Abstract
Background
Concurrent chemotherapy plus radiation therapy (chemoRT) is the standard treatment for stage IIIA(N2) non-small cell lung cancer (NSCLC), a common disease entity. Phase II studies demonstrated feasibility of resection after chemoRT with encouraging survival rates. This phase III trial compared both approaches.
Methods
Patients with stage T1-3pN2M0 NSCLC were randomized before induction chemoRT (2 cycles of cisplatin and etoposide [PE] concurrent with 45 Gy RT). If no progression, arm 1 underwent resection, and arm 2 continued RT uninterrupted to 61 Gy. Two additional cycles of PE were given. The primary endpoint was overall survival (OS).
Findings
Progression-free survival for 396 eligible patients was superior in arm 1: median 12.8 versus 10.5 months, p=0.017, hazard ratio (HR) 0.77 (0.62,0.96); 5-yr 22.4% versus 11.1%. Median OS was 23.6 versus 22.2 months, p=0.24, HR 0.87 (0.70,1.10). Five-year survivals were arm 1, 27.2% and arm 2, 20.3%; odds ratio 0.63 (0.36,1.10, p=0.10). N0 status at thoracotomy predicted median OS of 33.5 months (5-year, 41.8%). Major chemoRT toxicities were neutropenia and esophagitis. Treatment-related death occurred in 16 (7.9%) patients on arm 1, of which 14 were post-pneumonectomy; and in 4 (2.1%) on arm 2. An exploratory analysis showed improved OS for patients who underwent lobectomy versus a matched cohort on chemoRT alone, but not for those undergoing pneumonectomy (matched similarly).
Interpretation
There was no significant survival advantage to surgery after chemoRT, despite improved PFS. Both chemoRT with definitive RT and chemoRT followed by resection (preferably lobectomy) are options for patients with stage IIIA(N2) NSCLC.
Historically, patients with stage IIIA non-small-cell lung carcinoma (NSCLC) with clinically-evident, ipsilateral mediastinal nodal metastases (N2) had poor outcomes following single modality treatment with either surgical resection or radiotherapy (RT). (1-4) Addition of chemotherapy to RT (chemoRT) significantly improved survival for this stage subset and is now considered standard care. (5-8) Subsequent phase III trials demonstrated superior survival with concurrent chemoRT compared with sequential administration. (9-11)
Phase II pilot studies were conducted that tested the role of surgical resection after induction therapy with either chemotherapy or chemoRT in order to optimize local control after systemic treatment. The results were provocative with long-term survival rates higher than anticipated. (11-13) However, these studies demonstrated significant toxicity, postoperative morbidity and mortality, and were criticized because patients enrolled on these trials had heterogeneous substages of disease and seemed unusually fit as compared to the general stage III population.
We designed a phase III trial (R9309, INT0139) in patients with uniform, pathologically-documented stage IIIA(pN2) NSCLC (confirmed ipsilateral mediastinal nodal metastases), based on two previous phase II studies conducted by the Southwest Oncology Group (SWOG). (14, 15) A standard concurrent chemoRT induction with the RT dose to 45 Gy, followed by surgical resection, was compared to continuing to 61 Gy without surgery. The objectives were to 1) assess whether resection resulted in a significant improvement in survival outcomes compared to chemoRT alone, 2) evaluate the toxicity in each arm, and 3) report patterns of local and distant disease recurrence.
METHODS
All patients provided informed consent after local institutional review board approval. This trial was funded by the National Cancer Institute with high priority designation, and administered by the Radiation Therapy Oncology Group (RTOG, R9309), with participation by SWOG, National Cancer Institute of Canada Clinical Trials Group, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B and North Central Cancer Treatment Group. RTOG was responsible for data collection, analysis and publication.
Eligibility Criteria
Staging included a computed tomographic (CT) scan of the chest, liver and adrenal glands; bone scan; and CT or MRI of the brain. The International Staging System for lung cancer was employed. (1) All patients had stage IIIA(pN2) disease: T1, T2 or T3 primary NSCLC was required with pathologic proof of N2 involvement (via biopsy of ipsilateral mediastinal nodes visible on radiographs by any of a number of protocol-specified standard procedures). If contralateral mediastinal nodes larger than 1 cm were visible on the CT scan, biopsy was required to exclude N3 (stage IIIB) disease.
Patients were evaluated by a thoracic surgeon, radiation oncologist and medical oncologist (each approved to participate through a centralized questionnaire process) to establish that 1) N2 disease was present to the extent that chemoRT was considered the standard approach, not upfront resection; and 2) the disease was potentially technically resectable. Pulmonary function criteria were mandated (predicted post-resection FEV1 of at least 800 cc on quantitative perfusion scan if FEV1 overall was less than 2000 cc; standard formula specified in protocol). As needed, pulmonary medicine specialists were also consulted to confirm pulmonary fitness for a potential resection. The Karnofsky performance status (KPS) was either 90 or 100; or, if 70 or 80, the albumin of was least .85X normal with <10% weight loss within the previous 3 months.
