, consisting of an experimental group (n = 142) and a control group (n = 134). No additional inclusion or exclusion criteria were applied. Because the experimental intervention did not have a beneficial effect in reducing fatigue or sleep disturbance,23 both groups were combined and analyzed as a unit in the present study. However, the effect of experimental intervention on symptom experience at Time 2 was examined due to the possibility that it could be associated with subgroup creation. Missing data on latent class indicators and covariates were handled by performing a full-information maximum-likelihood estimation from complete raw data; thus, all data from the 276 patients were used to estimate a model for this study. Because the data had already been collected, the sample size could not be changed. The findings were carefully interpreted according to the size and nature of the sample.
Instruments
Pain intensity was measured by the intensity subscale of the Brief Pain Inventory.26 It is composed of four items: the worst and least pain in the past 72 hours, average pain, and pain at the present moment. The scale ranged from 1 = no pain to 10 = pain as bad as you can imagine. Validity and reliability of this measure have been well established.26,27 Cronbach’s α was 0.88 in the present sample.
The General Fatigue Scale (GFS), a fatigue measurement, was developed for the clinical assessment of cancer-related fatigue.28 The GFS consists of seven items that measure fatigue intensity, the level of distress caused by fatigue, and the impact of fatigue on daily activities in the present day, the past 48 hours, or the past week. The scale ranged from 1 = no fatigue to 10 = the greatest possible fatigue. Acceptable reliability and validity were reported.28 In the present study, Cronbach’s α was 0.92.
The interference subscale of the Brief Pain Inventory measures interference with daily life caused by pain for the past 72 hours. The parent study modified this subscale to measure the interference due to symptoms, not specifically pain. The present study used this subscale as a functional-limitation measure. The subscale contains seven items that measure interference in general activity, life enjoyment, mood, relationships, sleep, walking, and work. The scale ranges from 0 = did not interfere to 10 = completely interfered. Cronbach’s α was 0.91 in this sample.
Depressed mood was measured by the depression subscale from the Profile of Mood States—Short Form.29 Validity and reliability were found to be acceptable in various populations29 The depression subscale consists of five items on a Likert-type scale where 0 = not at all and 4 = extremely. Cronbach’s α was 0.90 in this sample.
Analysis
To identify subgroups, LPA with covariates was conducted at each data point, estimated in Mplus v.6.12. Models were evaluated based on Akaike’s information criterion, where smaller results were preferable;30 Bayesian information criterion, where a smaller number was preferable;31 the Bootstrap Likelihood Ratio Test, where the number of subgroups was tested against a smaller number of subgroups (p < 0.05 indicated that at least the present number of subgroups exist in the data); an entropy value higher than 0.8, which summarized the probability for the most likely latent class membership based on the estimated model; the interpretability of classes;20 and the model’s convergence on a stable solution. Where these criteria suggested different results, we subjectively assessed the preponderance of evidence.
Interpretability was determined by distinguishability of subgroups in indicator variables, nontrivial subgroups sizes, and subgroup meaningfulness. Model convergence was examined through replication of maximum log-likelihood values across iterations, using random starting values. Distributions of the indicator variables were examined for extreme skewness and kurtosis. Pain had marked skewness and the model was estimated as if the given variable were normally distributed beyond its measured scale by using the censored command.
Subgroup-membership influencing factors were examined by multinominal logistic regression predicting the latent nominal variable. Subgroup differences in functional limitation were examined using the limitations measure as an “auxiliary variable” associated with subgroup membership. This is analogous to a one–way analysis of variance (ANOVA) based on the identified subgroups, but minimizes measurement error in the predictor by using subgroups as a latent variable. For missing data on subgroup indicators and covariates, we performed full-information maximum-likelihood estimation from complete raw data; thus, all data were used to estimate this study’s model. We obtained approvals from institutional review boards.
Results
Sample Nature
Table 1 shows the demographic and clinical characteristics of the sample. Of note, 55% were recruited from the comprehensive cancer center at Fox Chase. Patients were largely female, married, Caucasian, and had post-high school education. Half of the total sample had breast cancer and early stage cancer (Stages I and II). Half received symptom-management intervention designed in the parent study. Approximately 90% were treated with CTX alone. The most frequent comorbid condition was hypertension (25%).
