Abstract
Events that disrupt the early development of the nervous system have lifelong, irreversible behavioral consequences. The environmental contaminant, methylmercury (MeHg), impairs neural development with effects that are manifested well into adulthood and even into aging. Noting the sensitivity of the developing brain to MeHg, the current review advances an argument that one outcome of early MeHg exposure is a distortion in the processing of reinforcing consequences that results in impaired choice, poor inhibition of prepotent responding, and perseveration on discrimination reversals (in the absence of alteration of extradimensional shifts). Neurochemical correlates include increased sensitivity to dopamine agonists and decreased sensitivity to gamma-aminobutyric acid (GABA) agonists. This leads to a hypothesis that the prefrontal cortex or dopamine neurotransmission is especially sensitive to even subtle gestational MeHg exposure and suggests that public health assessments of MeHg based on intellectual performance may underestimate the impact of MeHg in public health. Finally, those interested in modeling neural development may benefit from MeHg as an experimental model.
Keywords: methylmercury, perseveration, dopamine, development, aging, executive functions, discrimination reversal, choice
Nervous system development requires a delicately balanced chemical environment, one that can be disrupted by exogenous influences such as drugs or environmental contaminants. Drug exposure is voluntary in a sense, so its epidemiological pattern is restricted to intentional users. In contrast, individuals have little control over exposure to environmental contaminants when they are found in the air or the food or water supply. Lead presents a well-studied example. Because of its presence in the air and drinking water, everybody was exposed to lead in the 20th century, many at levels that, by current standards, were quit high. A striking pattern has been noted, in which an increase and then decrease in intellectual disabilities and violent crime tracked the rise and fall in environmental lead, respectively (Nevin, 2009; Reyes, 2007). The story of lead’s addition and removal from the environment had been hailed as one of the most important Public Health successes of the 20th century (Domestic Public Health Achievements Team, 2011), and its removal was supported by its reduction in its behavioral effects (Davis et al., 1993; Gilbert and Weiss, 2006; Schwartz, 1994).
Methylmercury (MeHg), like lead, is a ubiquitous neurotoxicant. Everybody who eats fish is exposed to MeHg (Mahaffey, 2004). It is found in the highest concentration in large, long-lived predatory fish, including several marine species and fresh-water fish from contaminated water bodies. The broader lessons learned from lead apply to MeHg, even if the pattern of deficits is quite different. Effects of low-level exposure can be detected only with the appropriate behavioral (in animals) or psychometric (in humans) evaluations, but otherwise the effects will rarely appear as overt morphological changes. Because of long half-lives of elimination from the brain, there is accumulation with continued exposure, the most common scenario. The consequent nervous system damage has behavioral manifestations that are often silent, significantly delayed, and irreversible (Rice, 1996; Weiss et al., 2002; Weiss and Reuhl, 1994). The darkest lesson, which came from Japanese experience with MeHg (Smith and Smith, 1975), is that the developing nervous system is especially vulnerable (Harada, 1995; Harada, 1968).
In the present review, we argue that MeHg exposure during rodent gestation, a developmental period that approximately models human neural development during the first two trimesters of gestation (Bayer et al., 1993; Rice and Barone, 2000), has long-lasting behavioral consequences that appear in adulthood and, in some cases, may not appear until aging. Such exposure produces a behavioral rigidity that appears as perseveration in discrimination reversals, disrupts the acquisition of choice as environmental demands change, and can be linked to, and may be caused by, an elevated impact of reinforcing consequences. Neural correlates include increased sensitivity to dopamine agonists, diminished sensitivity to inhibitory gamma-aminobutyric acid (GABA) agonists, and raise the hypothesis that the prefrontal cortex is especially sensitive to this developmental neurotoxicant. Since such a pattern of behavior change is only weakly correlated with changes in IQ scores, these effects suggests that a reliance on tests of academic achievement or IQ as a measure of altered development may underestimate MeHg’s developmental neurotoxicity. From a scientific perspective, these data suggest that MeHg may provide an excellent experimental model by which early cortical developmental might be studied.
1 Why Study Methylmercury in the Laboratory?
Fish are the sole source of MeHg exposure, and this presents a significant public health dilemma because of the well-known nutritional benefits of fish consumption (Oken et al., 2012; Ström, Helmfrid, Glynn, & Berglund, 2011). There is an extensive literature comparing adverse effects of MeHg with nutritional benefits, and the common conclusion is that large, long-lived predatory fish should be avoided (Mahaffey et al., 2011; Oken et al., 2012; Ström et al., 2011). Many of citations here represent studies of human populations, some of heavy fish consumers explicitly and others of broader populations. These investigations are important but they are necessarily correlational, and therefore contain the confounding variables and ambiguities about causality embedded in epidemiological studies. To tease out cause-effect relations, our laboratory has directly compared the benefits of fish nutrients selenium (Se) and docosahexaenoic acid (DHA) under controlled experimental conditions using prenatal and adult-onset MeHg exposure (Newland and Paletz, 2000; Newland et al., 2008).