Study Design
Eligible patients were stratified by primary T designation (T1 vs T2 vs T3), KPS (90 or 100 vs 70 or 80) and contralateral mediastinal nodal sampling (yes vs no). Randomization was either to arm 1, induction chemoRT followed by surgery; or arm 2, the same induction chemoRT with completion of definitive-dose RT. The induction chemoRT was cisplatin (50 mg/m2 days 1, 8, 29, 36), and etoposide (50 mg/m2 days 1-5 and 29-33), plus 45 Gy thoracic RT beginning day 1, in 1.8 Gy daily fractions. Disease re-evaluation by CT scan plus repeat pulmonary function tests was done 2-4 weeks after completion of RT in arm 1, and 7 days before completion of induction chemoRT in arm 2. If there was no disease progression and the patient remained medically fit, a complete surgical resection (with protocol-specified mediastinal lymph node sampling/dissection) was performed 3-5 weeks after completion of RT in arm 1, or the RT was continued to 61 Gy without interruption in arm 2. Patients received 2 cycles of consolidation chemotherapy (same doses and schedule as during induction). Dose reduction guidelines were specified for chemoRT, with central quality control.
A chest CT scan was scheduled 4-6 weeks after completion of the last chemotherapy cycle. Patients were followed every 2 months for 1 year, every 3 months for 2 years, then every 6 months indefinitely. CT scans of the thorax and upper abdomen and MRI or CT of the brain were done at 12, 18, and 24 months and annually thereafter.
Statistical Considerations
Un-blinded treatment arm assignment used Zelen's randomized permuted block within strata. (16) Intent-to-treat analyses used only eligible patients per RTOG policy. The primary endpoint was overall survival (OS), defined as time from randomization to death by any cause. Secondary endpoints were progression-free survival (PFS), defined as time from randomization to disease progression, secondary primary, or death by any cause; toxicity; and patterns of failure.
The sample size was calculated using a non-stationary Markov process to model survival using the Lakatos method (17), assuming a one-sided log-rank test with a Type I error rate of 0.05 and 93% statistical power, and minimum follow-up of 2.5 years. Only two-sided p-values are reported. Two interim analyses were specified after 33% and 67% of patients were followed for at least 2.5 years.
The target sample size was 612 patients (556 eligible) to observe 507 deaths to detect 10% absolute improvement in the surgical arm, assuming 25% 2-year OS with chemoRT. This was recalculated upon recommendation of the Data Safety and Monitoring Committee (DSMC), based on slower accrual than projected and updated survival rates from the two phase II trials that represented each arm. (14, 15) The revised sample size was 510 patients (484 eligible).
OS and PFS were analyzed by log-rank test and multivariate analyses used the Cox proportional hazards model. (18) The adjusted alpha at final analysis was 0.0487. Only the unadjusted estimates and confidence intervals are reported here, as the largest difference (between unadjusted and adjusted) was 0.002 for HRs and 0.04 for OS and PFS rates. All figures display Kaplan-Meier estimates. (19) Stepwise selection was used in Cox modeling, including sex, weight loss (<5 kg or ≥5 kg), number of positive nodal stations (1 or 2-3), T-stage (T1 vs T2 vs T3), histology (non-squamous vs squamous), age (≤ 60 vs > 60 years old), KPS (90 or 100 vs 70 or 80), and LDH (normal vs abnormal). Exploratory logistic regression for factors associated with 5-year survival was conducted.
An unplanned, exploratory OS analysis was added for hypothesis-generation, prompted by unexpectedly high postoperative mortality rates. Patients in arm 1 who underwent pneumonectomy were matched one-to-one to arm 2 patients based on age (+/− 5 years), sex, KPS (70 or 80 and 90 or 100), and clinical T-stage (exact match). Patients in arm 1 who had a lobectomy were matched one-to-one on the same characteristics to patients on arm 2.
RESULTS
Study Conduct and Reporting
Accrual occurred from March, 1994 through November, 2001. Due to the extended accrual period resulting in sufficient events, the DSMC (privy to the survival curves by arm) recommended closure at 429 randomized patients. A PFS analysis and initial OS were previously presented, with a subsequent update. (20, 21) Definitive estimates are now available for all endpoints, with median follow-up for all patients of 22.5 months (range: 0.9-125.1 months) and 69.3 months (range: 6.2-125.1 months) for patients still alive.