Table 1.
Demographic/Clinical Characteristics of the Sample (N = 276)
| Variables | Number of patients (%) | |
|---|---|---|
| Data collection sitea | Site A | 152 (55.1) |
| Site B | 87 (31.5) | |
| Others | 37 (13.4) | |
| Age (mean years ± SD) | 53.97 (±11.79) | |
| Gender | Female | 228 (82.6) |
| Male | 48 (17.4) | |
| Marital status | Married | 191 (69.2) |
| Single | 81 (29.4) | |
| Missing | 4 (1.4) | |
| Race | Caucasian | 242 (87.7) |
| Non-Caucasian | 30 (10.9) | |
| Missing | 4 (1.4) | |
| Education | High school or less | 70 (25.4) |
| Post-high school education | 201 (72.8) | |
| Missing | 5 (1.8) | |
| Employment status | Employed | 150 (54.3) |
| Unemployed | 122 (44.2) | |
| Missing | 4 (1.5) | |
| Study Groupb | Experimental group | 142 (51.4) |
| Control group | 134 (48.6) | |
| Cancer Type | Breast | 152 (55.1) |
| Lung | 47 (17.0) | |
| Gynaecologic cancer | 32 (11.6) | |
| Lymphoma | 23 (8.3) | |
| Others | 22 (8.0) | |
| Disease stage | Stage 1 | 44 (15.9) |
| Stage 2 | 90 (32.6) | |
| Stage 3 | 68 (24.6) | |
| Stage 4 | 44 (15.9) | |
| Not staged | 30 (10.9) | |
| Time lapse since the first CTX | 43–46 vs. | 159(57.6) |
| 57–60 day | 111(40.2) | |
| Missing | 6 (2.2) | |
| Surgery right before baseline | Yes | 198 (71.7) |
| No | 75 (27.2) | |
| Missing | 3 (1.1) | |
| Treatment modality during the study | CTX only | 250 (90.6) |
| Concurrent treatment with radiation treatment | 18 (6.5) | |
| Missing | 8 (2.9) | |
| Comorbid conditions | Hypertension | 69 (25.0) |
| Arthritis | 28 (10.1) | |
| Hemoglobin at Time 1 | 12.92 (±1.6) | |
| Hemoglobin at Time 2 | 11.75 (±1.6) |
Note.
Site A= Fox Chase Cancer Center, Site B= Utah.
The primary study was a randomized clinical trial of the effectiveness of a cognitive-behavioral intervention on fatigue and insomnia.
Subgroups of Cancer Patients with Unique Pain and Fatigue Experience
At Time 1, a model with three subgroups was selected based on the model selection criteria (see Table 2). Table 3 summarizes the mean values of symptom intensity for each subgroup. Pain was censored in the model estimation due to extreme skewness in distribution, so it carries no clinical meaning, but rather shows the relative distribution across subgroups. Subgroups were named based on pain and fatigue levels: high-pain/high-fatigue (HPHF; 41.2%), low-pain/high-fatigue (LPHF; 34.3%), and low-pain/low-fatigue subgroups (LPLF; 24.5%). According to the Wald tests, the subgroups differed from each other in both pain (all p < .0001) and fatigue intensity (all p < .05; see Table 3).
Table 2.
Comparisons of Models with Different Numbers of Subgroups at Each Time Point
| No. of classes |
Likelihood ratio G2 |
AIC | BIC (sample size adjusted) |
Bootstrapped Likelihood Ratio Test |
Entropy |
|---|---|---|---|---|---|
| Time 1 | |||||
| 2 | 6211.67 | 6127.86 | 6149.44 | 0.0000 | .75 |
| 3 | 4537.80 | 6095.35 | 6123.67 | 0.01 | .80 |
| 4 | 4537.80 | 6077.32 | 6112.38 | 0.6 | .84 |
| Time 2 | |||||
| 2 | 6646.84 | 6766.84 | 6793.81 | 0.000 | .60 |
| 3 | 4165.36 | 6729.39 | 6764.46 | 0.000 | .73 |
Note. Likelihood Ratio G2 is – 2 times of loglikelihood value; Smaller Akaike’s information criterion (AIC) is preferable; Smaller Bayesian information criterion (BIC) is preferable; p < 0.05 in Bootstrap Likelihood Ratio Test indicates that at least the present number of classes exist in given data; A higher than 0.8 entropy value (i.e., the summary of the latent class probability for most likely latent class membership based on the estimated model) is preferred.