2 The Developmental Window is Important
The consequences of MeHg exposure depends critically upon the developmental period during which exposure occurs, and this is one of many reasons that it can serve as a model of disrupted neural development. Adult-onset exposure produces sensorimotor deficits and accumulation of mercury in, and damage to, the cerebellum and sensory and motor regions of the cortex (Castoldi, Coccini, & Manzo, 2003; Gilbert & Maurissen, 1982; Harada, 1995; Heath et al., 2010). In contrast, the entire neocortex is vulnerable to pre- and perinatal MeHg exposure (Eto, 1997), suggesting that effects of developmental exposure could extend into domains mediated by that region. Moreover, exposure levels that produce toxicity are many-fold lower if exposure occurs during early development than during adulthood (Costa et al., 2004). For example, we have shown that chronic, adult-onset exposure produces overt neurological signs after about a year of exposure to 5 ppm of MeHg in drinking water, but not 0.5 ppm (Heath et al., 2010). In contrast, maternal exposure for only three weeks of gestation to 0.5 ppm produced subtle but irreversible effects in the adult and aging offspring, even though by all cage-side observations these animals appeared perfectly healthy. That is, exposed animals showed no neurological signs, weight loss, reproductive toxicity, or changes in physical appearance even as they showed significant, if subtle, neurotoxicity as adults (Newland, 2012; Newland et al., 2008). Such sensitivity has been reported with auditory, visual, and somatosensory deficits in monkeys (Rice, 1996) and in behavioral studies with rodents (Bourdineaud et al., 2008; Bourdineaud et al., 2011; Castoldi et al., 2008; Liang et al., 2009; Montgomery et al., 2008; Newland et al., 2008; Onishchenko et al., 2008; Weiss et al., 2005) and are linked to disturbances in the development of the dopamine systems (Rasmussen and Newland, 2001; Reed and Newland, 2009).
Some of the behavioral effects of gestational exposure that we have reported, such as response perseveration, were affected at the lowest exposure level examined, 0.5 ppm in drinking water, yielding about 40 μg/kg/day of Hg as MeHg. This dosing regimen produced brain mercury concentrations equivalent to those experienced by many people (Newland et al., 2008). It is also relevant to public-health policy regarding MeHg exposure. The exposure level considered to be unlikely to be harmful is established by public-health agencies such as the Environmental Protection Agency or the World Health Organization after reviewing human and animal studies. These agencies extrapolate from experimental models using laboratory animals, which typically use exposures that exceed those experienced by humans, to estimate human daily intakes that are unlikely to present a significant risk. Using the Environmental Protection Agency’s approach to extrapolation, the 40 μg/kg/day level described in our experimental model translates into a “reference dose” (a level unlikely to be harmful) of 0.4 ug/kg/day, which is quite close to the current reference dose of 0.1 μg/kg/day that was established in 1997 (Keating et al., 1997). This leaves little room for error. Thus, based on brain concentrations and estimated acceptable daily exposure levels, this dosing regimen models human exposure.
3 Methodological Considerations
Rodents give birth to multiple offspring, which means that littermates experience the same uterine environment and, of course, half of their genes. When examining the impact of gestational exposure to a chemical, drug, or toxicant, this also means that assigning all same-sex littermates to a single treatment group confounds prenatal experience and genetic background with exposure. Because of this, it is poor practice to assign littermates to the same experiment, but the presence of multiple births generate creates an efficient and sensitive experimental design: assign littermates to separate studies. The litter becomes the statistical unit, but each litter can contribute to multiple experiments. Therefore, only one same-sex pup is assigned to a single experiment, although when examining sex differences some investigators treat male-female siblings as a repeated measure (Maurissen, 2010; Spyker and Spyker, 1977).
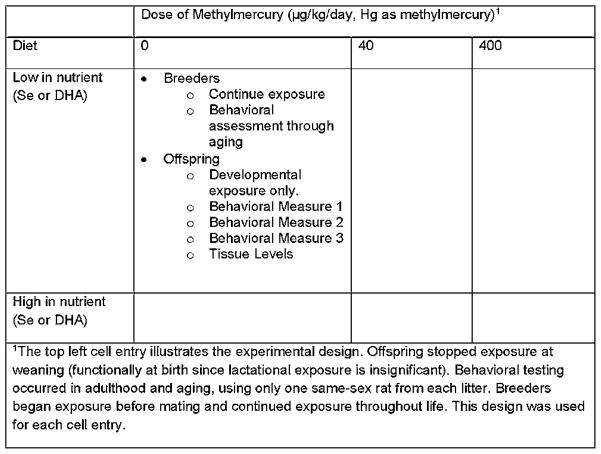
Human MeHg exposure is almost exclusively through fish consumption (Mahaffey, 2004), raising a scientific and regulatory dilemma because fish are also the source of a number of important nutrients, including selenium (Se) and the n-3 polyunsaturated fatty acid, DHA (Budtz-Jorgensen et al., 2007; Mahaffey et al., 2011; Rice, 2008). Our laboratory was interested in whether nutrients found in fish influenced the impact of MeHg exposure during gestation. To address this issue, we exposed pregnant rats to one of three MeHg levels, 0, 0.5 or 5 ppm in their drinking water using a full factorial design (Figure 1). Half of the rats consumed a diet rich in a nutrient (Se or DHA) and half consumed a diet that was low. In each case, the nutrient level was within the bounds of what was recommended for laboratory rodents (National Research Council, 1995). This approach allowed us to determine whether MeHg was toxic under both dietary conditions, as would be revealed in a main effect of MeHg. A main effect of diet allowed us to identify potential benefits of Se or DHA and an interaction would reveal protection by these nutrients.
Figure 1.