Study Population and Treatment Delivered
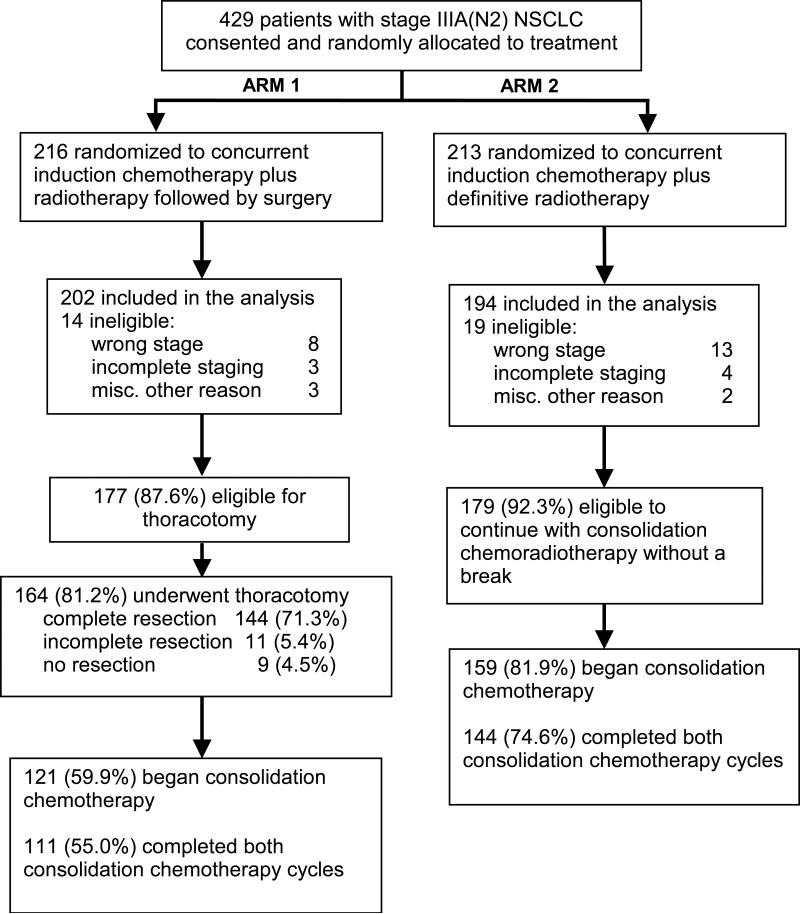
Of 429 patients randomized (Figure 1), 396 patients (92%) were eligible (202, arm 1 and 194, arm 2). The primary reasons for ineligibility were wrong stage or incomplete staging on central review. The ineligibility rate and reasons did not differ significantly by arm. Patient and tumor characteristics were well balanced across the treatment groups (Table 1).
Figure 1.
Study R9309 (INT0139) design, consort diagram and treatment delivered. NSCLC, non-small cell lung carcinoma
Table 1.
Pretreatment Characteristics of Eligible Patients
| Arm 1 CT/RT/S (n=202) | Arm 2 CT/RT (n=194) | Total (n=396) | |
|---|---|---|---|
| Age | |||
| Median (years) | 59 | 61 | 60 |
| Range (years) | 31-77 | 32-78 | 31-78 |
| ≤ 60 | 113 (55.9%) | 95 (49.0%) | 208 (52.5%) |
| > 60* | 89 (44.1%) | 99 (51.0%) | 188 (47.5%) |
| Sex | |||
| Male | 131 (64.9%) | 121 (62.4%) | 252 (63.6%) |
| Female | 71 (35.1%) | 73 (37.6%) | 144 (36.4%) |
| Karnofsky Performance Status | |||
| 70-80 | 23 (11.4%) | 25(12.9%) | 48 (12.1%) |
| 90-100 | 179 (88.6%) | 169 (87.1%) | 348 (87.9%) |
| Estimated weight loss in previous 6 months | |||
| <5 kg | 154 (76.2%) | 146 (75.3%) | 299 (75.5%) |
| 5-10 kg | 36 (17.8%) | 30 (15.5%) | 67 (16.9%) |
| >10 kg | 7 (3.5%) | 10 (5.2%) | 17 (4.3%) |
| Unknown | 5 (2.5%) | 8 (4.1%) | 13 (3.3%) |
| LDH | |||
| Normal | 148 (73.3%) | 147 (75.8%) | 295(74.5%) |
| Abnormal | 39 (19.3%) | 33 (17.0%) | 72 (18.2%) |
| Not done | 15 (7.4%) | 14 (7.2%) | 29 (7.3%) |
| Histology | |||
| Squamous cell carcinoma | 64 (31.7%) | 65 (33.5%) | 129 (32.6%) |
| Adenocarcinoma | 79 (39.1%) | 82 (42.3%) | 161 (40.7%) |
| Large cell | 29 (14.4%) | 24 (12.4%) | 53 (13.4%) |
| Mixed/other NSCLC | 30 (14.9%) | 23 (11.9%) | 53 (13.4%) |
| T-stage | |||
| T1 | 50 (24.8%) | 47 (24.2%) | 97 (24.5%) |
| T2 | 130 (64.4%) | 121 (62.4%) | 251 (63.4%) |
| T3 | 22 (10.9%) | 26 (13.4%) | 48 (12.1%) |
| Number of positive nodal stations reported ** | |||
| 1 | 153 (75.7%) | 146 (75.3%) | 299 (75.5%) |
| 2 | 39 (19.3%) | 39 (20.1%) | 78 (19.7%) |
| 3 | 4 (2.0%) | 4 (2.1%) | 8 (2.0%) |
| Unknown | 6 (3.0%) | 5 (2.6%) | 11 (2.8%) |
63 (15.9%) of patients were ≥ 70 years
Not all patients had mediastinoscopy, since method of documentation of N2 disease was at the discretion of the investigator
Upon completion of induction chemoRT and re-evaluation, 177 patients (87.6%) in arm 1 were eligible for surgery, and 164 (81.2%) underwent thoracotomy (Figure 1). A complete resection was accomplished in 144 patients (71.3%), incomplete resection in 11 (5.4%) and no resection in 9 (4.5%). Of the 155 resections, there were 3 wedge resections, 98 lobectomies and 54 pneumonectomies (29, right; 25, left). Consolidation chemotherapy was started as specified in 121 (59.9%) patients. At the reevaluation point during the last week of induction chemoRT in arm 2, 179 (92.3%) patients were eligible to continue consolidation chemoRT without a break, and 155 (79.9%) began consolidation chemotherapy per protocol guidelines (Figure 1).