Table 3.
Subgroups of Pain and Fatigue Experience at Each Time Point
| (N = 276) | ||||
|---|---|---|---|---|
| Subgroups at Time 1 Mean (SD) | ||||
| Symptoms | HPHF (n = 114, 41.2 %) |
LPHF (n = 90, 34.3 %) |
LPLF (n = 68, 24.5 %) |
Wald tests for pairwise comparisonsa (df = 1) |
| Painb | 3.91(1.65) | −16.36(7.56) | −0.07 (2.11) | All p < .0001 |
| Fatigue | 7.08(1.52) | 6.26(1.59) | 3.30(0.96) | All p < .05 |
| Subgroups at Time 2 Mean (SD) | ||||
| Symptoms | HPHF (n = 101, 36.5%) |
LPLF n = 175, 63.5%) |
Wald test df = 1 |
|
| Painb | 4.48 (1.52) | −0.82 (2.99) | p < .0001 | |
| Fatigue | 6.59(1.96) | 5.27 (1.78) | p < .001 | |
Note:
Bonferroni correction was done for multiple comparisons;
Pain was censored and thus the value in this table does not carry clinical meaning but it shows relative distribution across groups;
HPHF = high pain and high fatigue subgroup; LPLF = low pain and low fatigue subgroup; LPHF = low pain and high fatigue subgroup.
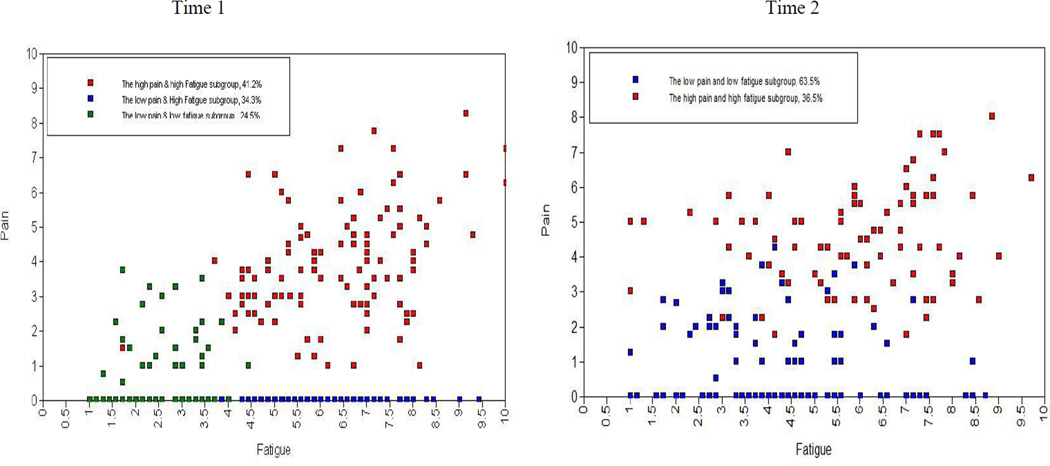
The left panel of Figure 2 is a scatterplot of raw scores of respondents’ pain and fatigue at Time 1. Although in analytic models, subgroup membership is latent—respondents are never assigned to subgroups—points in the scatterplot are color coded by most likely subgroup membership of each respondent, to show the approximate loci of each subgroup. Clinically, fatigue level was high in the HPHF subgroup; moderate in LPHF; and low in LPLF. Pain level was moderate in HPHF; low in LPLF; and was not measurably present in LPHF (see the left panel of Figure 2). Expected a posteriori classification based on the selected LPA model would have correctly classified 92% of HPHF, 93% of LPHF, and 88% of LPLF, corresponding to an entropy value of 0.80.
Figure 2.