Experimental Design for studies of gestational MeHg exposure. The effects of MeHg, Diet, and their combination were investigated using a full factorial design. Offspring, which received only developmental exposure, were assigned such that only one same-sex littermate was represented in each experimental group. The exposed dam was also tracked after giving birth.gr1
The distribution of rats to experiments is illustrated in the top left cell. The female breeders continued exposure so we could examine chronic, adult-onset exposure. Offspring were assigned to different experiments, so each experiment had only one representative, or one Male-Female pair when sex differences was of interest, from each litter.
A final set of methodological considerations is embedded in the behavioral domains and tasks employed. One approach is to use rapid assessment techniques, many of which employ naturalistic proclivities of the species under study. Examples are locomotor activity, elevated- plus mazes, forced-swim tasks, and many others that could be identified (Kulig and Jaspers, 1999; Norton, 1989; Tilson and MacPhail, 1994). These tasks have the advantage that they can be conducted rapidly, with untrained animals and with technicians that require little training, and sometimes can predict the actions of clinical pharmaceutics. Because these tasks often involve a limited number of observations of an animal, sometimes only one, they can be economical to conduct but there is often considerable variability associated with them. A second approach is to apply our understanding of conditioning and learning to assess the behavioral impact of neurotoxicant exposure. While this requires much more handling of animals, this refined testing has many advantages. By explicitly controlling the antecedents and consequences of behavior, it is possible to reduce variability considerably. There is a long history of the study of basic conditioning processes, and of the effects of drugs with known actions, that can be drawn upon to help interpret effects. Finally, and perhaps the strongest advantage, is that in skilled hands, the reinforcement contingencies can be tailored to produce the type of behavior of interest. Specific operant or Pavlovian procedures can be employed to examine memory, choice, motor function, sensory function, anxiety, or the acquisition of stimulus control (Cory-Slechta and Weiss, 2014; Newland, 1995; Newland, 2010; Weiss and O’Donoghue, 1994).
4 Acquisition of Choice Under a Concurrent Schedule
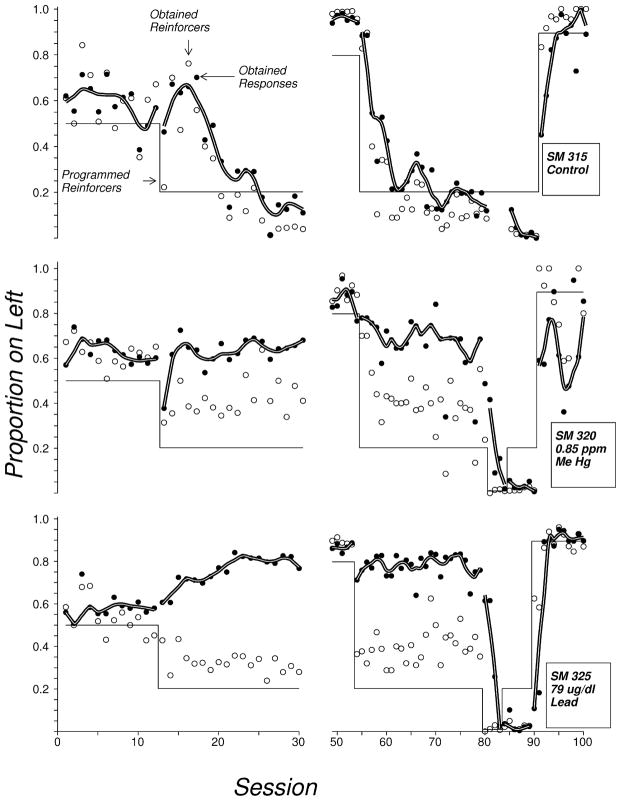
The expression and acquisition of choice is a fundamental process underlying effective behavior. It is of interest here because it reflects the function of the prefrontal cortex (Tremblay and Schultz, 1999), a region sensitive to MeHg exposure (Barone et al., 1998). In an early study, squirrel monkeys were exposed to MeHg during gestation and trained to lever-press under concurrent schedules of reinforcement when they were five to six years of age (Newland et al., 1994). They were first trained to lever-press under a Concurrent Random Interval 30″ Random Interval 30″ schedule of reinforcement (Conc RI 30″ RI 30″) in which responding on either of two levers (left or right) was reinforced unpredictably but on the average of two reinforcers per minute. The acquisition of lever-pressing and the initial acquisition and expression of choice proceeded similarly for exposed and unexposed monkeys. When the schedule changed such that one lever produced reinforcers at four times the rate of the others, the control monkeys’ behavior changed quickly: after six or seven sessions, most responding occurred on the richer alternative. The MeHg-exposed monkeys showed little change (Figure 2). This insensitivity to the difference in reinforcement rates persisted through several transitions. Behavior eventually changed, but only when one lever ceased producing reinforcers. Incidentally, lead-exposed monkeys (illustrated in the bottom panel) also showed quite severe deficits, and the one illustrated shifted most behavior to the leaner lever.
Figure 2.