There was no significant difference between arms in the amount of chemotherapy delivered per protocol during induction chemoRT (arm 1, 95.0%; arm 2, 91.7%). Consolidation chemotherapy was completed in 111 (55.0%) on arm 1 and 144 (74.6%) on arm 2, p<0.0001 (Figure 1). RT was delivered per protocol or with acceptable variation in 193 (95.5%), arm 1; and 154 (79.4%), arm 2, p<0.0001.
Morbidity and Treatment-Related Mortality
Toxicities are summarized in Table 2. The most common grade 3 or 4 toxicity was neutropenia, which occurred in 77 (38.1%) patients on arm 1 and 80 (41.2%) on arm 2. Infections were infrequent. Grade 3/4 esophagitis was reported in 20 (9.9%) patients on arm 1 and 44 (22.7%) on arm 2 (p=0.0006). Pneumonitis or other grade 3/4 respiratory complications occurred in 18 (8.9%) patients on arm 1 and 28 (14.4%) on arm 2 (p=NS). Grade 3/4 nausea and/or emesis was reported in 29 (14.4%) patients on arm 1 and 26 (13.4%) on arm 2 (p=NS). There was no difference between arms in grade ≥3 toxicity during induction chemoRT, whereas hematologic toxicity was greater in arm 2 during consolidation chemotherapy (56% versus 36%).
Table 2.
Overall Worst Toxicities at Any Time
| Toxicity | Arm 1 (n = 202) | Arm 2 (n = 194) | ||||
|---|---|---|---|---|---|---|
| Grade | Grade | |||||
| 3 | 4 | 5* | 3 | 4 | 5* | |
| Leukopenia | 82 | 15 | 0 | 76 | 31 | 0 |
| Neutropenia | 54 | 23 | 0 | 47 | 33 | 0 |
| Anemia | 25 | 1 | 0 | 42 | 5 | 0 |
| Thrombocytopenia | 10 | 4 | 0 | 12 | 11 | 0 |
| Worst hematologic toxicity per patient | 89 | 28 | 0 | 75 | 50 | 0 |
| Nausea and/or emesis | 17 | 3 | 0 | 37 | 7 | 0 |
| Neuropathy | 10 | 0 | 0 | 4 | 3 | 0 |
| Esophagitis | 17 | 3 | 0 | 37 | 7 | 0 |
| Stomatitis and/or mucositis | 6 | 0 | 0 | 4 | 1 | 0 |
| Pulmonary* | 17 | 1 | 13 | 24 | 4 | 3 |
| Other gastrointestinal or renal | 6 | 4 | 0 | 5 | 2 | 0 |
| Cardiac | 4 | 3 | 3** | 7 | 2 | 0 |
| Miscellaneous infection | 5 | 1 | 0 | 8 | 0 | 0 |
| Hemorrhage | 0 | 0 | 1 | 0 | 1 | 0 |
| Fatigue | 11 | 0 | 0 | 9 | 0 | 0 |
| Anorexia | 3 | 0 | 0 | 4 | 3 | 0 |
| Allergy | 1 | 0 | 0 | 3 | 0 | 0 |
See text for details regarding grade 5 events
One patient also included in grade 5 pulmonary
There were no treatment-related deaths during induction chemoRT on either arm. Subsequently, 16 (7.9%) patients died of causes not due to cancer on arm 1, 10 of which occurred within 30 days of thoracotomy. Of these 16 deaths, 14 were after pneumonectomy, 1 followed lobectomy, and 1 occurred in a patient who did not undergo thoracotomy. Causes were ARDS, 9; other respiratory, 4; cardiac, 2; hemorrhage, 1. There were 4 (2.1%) patients on arm 2 who died of treatment-related causes (3, non-ARDS respiratory; 1, other) during or after consolidation chemoRT.