Pain and Fatigue Subgroups Based on the Most Likely (expected a posteriori) Subgroup Membership of Each Respondent
Note: Intensity of each symptom is raw score and patients are classified based on the estimated model; The ranges of scores for fatigue and pain were 1–10; Higher scores indicated more intense symptoms.
At Time 2, a model with two subgroups was selected: HPHF (36.5%) and the LPLF (63.5%), graphically depicted in the right panel of Figure 2. Baseline model-testing statistics indicated the possibility of the three subgroups (see Table 2), appearing to be similar to the model at Time 1. However, the three-subgroup model was not selected, due to the failure of parameter estimation for several variables, and because the maximum log-likelihood value was not replicated, indicating a convergence issue. Expected a posteriori classification based on the selected LPA model would have correctly classified 89% of LPLF, and 84% of HPHF, corresponding to an entropy value of 0.60. Although the low-entropy value was concerning in model selection, this two-subgroup model was best replicated using the maximum log likelihood value and was selected as the best model for Time 2. In the two-subgroup model, pain and fatigue levels significantly differed, according to the Wald tests (all p < .001). HPHF had clinically moderate-to-high levels of pain and fatigue; LPLF had clinically low levels of pain and fatigue (see the right panel of Figure 2).
Influencing Variables of Subgroup Memberships
Multinomial logistic regression analyses were conducted with 10 variables at Time 1 and 13 at Time 2 (see Table 4 for odds ratios). The three additional variables at Time 2 were the time lapsed since the first CTX (i.e., 43–46 days vs. 57–60 days), symptom management intervention given by the parent study, and employment status. Employment status was included only because this variable had an interaction with intervention in the parent study. At Time 1, only depressed mood significantly influenced subgroup memberships. Patients with higher depressed mood were more likely to be in HPHF (odds = 57.89, p <.0001) or LPHF (odds = 67.22, p <.0001), rather than LPLF.
Table 4.
Odds Ratios and 95% Confidence Interval for Influencing Variables of Subgroup Membership
| Time 1(n = 276)a | Time 2 (n = 276) | |||
|---|---|---|---|---|
| Comparison versus reference | HPHF vs. LPLF | LPHF vs. LPLF | HPHF vs. LPHF | HPHF vs. LPLF |
| Site A | 6.57 (0.70, 61.33) | 9.04 (0.86, 94.64) | 0.73 (0.24, 2.22) | 4.44 (0.50, 39.81) |
| Site B | 7.69 (0.83, 71.14) | 7.38 (0.71,76.12) | 1.04 (0.30, 3.69) | 3.09 (0.25, 38.57) |
| Age | 0.77 (0.50, 1.16) | 0.83 (0.53,1.29) | 0.93 (0.69, 1.26) | 0.96 (0.58, 1.58) |
| Cancer type (breast vs. others) | 1.94 (0.47,8.11) | 4.78 (0.98, 23.24) | 0.41 (0.17,0.94) | 1.45 (0.37, 5.68) |
| Disease stage (stage I &II vs.III &IV) | 0.96 (0.48, 1.91) | 1.02 (0.48, 2.15) | 0.94 (0.63, 1.41) | 1.74 (0.96,3.16) |
| Comorbid-arthritis | 2.69 (0.59, 12.30) | 1.94 (0.28, 13.37) | 1.39 (0.44, 4.21) | 3.30 (0.45, 24.01) |
| Comorbid- hypertension | 0.72 (0.17,3.06) | 0.64 (0.11,3.62) | 1.13 (0.50, 2.60) | 5.29 (1.49, 18.84)* |
| Hemoglobin level | 0.92 (0.59,1.43) | 0.93 (0.57,1.51) | 0.99 (0.81, 1.21) | 0.87 (0.61, 1.23) |
| Previous surgery (yes vs.no) | 1.64 (0.40,6.61) | 2.89 (0.61,13.70) | 0.56 (0.24, 1.30) | 0.72 (0.14, 3.47) |
| Depressed mood | 57.89 (6.59, 509.09)*** | 67.22 (7.72, 585.13)*** | 0.86 (0.56, 1.33) | 4.33 (1.82, 10.26)** |
| Time lapse since the first CTX (43–46 vs. 57–60 day) | 0.14 (0.04, 0.47)** | |||
| Symptom managementb (yes vs.no) | 1.45 (0.52, 4.02) | |||
| Employment statusc (employed vs. un-employed) | 1.26 (0.40, 3.96) | |||
Note.