Three squirrel monkeys exposed to no contaminant, MeHg, or Lead (top to bottom panels) lever-pressed under concurrent schedules of reinforcement. The thin solid line shows programmed reinforcers, the open circles show obtained reinforcer and the filled circles show response proportion, all as a proportion of events on the left lever. The thick line is a LOWESS smoothed curve fitted to the response proportion. A “behavior therapy” condition commenced at about session 80: all reinforcers came from the right lever. Note that response proportions approximately matched reinforcer proportions but exposed monkeys’ behavior was relatively insensitive to changes in the source of reinforcement until the relative difference in reinforcer allocation became extreme.gr2
As fleshed out below, these data were interpreted as suggesting that both MeHg’s and lead’s behavioral toxicity was linked to a common behavioral mechanism, insensitivity to the reinforcing consequences of behavior, even if the neurotoxicants’ damage may have their origins in different processes. The identification of a behavioral mechanism underlying impairment can help reveal therapeutic interventions to increase that behavior and decrease untargeted behavior. It can also point to neural mechanisms that may be common to different forms of neurotoxicant injury. The success of this approach has been shown repeatedly in clinical settings with individuals with common problem behavior that likely result from very different chemical or genetic histories. (For a similar argument with stroke victims see (Taub et al., 1994)).
Perseveration
The sort of behavioral rigidity seen under concurrent schedules with monkeys was reexamined using discrimination reversal procedures with rodents. In a spatial discrimination reversal (SDR), animals acquire a simple discrimination based on spatial location (e.g., left lever-pressing is reinforced). When responding stabilizes on the left lever (in this example), a reversal is implemented such that only right lever-pressing is reinforced. The reversal procedure need not be limited to spatial stimuli; non-spatial stimuli can also be used. In the non-spatial visual discrimination reversal (VDR) procedure, only lever-pressing on the lever beneath a lit LED (original discrimination) is reinforced (Paletz et al., 2007). After criterion, lever presses are reinforced only when they occur on the side beneath the unlit LED (first reversal). Because the reversals in the SDR and VDR are along the same dimension, spatial location or illumination, respectively, these procedures are sometimes called an intradimensional shift. The SDR and VDR are similar to the intradimensional shift tasks as measured on the CANTAB, a neuropsychological testing battery derived, in part, from procedures used in the animal literature (De Luca et al., 2003; Fray and Robbins, 1996) as well as studies using laboratory animals (Dias et al., 1997).
A related procedure, but one that is thought to tap a different cortical region, is the extradimensional shift. Training is established under one stimulus dimension (e.g., spatial) and then that spatial dimension becomes irrelevant and a new dimension (e.g., visual) forms the basis for new discriminative stimuli. In neuropsychological testing, this is said to test set shifting or cognitive flexibility. Extradimensional shifts are tested in humans using the Wisconsin Card Sort Task (Boone et al., 1993; Dias et al., 1997) or the extradimensional shift test of the CANTAB (Dias et al., 1997; Fray and Robbins, 1996). Both extra- and intra- dimensional shifts are mediated by the prefrontal cortex, but by different subregions, supportive of the claim that these procedures reflect different behavioral domains. The intradimensional shift is disrupted by lesions of the orbitofrontal cortex, while the extradimensional shift is disrupted by lesions of the dorsolateral cortex in monkeys and the medial frontal cortex in rodents (Dalley et al., 2004).
Using a response-initiated SDR and VDR procedure, we (Paletz et al., 2007; Reed et al., 2006) and others (Gilbert and Rice, 1987; Rice, 1985; Schantz et al., 1989; Widholm et al., 2001) have shown the first reversal to be particularly sensitive to neurotoxicant exposure, even as the original acquisition of the discrimination shows no deficits and subsequent reversals show no or diminished disruption. Figure 3 illustrates this point with representative data from individual MeHg-exposed rats (from (Reed et al., 2006)). A representative control (top left panel) and exposed rat (top right panel) acquired the original discrimination rapidly, within three to five sessions. Subsequent reversals proceeded smoothly for the control rat, but the MeHg-exposed rat experienced five consecutive sessions, 300 trials, without a single correct response. It did produce numerous incorrect responses, i.e., perseveration on the previously correct lever, and eventually response omissions. An exposed outlier rat is illustrated in the bottom panel. This rat was slow to acquire the original discrimination, and it experienced 17 sessions (1020 trials) without a single correct response. This rat lever-pressed correctly only when the incorrect lever was removed from the chamber (“therapy”). With MeHg-exposed rats, no differences in the acquisition are noted among the groups for the original discrimination, yet for the first and third reversals, which are away from the original lever, MeHg-exposed rats make more errors than controls (Reed et al., 2006). Interestingly, once correct responding began, it developed rapidly in the first and in subsequent reversals.
Figure 3.

Spatial discrimination reversals for three representative rats exposed to MeHg during gestation. Correct responses, incorrect responses, and response omissions are shown. A reversal is indicated by a break in the lines. The top left and top right are typical of unexposed and exposed rats, respectively. The bottom panel represents extreme cases sometimes seen. Note that exposed rats perseverated on the formerly correct lever after a reversal.gr3
In a separate experiment with a different breeding cohort, the effects on SDR (including the presence of an outlier) were replicated (Paletz et al., 2007). In that experiment, an extradimensional shift from SDR to VDR was imposed and there were no MeHg-related deficits on that shift. Interestingly, however, a subsequent discrimination reversal on the VDR revealed a dose-related effect of MeHg. The VDR task is more difficult to acquire and train than the SDR since rats’ behavior comes under control of visual stimuli with greater difficulty than spatial stimuli (Paletz et al., 2007; Widholm et al., 2003) but these differences in difficulty did not undermine the reproducibility of the phenomenon.