Progression-Free Survival and Overall Survival
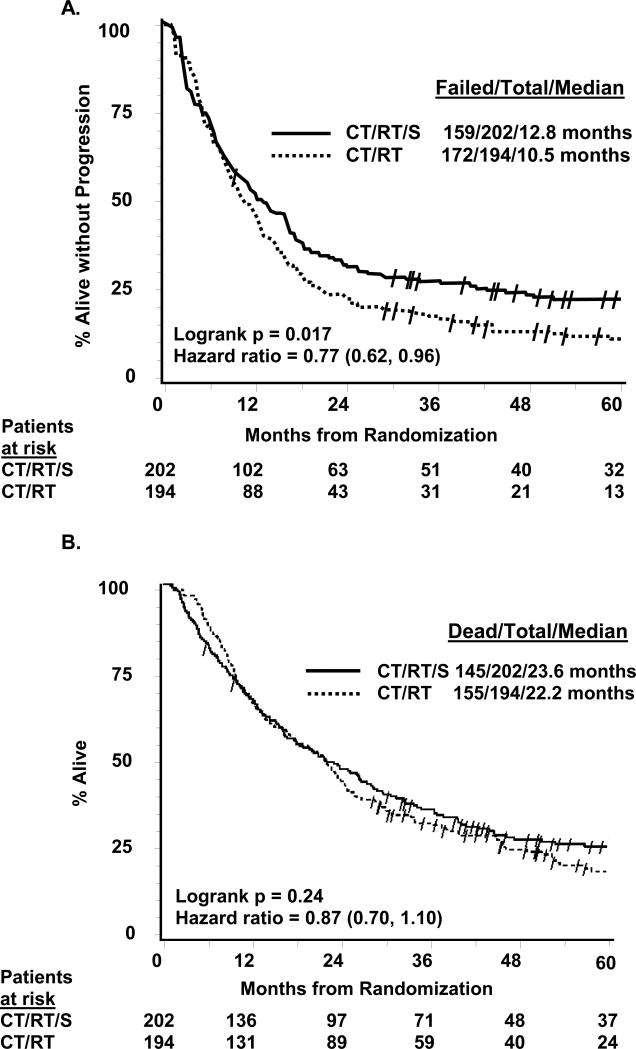
PFS was significantly prolonged in arm 1 vs arm 2 (Figure 2A): median 12.8 vs 10.5 months, logrank p=0.017, hazard ratio 0.77 (95% CI 0.62,0.96). The 5-year PFS (+/− 95% CI) was 22.4% (6) on arm 1 vs 11.1% (5) on arm 2. OS (Figure 2B) was not significantly improved in arm 1 vs arm 2: median 23.6 vs 22.2 months, logrank p=0.24, hazard ratio 0.87 (95% CI 0.70, 1.10). There were more patients on arm 1 alive without progression (21.3% vs 11.3%, p=0.008), but more died without progression on arm 1 (17.8% vs 9.8%, p=0.02). By year 5, there was a 7% absolute difference favoring the surgical arm, with 27.2% (6) alive vs 20.3% (6) and an odds ratio of 0.63 (0.36,1.10). No other factor predicted 5-year survival by logistic regression.
Figure 2.
Panel A. Progression-free survival (intent-to-treat). Panel B. Overall survival (intent-to-treat). Slash marks represent censored observations. CT/RT/S, chemotherapy plus radiotherapy followed by surgery (arm 1); CT/RT, chemotherapy plus radiotherapy (arm 2)
The Cox OS model found several independent predictors of outcome, including absence of major weight loss (p=0.003), female sex (0.009), and one N2 nodal station positive at diagnosis vs more (p=0.024). Treatment arm, age, KPS, T stage, LDH, and histology were not retained in the model. Because different factors determined whether a pneumonectomy or lobectomy was chosen, a survival comparison of the pneumonectomy vs lobectomy cohorts was not conducted.