Bonferroni correction was done for multiple comparisons.
At Time 1, the intervention was not given and thus this variable was not entered in the model at Time 1.
Employment status interacted with the intervention effect on the secondary outcome of pain at Time 2 in the parent study and thus, this variable was included in the model at Time 2 model estimation;
HPHF = high pain and high fatigue subgroup; LPLF = low pain and low fatigue subgroup; LPHF = low pain and high fatigue subgroup;
p < .05.
p < .01.
p < .0001.
An odds ratio >1 would mean that the higher-coded category (or higher values in a continuous variable) was associated with the greater odds of being in a given subgroup as opposed to the reference subgroup. A p-value < .05 indicated that the effect of a variable was significant after controlling for the rest of the variables in a model.
At Time 2, depressed mood was the most important predictor, showing similar tendencies as observed at Time 1. Patients with shorter time-lapses since first treatment (43–46 days vs. 57–60 days) were less likely to be in HPHF (odd ratio = 0.14, p =.002). The comorbid conditions of hypertension and arthritis were included in the model because they were the most prevalent. Patients with comorbid hypertension were more likely to be in HPHF (odds ratio = 5.29, p =.01).
Subgroup Differences in Concurrently Measured Functional Limitation
Although the subgroup-mean levels of functional limitations (i.e., 5.36 vs. 4.33 vs. 1.49 at Time 1; 5.21 vs. 3.02 at Time 2) were moderate, statistically and clinically meaningful differences existed between subgroups (see Table 5). Patients in HPHF had the most serious functional limitations in both times (post hoc contrasts, all p < .01). At Time 1, functional limitation in LPHF was higher than in LPLF, but lower than in HPHF (post hoc contrasts, p < .01).
Table 5.
Differences in Functional Limitations by Subgroup at Each Time Point
| (N = 276) | ||||
|---|---|---|---|---|
| Time 1 Mean (SD) | ||||
| Total (n = 276) |
HPHF (n = 114, 41.2%) |
LPHF (n = 90, 34.3%)) |
LPLF (n = 68, 24.5%) |
Overall test X2 (df=2) = 137.39 (p < .0001) |
| Functional limitations | 5.36 (.22) | 4.33 (.36) | 1.49 (1.19) | All p < .01 (df = 1)a |
| Time 2 Mean (SD) | ||||
| Total (n = 276) |
HPHF (n = 101, 36.5%) |
LPLF (n = 175, 63.5%) |
X2 (df=1) = 39.05 (p < .0001) |
|
| Functional limitations | 5.21(0.28) | 3.02(0.19) | ||
Bonferroni correction was performed for multiple comparisons;
HPHF = high pain and high fatigue subgroup; LPLF = low pain and low fatigue subgroup; LPHF = low pain and high fatigue subgroup.
Discussion
LPA successfully identified subgroups with pain and fatigue that were replicable in a given sampling distribution. Two subgroups with the same direction of pain and fatigue intensity (HPHF and LPLF) are comparable to previous research findings on symptom clusters: pain and fatigue formed a symptom cluster in some cancer patients. HPHF had the most serious limitation in functional status. Thus, there is a clinical implication of a pain and fatigue cluster, supporting cumulative effects of multiple symptoms on functioning.12–14,32,33
In addition, experiencing both pain and fatigue at a high intensity was independent of most selected situational and physiological variables. A higher level of depressed mood increased the risk to be HPHF, independent of other variables; depressed mood may be a sentinel to detect subgroup with high pain and fatigue. Depressed mood did not differentiate between the two subgroups with high fatigue at Time 1, and is more closely associated with fatigue than pain.
LPHF was found only at Time 1. In the present study, we anticipated that patients with lower hemoglobin would fall into LPHF, but this was not supported. The effect of hemoglobin was inconclusive in this study due to limited variance and lack of control over the time lapsed since blood sampling. As shown in Figure 2, symptom patterns appear to be similar over two chemotherapy cycles with slightly more diversity at Time 2. However, LPHF was not separable from LPLF, possibly due to the very small number of subjects experiencing the LPHF pattern. Low pain and high fatigue experience may occur in patients with a particular condition during CTX, and the condition should be further examined.