Exposure to MeHg during gestation resulted in significant and dose-related perseveration on discrimination reversal in three replications: two using SDR and one using VDR. This was specific, since no such effect was reported on an extradimensional shift from SDR to VDR. Since reversal learning is linked to prefrontal cortical damage, these results are consistent with reports that low-level gestational MeHg exposure results in cortical damage (Barone et al., 1998; Castoldi et al., 2003). While speculative, they also raise the interesting possibility that it is the orbitofrontal cortex that is especially sensitive, since that region is linked to reversal learning (Dias et al., 1997).
The results from the SDR study resemble those from the concurrent-schedule study described in the previous session. The SDR offers the advantage of simplicity. The concurrent schedule offers some advantages since it maintains responding on both levers throughout the study and the magnitude of a transition can be manipulated. In contrast to the SDR procedure, in which reinforcement is all-or-nothing, the concurrent schedule arrangement permits transitions to larger or smaller discrepancies in reinforcer rates.
5 Motor perseveration and the Low-Rate Challenge
A different form of perseveration was reported recently (Newland et al., 2013). In the procedures described above, responding had to shift from one response device to another. With motor perseveration, the response device does not change but, instead, a specific response pattern (e.g., high-rate responding) persists even though it is no longer reinforced. That is, intrusion by a previously-established response pattern prevents behavioral contact with existing reinforcers. In the clinical literature, this is viewed as resulting from a loss of inhibition or, more concretely, the intrusion of a prepotent response pattern, and is a component of behavioral disorders such as ADHD (Barkley, 1997). One approach to studying these intrusions is the use of time-based schedules like the Differential Reinforcement of Low-Rate responding (DRL) or Fixed-Interval (FI) schedule, as has been done in the clinical (Barkley, 1997; Pattij et al., 2004) and basic (Hirai et al., 2011; LeFrancois and Metzger, 1993; Urbain et al., 1978; Weiner, 1969) literature.
To examine the generality of the perseveration described above, rats were transitioned from a high-rate to a low-rate reinforcement contingency, a sort of low-rate challenge. In an earlier study, rats prenatally exposed to MeHg acquired DRL responding more slowly than control rats after a history of high-rate responding under fixed-ratio schedules of reinforcement (Paletz et al., 2006). In a subsequent study, it was reasoned that the high-rate response pattern established during the fixed-ratio phase may have intruded during the DRL phase and prevented the collection of reinforcers (Newland et al., 2013). To examine this possibility, a different approach was taken to establish high-and low-rate responding and an analytic strategy, log-survivor analysis, was applied to reveal the microstructure of high-rate responding. High-rate responding was established by reinforcing short interresponse times while dynamically adjusting the response criterion according to the rat’s most recent responding. Criterion interresponse times, those that were shorter than fifteen of the previous twenty, were reinforced randomly, with an average rate of 2 reinforcers/min. This approach severed the link between overall reinforcer rate and the pattern (high- or, immediately below, low-rate responding), making it quite different from both the fixed ratio- and the DRL schedule used in the earlier study. After responding stabilized under the percentile schedule, the criterion was inverted, so that instead of reinforcing short IRTs, those that were longer than fifteen of the previous twenty were eligible for reinforcement, a low-rate percentile schedule. In both the high- and low-rate percentile schedules, the criterion adjusts continuously so it is sensitive to recent performance.
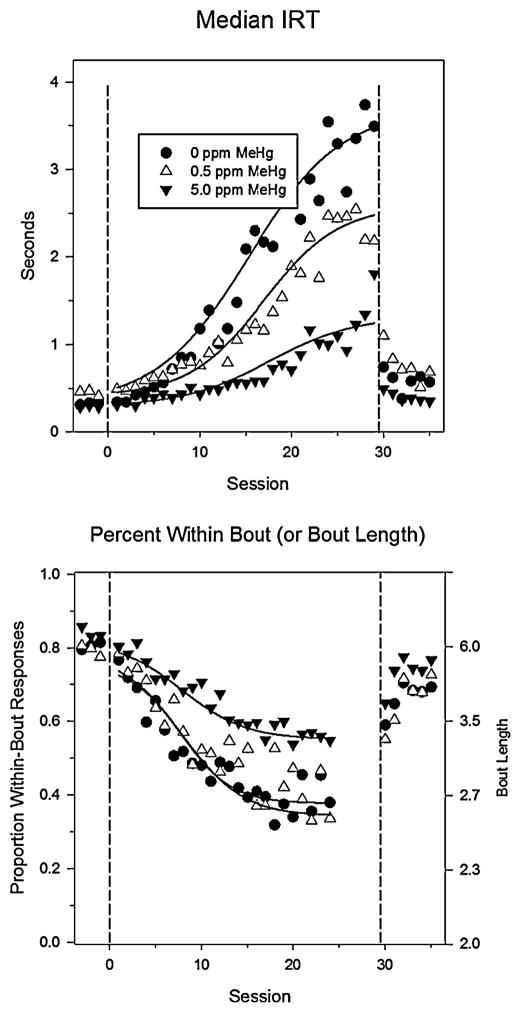
The high-rate schedule generates bouts of high-rate responding separated by inter-bout intervals (Johnson et al., 2011, 2009). If developmental MeHg exposure results in motor perseveration, as suggested by the poor acquisition of DRL responding, and this is due to the persistence of a prepotent response pattern, then exposed rats should acquire the long IRTs more slowly than controls and the difference should be due to the persistence of high-rate response bouts in exposed animals. This is exactly what happened. For unexposed rats, the median IRT quickly lengthened from a value of about 0.5 seconds under the high-rate percentile schedule to greater than 3.5 seconds, a 7-fold increase (Figure 4, top panel). In contrast, the median IRT for exposed rats was far shorter. This was because of the persistence of high-rate response bouts. As shown in the bottom panel of Figure 4, the length of response bouts shortened dramatically for controls until they were too short to estimate using log-survivor analysis, while the exposed rats continued to respond in bursts.