Pathology Findings and Patterns of Disease Recurrence
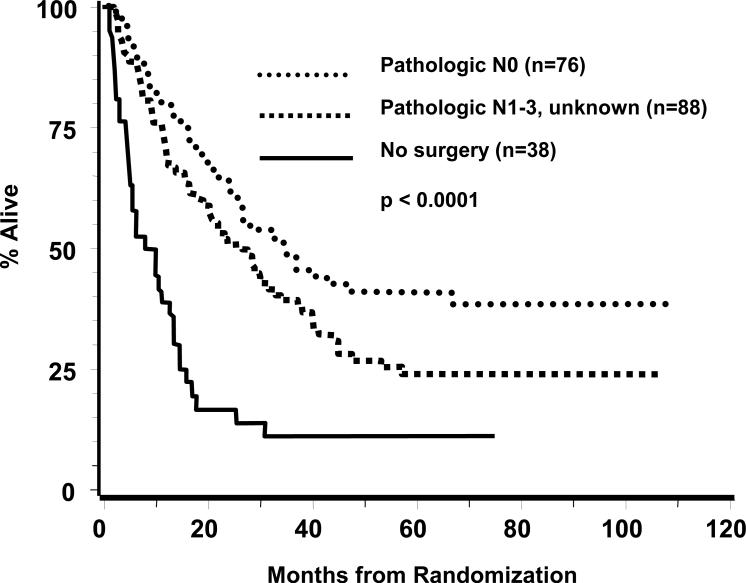
The post-induction pathology findings in arm 1 by T and N category were tabulated according to percent of the 164 thoracotomies performed as well as percent of the total patient enrollment of 202, respectively, for each category. These were T0N0, 29 (17.7, 14.4); T1N0, 31 (18.9%, 15.3%), T2-4N0, 16 (9.8%, 7.9%); N1-3, 85 (51.8%, 42.1%); unknown, 3 (1.8%, 1.5%). Of the 29 T0N0 specimens, a pneumonectomy had been performed in 13 (44%). Figure 3 depicts OS by grouping pathologic stage according to N status. Median and 5-year survivals were T(any)N0, 34.4 months and 41.0%; T(any)N1-3 or unknown, 26.4 months and 23.8%; and no surgical resection, 7.9 months and 8.3% (p<0.0001). The T0N0 subset had a median survival of 39.8 months and 5-year survival of 41.9%. Post-induction pathologic categories are unknown in arm 2, thus comparisons between arms are not feasible within TN subsets.
Figure 3.
Overall survival within arm 1 by pathologic substage determined following thoracotomy.
There were no differences in sites of first progression except there were fewer local-only relapses in arm 1 (10.3%) than arm 2 (22.2%). These occurred at the primary tumor site only (2.5% vs 14.4%); hilar, mediastinal or supraclavicular nodes only (6.9% vs 3.1%), and both (1.0% vs 4.6%). The brain was the sole initial site of relapse in 11.4% vs 14.9%. Other distant sites of recurrence occurred in 37.1% vs 41.8%, respectively.
Unplanned, Exploratory Matching Analysis
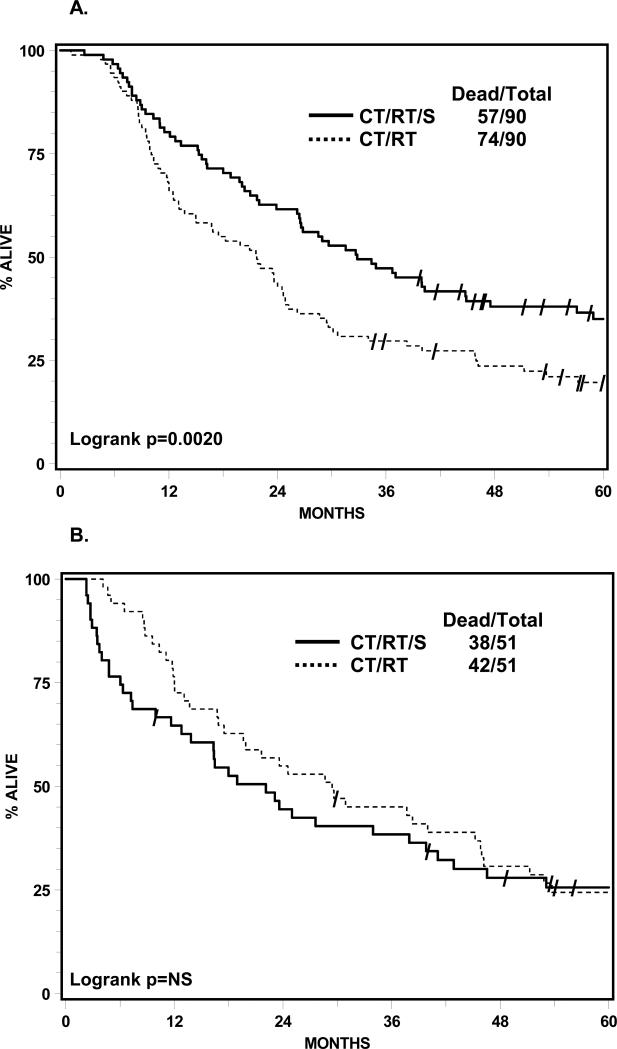
The OS matching analysis on four prestudy factors for arm 1 against arm 2 subsets was feasible for 90 of the 98 lobectomies and 51 of the 54 pneumonectomies. OS was significantly improved on the surgical arm if a lobectomy was performed compared to the OS of the matched cohort in the chemoRT arm (Figure 4A). Median survivals were 33.6 vs 21.7 months, logrank p=0.002, with 5-year survivals of 36.1% vs 17.8%. There was a nonsignificant trend toward worse OS in arm 1 for the pneumonectomy group vs the OS for the matched cohort in arm 2 (Figure 4B). Median survivals were 18.9 vs 29.4 months, 3-year 36.3% vs 45.0%, and 5-year 21.9% vs 23.6%.