Previous studies reported 15–30% of participants had high pain and low fatigue.12,13 It was anticipated that this subgroup would be associated with surgery, because surgery-site pain can occur without serious fatigue. However, the present study did not find this subgroup, and furthermore, did not find the surgery experience to influence the symptom experience. Considering that Time 1 was 4 days after the first CTX, both measurement time points consisted of long periods after surgery for most patients in this study. In Kim et al.,12 surgery experience predicted symptom experience only at the time point before CTX or radiation treatment. Given et al.16 reported that the pain and fatigue experience was influenced only when surgery was completed within 40 days. Surgery-related pain may occur without fatigue within a short period after surgery, may be different from pain with fatigue, and should be treated differently. Separating types of pain based on symptom experience may have important implications for symptom management.
At Time 2, having a longer time lapse from the first CTX or a comorbid condition of hypertension predicted a high-pain and high-fatigue experience. Although the reason is not yet clear, the symptom experience could have been affected by medications for hypertension, not hypertension per se, and by various events that occurred after the first CTX (e.g., medications), not the time lapse per se.
LPA was recently used to examine an association between cytokines and a symptom cluster of pain, fatigue, sleep disturbance, and depression in a sample of cancer patients and their family caregivers.34 Their findings are not comparable to the present study findings because their subgroups were characterized by depression level, not by pain and fatigue level, due to the nature of the sample: their sample included family caregivers who may not experience serious pain and/or fatigue. Nonetheless, this particular study shows applicability of LPA to investigate a possible mechanism of symptoms.
Limitations of this study include the use of many categorical variables, and cross-sectional analysis of longitudinal data (i.e., separate analysis at each time point). Having too many categorical variables hampered analyses due to zero variance in cells of multiple categorical variables. Including more continuous variables, if possible, is recommended for future study when working with a small sample size. Also, measurement time points for influencing variables should be carefully selected to yield a clear conclusion. This study sample included patients undergoing concurrent treatments of CTX and RT. However, inclusion of those subjects would not influence study findings because the number is too small (n = 18) and the measurement time point was set to capture the symptom experience after CTX treatment. Using the longitudinal nature of the data, we are currently examined the transition patterns of patients in pain and fatigue experience over two different time points.
Conclusion
This study provides evidence of the existence of subgroups with unique pain and fatigue experiences in diverse cancer patients during CTX. Patients with both pain and fatigue at higher intensities had the worst patient outcomes, and tended to experience more severe depressed mood. Clinicians need to pay special attention to this group of patients. Opposing directions of pain and fatigue experience appeared to exist only at a specific time point in the illness trajectory or with respect to patients with a particular cancer. Future studies should investigate the assessment/management strategies targeting pain or fatigue based on two main criteria: (a) the population where one can find a certain combination of pain and fatigue, and (b) the possible reasons behind unique pain and fatigue-symptom experience.
Acknowledgement
The primary study was supported by the National Institute of Nursing Research (R01NR04573)(USA). The present study was supported by the Catholic Medical Center Research Foundation made in the program of 2011(5-2012-b0001-00005) (South Korea) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1009672).
Footnotes
There is no conflict of interest for this work.
Contributor Information
Hee-Ju Kim, Catholic University of Korea, College of Nursing, Seoul, Korea..
Patrick S. Malone, Department of Psychology, University of South Carolina, SC, USA.
Andrea M. Barsevick, Department of Medical oncology, Thomas Jefferson University, Philadelphia, USA..