Figure 4.
A “low-rate challenge” indicated perseveration of a high-rate pattern of lever-pressing. Rats were trained under a percentile that reinforced short interresponse times (IRTS) and, therefore, high response rates. At “session 0,” long IRTs were reinforced instead and at Session short ones were again reinforced. The solid, curved line shows logistic fits to the data. The median IRT lengthened for all groups but faster and with a greater magnitude for unexposed rats. The bottom panel shows that exposed rats persisted in producing high-rate response bouts while unexposed rats’ bouts were shorter.gr4
The slower extinction of a high-rate response pattern may be responsible for this phenomenon. Exposed rats showed slower extinction from fixed-interval (Reed and Newland, 2007) and fixed ratio schedules (Paletz et al., 2006), and could form the basis for interpretations based on the construct of loss-of-inhibition. Methodologically, this also suggests that the sensitivity of a low-rate challenge will be enhanced by first generating a stable pattern of prepotent responding first.
6 Reinforcer impact
Is there a consistent link that binds the effects described above? A MeHg-induced enhancement of reinforcer efficacy may explain the perseverative responding and behavioral rigidity observed following gestational MeHg exposure. Such a mechanism could even account for a counterintuitive effect of MeHg: developmentally MeHg-exposed rats acquire large fixed ratio (FR) responding more rapidly than controls. This was shown by transitioning them from a FR 1 (one response/reinforcer) to FR 5, 25, and then 75 over the course of only a few sessions (Paletz et al., 2006). The control rats exhibited the expected erratic and low-rate responding at large FRs, a phenomenon commonly referred to as “ratio strain” and suggestive of extinction (Perone & Courtney, 1992; Shull, 1979). In contrast, rats exposed during gestation to MeHg showed a rapid and smooth acquisition of lever-pressing, even when 75 responses were required for a single reinforcer. Interestingly, in an earlier report, a similar effect was reported in rats exposed during early development to another metal, cadmium, that caused cortical damage (Newland et al., 1986). The robust responding by MeHg-exposed rats seems paradoxical, neurotoxicants are supposed to interfere with behavior, not “make it better,” but it does resemble other reports of distorted sensitivity of behavior to a change in the reinforcement contingency.
The “improved” performance under rapidly changing fixed-ratio schedules supports a hypothesis that there is a disruption of reinforcer efficacy. This is because a standard approach to evaluating efficacy is the use of gradually changing progressive ratio (PR) schedules. Under the PR schedule, the response requirement starts small and increases gradually throughout a session until responding deteriorates or stops all together. The ratio at which this occurs is called the breakpoint. PR schedules are commonly used to measure the reinforcing efficacy of abused drugs (Stafford et al., 1998), with highly reinforcing drugs producing breakpoints upwards of thousands of responses. In agreement with the FR studies, breakpoints are higher in PR schedules for MeHg-exposed rats compared to controls, and this was reported in two separate studies using separate groups of rats (Paletz et al., 2006; Reed et al., 2008).
Reinforcement omission trials are another way to assess reinforcer efficacy and response perseveration. Following stabilization of responding under a fixed interval (FI) schedule of reinforcement, reinforcement omission trials are inserted such that responses at the end of an interval are not followed by reinforcement, but responding continues to be measured (Reed and Newland, 2007). These trials permit the evaluation of MeHg’s effects on resistance to change, or perseverative responding. Response rates are higher for MeHg-exposed rats than controls during omission trials (Reed and Newland, 2007). Together with findings of MeHg-induced behavioral rigidity in discrimination reversals, the FR, PR, and reinforcement omission studies suggest MeHg increases reinforcer efficacy, leading to a persistence of responding. In the case of the FR schedules, this persistence in responding would manifest as a facilitation in acquisition, whereas in the case of discrimination reversals and reinforcement omission studies, this increased persistence would appear as perseveration.
Enhanced reinforcer efficacy due to gestational MeHg exposure could be related to perseveration reported here but other possibilities might be noted. Overtraining of response sequences can make animals insensitive to distortions in reinforcer parameters (Dezfouli and Balleine, 2012; Nevin and Grace, 2000). These phenomena, like those associated with MeHg exposure, have been linked to altered function in cortical and striatal regions, including changes in dopamine activity (Dezfouli and Balleine, 2012; Smith and Graybiel, 2014) as noted below. It is difficult at present to sort out which of these mechanisms may be at play in the studies describe here. In fact, it is possible these mechanisms are not incompatible but rather identify different perspectives on a similar phenomenon.