Figure 4.
Panel A. Lobectomy subset from arm 1 vs matched cohort in arm 2, overall survival (intent-to-treat). Panel B. Pneumonectomy subset from arm 1 vs matched cohort in arm 2, overall survival (intent-to-treat). Slash marks represent censored observations. CT/RT/S, chemotherapy plus radiotherapy followed by surgery (arm 1); CT/RT, chemotherapy plus radiotherapy (arm 2)
DISCUSSION
This goal of this landmark phase III study was to evaluate the potential benefit of surgical resection after chemoRT for a common disease entity, stage IIIA(N2) NSCLC. These patients were judged fit for a rigorous treatment regimen and had disease for which chemoRT alone was deemed standard therapy, yet was technically resectable. This population stands in marked contrast to another group of patients with less extensive N2 disease who were included in the randomized trials of induction chemotherapy followed by surgery against a surgical-only control arm. (12, 13, 22-24) The results of our study demonstrating no statistical improvement in the primary OS endpoint, despite significantly prolonged PFS, for patients who underwent trimodality therapy with chemoRT followed by surgical resection have broad clinical implications.
Both treatment regimens employed in this trial resulted in median and 5-year survivals better than expected from phase II data in stage IIIA(N2) NSCLC. (11,12) Although OS was not improved by the addition of surgery, there was a trend for increased 5-year survival after trimodality therapy. Potential reasons for lack of OS benefit despite significant prolongation in PFS include inadequate power and less delivery of cycles 3 and 4 of chemotherapy in the surgical arm. However, it is unclear whether this additional chemotherapy has any value, based on randomized data in the non-surgical setting.
The lack of OS benefit by surgery in our trial may relate in large part to the high postoperative death rate following pneumonectomy, predominantly due to ARDS and other respiratory causes. This observation led to the exploratory matching analysis, which generated the hypothesis that trimodality therapy may be beneficial if a complete resection by lobectomy can be performed after chemoRT or if mortality from pneumonectomy can be avoided. There are obvious limitations to this type of analysis, so it should not be used as the sole basis to select therapy. The necessity for pneumonectomy in many patients was probably related to other adverse prognostic factors. However, it is revealing that 44% of the pT0N0 specimens were resected via pneumonectomy. Thus, this exploratory analysis could be useful as an adjunct in decision-making, to raise a caution flag when considering a trimodality prescription with pneumonectomy.
Our trial is unique in posing the question of surgical resection in stage IIIA(N2) NSCLC after induction chemoRT compared to the present standard of concurrent chemoRT in a population in which up-front surgery cannot be recommended. Although the EORTC conducted a phase III trial in stage IIIA(N2) NSCLC that randomized patients to surgery or RT after response to induction chemotherapy (25), the study differed from ours in several important ways. The EORTC control arm of chemotherapy followed by RT is no longer considered standard (9-11), and the fate of the entire denominator of patients is not known (only the responding patients were randomized), in contrast to the upfront randomization in our trial. Similar to our experience, accrual was protracted because of the difficult randomization and no OS benefit was observed. The median and 5-year survivals in both arms of the EORTC study were inferior to the present study, although this could be explained in part by different entry criteria between the two trials.
The North America Intergroup study provides the first broad application of both the concurrent chemoRT control and trimodality approaches deemed promising in phase II studies (12-15), to allow comparison of treatment delivery and toxicity profiles between arms. The induction chemoRT was well tolerated in both arms, with excellent treatment compliance. Treatment-related mortality due to respiratory causes was worse in the surgical arm and fewer patients could complete consolidation chemotherapy after surgery. However, patients enrolled on the chemoRT arm had a greater rate of severe esophagitis and other toxicities during consolidation chemoRT and were less likely to complete the prescribed RT.
Given the prolonged accrual period needed to complete trials that address whether there is benefit to inclusion of surgery versus a non-surgical approach, it is highly unlikely that a prospective trial will be done to validate the hypothesis raised by our exploratory analysis (that trimodality approaches are superior if lobectomies can be performed). Thus, medically fit patients with stage IIIA(N2) NSCLC deserve evaluation by a team with expertise in multimodality therapy, during which treatment options can be considered. Based on this study, patients should be counseled regarding risks versus potential benefits of both definitive chemoRT alone as well as a surgical resection (preferably by lobectomy) after chemoRT.
ACKNOWLEDGEMENTS
The authors thank the additional North American Intergroup discipline chairs and others who provided advice or assistance during the design or conduct of this study: Roger Byhardt, MD, Yvon Courmier, MD, Claude Deschamps, MD, Bahman Emami, MD, David Ettinger, MD, Mark Krasna, MD, Randolph Marks, MD, Thomas Rice, MD, Stephen Seagren, MD, and Henry Wagner, MD. Also, we thank the membership of the Lung Cancer Committees of the Southwest Oncology Group, National Cancer Institute of Canada Clinical Trials Group, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B, and the North Central Cancer Treatment Group for their support of this study over the long accrual period. We especially acknowledge the lung cancer survivors who were treated and followed on this protocol and the lay advocates who supported this trial design.