References
- 1.Oncology Nursing Society. 2009–2013 ONS research agenda. [Retrieved November 20, 2010];2009 Available from http://www.ons.org/media/ons/docs/research. [Google Scholar]
- 2.Plante GE, VanItallie TB. Opioids for cancer pain: the challenge of optimizing treatment. Metabolism. 2010;59(Suppl 1):S47–S52. doi: 10.1016/j.metabol.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Cella D, Davis K, Breitbart W, Curt G. Fatigue Coalition. Cancer-related fatigue. J Clin Oncol: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. 2001;19(14):3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 4.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 5.Torvinen S, Färkkilä N, Sintonen H, Saarto T, Roine RP, Taari K. Health-related quality of life in prostate cancer. Acta Oncol. 2013 doi: 10.3109/0284186X.2012.760848. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282. doi: 10.1097/00002820-200507000-00005. quiz 283-274. [DOI] [PubMed] [Google Scholar]
- 7.Chen ML, Lin CC. Cancer symptom clusters: a validation study. J Pain Symptom Manage. 2007;34(6):590–599. doi: 10.1016/j.jpainsymman.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Barsevick AM, Tulman L, McDermott PA. Treatment-related symptom clusters in breast cancer: a secondary analysis. J Pain Symptom Manage. 2008;36(5):468–479. doi: 10.1016/j.jpainsymman.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Wang SY, Tsai CM, Chen BC, Lin CH, Lin CC. Symptom clusters and relationships to symptom interference with daily life in Taiwanese lung cancer patient. J Pain Symptom Manage. 2008;35(3):258–266. doi: 10.1016/j.jpainsymman.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs. 2010;33(1):3–10. doi: 10.1097/NCC.0b013e3181b4367e. [DOI] [PubMed] [Google Scholar]
- 11.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14(8):831–836. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Barsevick AM, Beck SL, Dudley W. Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: a secondary analysis. Oncol Nurs Forum. 2012;39(1):E20–E30. doi: 10.1188/12.ONF.E20-E30. [DOI] [PubMed] [Google Scholar]
- 13.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 14.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35(2):162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kozachik SL, Bandeen-Roche K. Predictors of patterns of pain, fatigue, and insomnia during the first year after a cancer diagnosis in the elderly. Cancer Nurs. 2008;31(5):334–344. doi: 10.1097/01.NCC.0000305769.27227.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage. 2001;21(6):456–466. doi: 10.1016/s0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 17.Chung H, Flaherty BP, Schafer JL. Latent-class logistic regression. J R Stat Series A: Application to marijuana use and attitudes among high-school seniors. 2006;169:723–743. [Google Scholar]
- 18.Wade TD, Crosby RD, Martin NG. Use of latent profile analysis to identify eating disorder phenotypes in an adult Australian twin cohort. Arch Gen Psychiatry. 2006;63(12):1377–1384. doi: 10.1001/archpsyc.63.12.1377. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Abraham I, Malone PS. Analytical methods and issues for symptom cluster research in oncology. Curr Opin Support Palliat Care. 2013;7(1):45–53. doi: 10.1097/SPC.0b013e32835bf28b. [DOI] [PubMed] [Google Scholar]
- 20.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Struct Equ Modeling. 2007;14(4):671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Geinitz H, Zimmermann FB, Thamm R, Keller M, Busch R, Molls M. Fatigue in patients with adjuvant radiation therapy for breast cancer. J Cancer Res Clin Oncol: long-term follow-up. 2004;130(6):327–333. doi: 10.1007/s00432-003-0540-9. [DOI] [PubMed] [Google Scholar]
- 23.Barsevick A, Beck SL, Dudley WN, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40(2):200–216. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25(1):51–62. [PubMed] [Google Scholar]
- 25.Meek PM, Nail LM, Barsevick A, et al. Psychometric testing of fatigue instruments for use with cancer patients. Nurs Res. 2000;49(4):181–190. doi: 10.1097/00006199-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 27.McGuire DB, Kim HJ, Lang X. Measuring pain. In: Frank-stromborg M, Olsen SJ, editors. Instruments for Clinical Health-Care Research. 3rd ed. Sudbury Massachusetts: Jones and Bartlett Publishers; 2004. pp. 603–644. [Google Scholar]
- 28.Meek PNLJL. Internal consistency reliability and construct validity of a new measure of cancer treatment related fatigue: the General Fatigue Scale (GFS) Oncol Nurs Forum. 1997;24(2):334. [Google Scholar]
- 29.McNair DMLMDL. Profile of mood states. Revised ed. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 30.Akaike H. A new look at the statistical mode identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 31.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 32.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):202–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 33.Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Support Care Cancer. 2008;16(8):925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]