7 Neurochemical mechanisms
Can a neurochemical mechanism be sensibly linked to enhanced reinforcer and perseverative behavior produced by gestational MeHg exposure? One approach to addressing this question is the use of drug challenges. Such challenges were designed to examine the dopamine neurotransmitter system because of its close relation with reinforcement processes. Drugs selective for different neurotransmitter systems, dopaminergics because of the hypothesis and others to test selectivity, were administered acutely prior to the start of a behavioral task. A wide range of doses, from inactive to behaviorally disruptive, was used both to determine whether there is a systematic relation between dose and behavior change and to avoid missing an important dose. Combined with behavioral tasks measuring specific behavioral processes, questions regarding the influence of a particular neurotransmitter system on a behavioral task can be addressed. Thus, acute drug challenges may unmask or amplify MeHg-induced changes in behavior.
In one study (Rasmussen and Newland, 1999), rats were exposed throughout gestation to 0, 0.5 ppm, or 6 ppm MeHg. At four months of age, lever-pressing was established under a differential reinforcement of high rate (DRH) 9:4 schedule of reinforcement, in which nine responses emitted in 4 seconds produced a food reinforcer. No differences were apparent in DRH performance among exposure groups under baseline conditions. When acute doses of amphetamine, an indirect dopamine agonist, were administered before some sessions, all rats demonstrated a dose-dependent reduction of reinforcement rate. Importantly, however, rats in the 6 ppm MeHg-exposed group demonstrated a 2–3 fold sensitivity to lower doses of amphetamine, suggesting dopamine-related changes in the brain.
In the same study (Rasmussen and Newland, 2001), pentobarbital (a GABA agonist) unmasked GABAergic alterations involved with MeHg exposure. Acute moderate doses of pentobarbital administered before DRH sessions resulted in a rate-increasing effect in rats exposed to the higher MeHg concentration; rats in the control and lower exposure group demonstrated no drug effects at similar doses. High, nearly sedative, pentobarbital doses reduced reinforcement rate for all rats, but the dose required to reduce response rates was three times higher for MeHg-exposed rats than for controls. That is, gestational MeHg exposure reduced the sensitivity of rats to this GABA agonist. Other studies support GABAergic and dopaminergic alterations at the level of behavior and receptor that are related to developmental MeHg exposure (Araki et al., 1981; Castoldi et al., 2003; Daré et al., 2003; Giménez-Llort et al., 2001; Su & Okita, 1986).
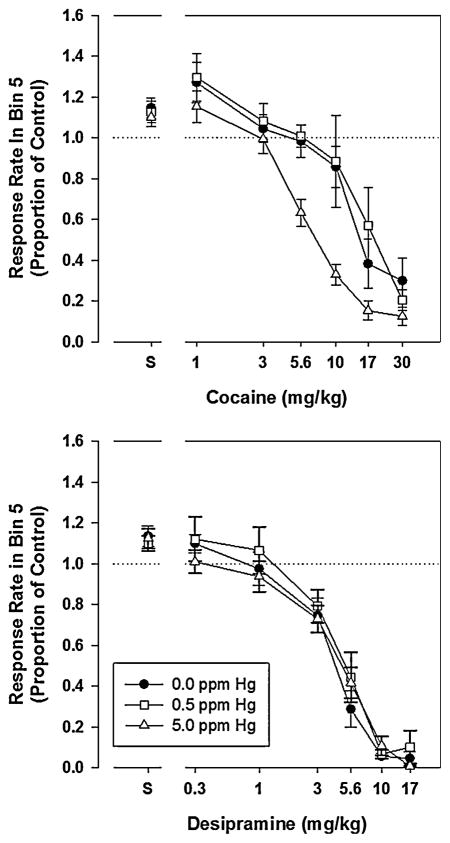
A later study was designed to replicate and extend the dopamine results just described. Here, behavior under a fixed interval (FI) schedule of reinforcement was selected for study because both low and high rates of responding can be examined in the first and last portion of the interval, respectively. Acute dose-effect curves were generated with dopamine (cocaine) and norepinephrine (desipramine) reuptake inhibitors, as well as a direct D1 or D2 agonist and antagonists (Reed and Newland, 2009). For measures of low-rate behavior, higher doses of cocaine increased response rate, by as much as 10-to 15-fold, for all but the 5 ppm MeHg group. For high-rate behavior, MeHg-exposed rats were 2 to 3 times more sensitive to the rate-reducing effects of high doses of cocaine, similar to the amphetamine results. No differential effects of MeHg were seen with desipramine, suggesting MeHg’s effect is specific to dopaminergic receptors (Figure 5). No differential effects were seen with the specific D1 and D2 agonists.
Figure 5.
Sensitive of rats exposed during gestation to acute doses of cocaine (top) and desipramine (bottom). Note that exposed rats (5 ppm via drinking water) were more sensitive to the dopamine reuptake inhibitor cocaine but no differential sensitive occurred to the norepinephrine uptake inhibitor, desipramine.gr5
The results of these two studies are informative. With drug challenges, we identified and then replicated disruptions in dopamine signaling resulting from development MeHg exposure. The specifics of the effects were similar between the two studies: high-rate responding in MeHg-exposed rats was 2–3 times more sensitive to two dopamine reuptake inhibitors, cocaine and d-amphetamine. Like the behavioral results above, these disruptions were apparently formed during gestational exposure and persisted into adulthood. This was selective, in that a norepinephrine reuptake inhibitor had no differential effect on the MeHg-exposure groups. The absence of differential sensitivity to D1 or D2 agonists alone suggests coactivation of D1 and D2 receptors may be needed to activate signaling pathways not activated by either receptor alone or that presynaptic mechanisms may be at play.