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA21661, CA37422, CA32115, CA46441, CA77202, CA49957, CA03927, CA25224; Canadian Cancer Society NCIC10362.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS AND CONFLICT OF INTEREST
The corresponding author (KSA) as the principal investigator participated in all phases of this study, including design and writing of the protocol, study and data/toxicity monitoring, analysis and manuscript preparation. The co-principal investigators (VRR and ATT) reviewed the final protocol and participated on ongoing data analyses for thoracic surgery and radiation oncology components of the trial, as well as data analysis. The study biostatistician (RSS) conducted all the analyses. Coauthors reviewed the manuscript contents and approved submission. There are no relevant conflicts of interest for any author.
REFERENCES
- 1.Mountain CF. Prognostic implications of the International Staging System for lung cancer. Semin Oncol. 1988;3:236–245. [PubMed] [Google Scholar]
- 2.Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Amer. 1987;67:1037–49. doi: 10.1016/s0039-6109(16)44341-0. [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DH, Einhorn LH, Bartolucci A, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non-small cell lung cancer. Annals Int Med. 1990;113:33–38. doi: 10.7326/0003-4819-113-1-33. [DOI] [PubMed] [Google Scholar]
- 5.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell-lung cancer. N Engl J Med. 1990;323:940–5. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 6.Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87:198–205. doi: 10.1093/jnci/87.3.198. [DOI] [PubMed] [Google Scholar]
- 7.LeChevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in non-resectable non-small cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991:417–423. doi: 10.1093/jnci/83.6.417. 1991. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer – a meta-analysis. Ann Intern Med. 1996;125:723. doi: 10.7326/0003-4819-125-9-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 10.Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential versus concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer: RTOG 9410. Proc Amer Soc Clin Oncol. 2003;22:621. [Google Scholar]
- 11.Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:3257–69. doi: 10.1200/JCO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Eberhardt WE, Albain KS, Pass H, Putnam JB, Gregor A, Assamura H, Mornex F, Senan S, Belderbos J, Westheel V, Thomas M, Van Schill P, Vansteenkiste J, Manegold C, Miramanoff RO, Pignon J, Roccmans P, Shepherd FA. Induction treatment before surgery for non-small cell lung cancer: IASLC consensus report. Lung Cancer. 2003;42:S9–14. doi: 10.1016/s0169-5002(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 13.Pisters K, Mirkovik N, Pass H, Albain KS. Lung Cancer: Principles and Practice. Third Edition JB Lippincott Williams and Wilkins; 2005. Preoperative chemotherapy/radiation therapy for early stage and locally advanced non-small cell lung carcinoma. pp. 626–649. [Google Scholar]
- 14.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR, Miller TP, Taylor SA, Stelzer KJ, Beasley KR, Livingston RB. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA(N2) and IIIB non-small cell lung cancer: Mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–92. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 15.Albain KS, Crowley JJ, Turrisi AT, Gandara DR, Farrar WB, Clark JI, Beasley KR, Livingston RB. Concurrent cisplatin, etoposide and chest radiotherapy in pathologic stage IIIB non-small cell lung cancer: a Southwest Oncology Group Study (SWOG-9019). J Clin Oncology. 2002;20:3454–60. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 16.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 17.Lakatos E. Sample size based on the long-rank statistic in complex clinical trials. Biometrics. 1988;44:229–241. [PubMed] [Google Scholar]
- 18.Cox D. Regression models and life-tables (with discussion). (Series B).J Royal Statist Soc. 1972;34:187–220. [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–81. [Google Scholar]
- 20.Albain KS, Scott CB, Rusch VR, Turrisi AT, Shepherd FA, Smith C, Gandara DR, Johnson DH, Green MR, Miller RC, for RTOG, SWOG, NCIC-CTG, ECOG, CALGB. NCCTG Phase III comparison of concurrent chemotherapy plus radiotherapy (CT/RT) and CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer: initial results from intergroup trial 0139 (RTOG 93-09). Proc Amer Soc Clin Oncol. 2003;22:621. [Google Scholar]
- 21.Albain KS, Swann RS, Rusch VR, Turrisi AT, Shepherd FA, Smith CJ, Gandara DR, Johnson DH, Green MR, Miller RC. Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) vs CT/RT followed by surgical resection for stage IIIA(pN2) non-small cell lung cancer: Outcomes update of North American Intergroup 0139 (RTOG-9309). J Clin Oncol. 2005;23:624s. [Google Scholar]
- 22.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing preoperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small cell lung cancer. J Natl Cancer Inst. 1994;86:673–630. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 23.Rosell R, Gomez-Codina J, camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 24.Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20:247–253. doi: 10.1200/JCO.2002.20.1.247. [DOI] [PubMed] [Google Scholar]
- 25.van Meerbeeck JP, Kramer GWPM, Van Schil PEY, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]