Pentobarbital, a sedative-hypnotic and anticonvulsant, acts on the inhibitory neurotransmitter GABA. The results with pentobarbital are consistent with reports diminished sensitivity to hexobarbital (Su and Okita, 1986) and a reduction in GABA receptors (O’Kusky and McGeer, 1985). In contrast to the effects with the dopaminergics, the results suggest a diminished sensitivity or, alternatively, an elevated threshold for inhibition, consistent with hypotheses relating MeHg exposure to epilepsy (Dasari and Yuan, 2010). Further examination of the connection between the disruption of GABA neurotransmission and MeHg exposure could help uncover the role of GABA neurotransmission in reversal learning.
8 Aging
It is remarkable enough that gestational MeHg exposure produces such profound effects in adults, but there is evidence that it also causes some effects that do not even appear until aging. Some evidence derives from the study of individuals from the fishing village of Minamata, Japan, who consumed fish containing very high concentrations of MeHg that was present because of the industrial dumping of mercury in Minamata Bay (Smith and Smith, 1975). Some members of this population began to show deficits on an Activities of Daily Living scale (e.g., ability to bath, eat, or toilet without assistance) only after they reached 50 years of age (Kinjo et al., 1993). In an experimental study, monkeys exposed during early development, including but not limited to gestation, showed sensorimotor deficits only after they reached about 13 years of age (Rice, 1996).
There is also support from rodent models that low-level gestational MeHg exposure has delayed consequences that begin emerging in later adulthood, suggesting that this exposure accelerates processes that are inherent in aging. In one study, rats exposed developmentally to low-level MeHg showed no differences at birth, or later at 4 months of age when lever-pressing under a DRH schedule of reinforcement (Newland and Rasmussen, 2000). As the rats aged, however, differential declines in reinforcement rate, a marker of their success in meeting the DRH contingency, became evident. Unexposed rats functioned at about 80% of baseline by 980 days of age, quite old for a rat. That is, they continued to lever-press and did so at high rates but collected about 80% as many reinforcers as they did as young adults. Rats exposed to MeHg demonstrated a 50% or greater decline in reinforcement rate that usually resulted in a complete loss of lever-pressing. This began at about 500 or 800 days of age for high- and low-dose exposure, respectively. These declines were sometimes gradual and sometimes quite precipitous, but they were not related in any obvious way to the health of the animal: the rats appeared healthy by all other measures. It was suggested that the nature of the DRH schedule helped to amplify MeHg’s effects: difficulty in meeting the high-rate requirement could decrease reinforcement rate, which could further lower response rate, resulting in a descending spiral until responding ceased.
The structure of the decline observed in the aged rats can also shed light on behavioral mechanisms involved in delayed effects of MeHg exposure. The DRH schedule of reinforcement allows motor vs. motivational effects of behavior to be characterized as changes in dimensions of the response units shaped by the schedule. A within-unit disruption, in which the nine responses are not produced rapidly enough to result in a reinforcer, would suggest motor dysfunction. A between-unit decrement, in which the 9-response unit is reliably emitted, though less frequently, may signal changes in reinforcer efficacy (Shull, 1979) or suggest that the upcoming ratio is perceived as more effortful, even if it still occurs with a high response rate (Perone and Courtney, 1992; Shull et al., 2001). In the Newland and Rasmussen (2000) study, the aging effects were demonstrated by a decrease in the between-unit dimension of reinforcement rate; the integrity of the nine-response unit held across time. Therefore, the age effects appear to relate to a change in reinforcer efficacy or an increase in the subjective effort required to produce the nine-response response bout.
Another study shows MeHg’s age-related effects on reinforcer efficacy (Newland et al., 2004). In this report, behavior was placed under concurrent schedules of reinforcement, in which the relative rates of reinforcement programmed on two levers changed across session. As expected, response allocation matched the relative reinforcer rate during steady states. In some sessions, however, the reinforcer ratios for the two response alternatives changed from 1:1 for the first 30 minutes to one in which one of the response alternatives was considerably richer than the other. Behavior in transition from one schedule to the next was compared at 1.7 years and 2.3 years. All rats showed sensitivity to changing reinforcement densities when they were younger and older. In the aged rats (2.3 years), however, mercury-exposed rats required twice as many reinforcers as controls to complete the transition to the new schedule. This insensitivity to reinforcement in the aged MeHg-exposed rat is consistent with the reduction of reinforcement rates observed in aged rats in the Newland and Rasmussen (2000) report. This suggests that even chronic low-level exposure that begins and ends early in life has latent impact that is not visible until aging, and this impact may reflect a reduced sensitivity to reinforcement.
9 Summary and Implications
Two sets of implications can be discussed, one pertaining to hypotheses regarding MeHg’s actions and their public health implications and a second to modeling the impact of early life events on behavior. The studies reviewed here were designed to model low-level, environmentally-relevant exposure to MeHg. The brain concentrations reported in rats and monkeys described in this review were similar to those reported in epidemiological studies of humans consuming MeHg because of a fish-rich diet (reviewed in Newland et al., 2008). The behavioral domains affected, and especially the specific sensitivity of discrimination reversal, suggest involvement of the prefrontal cortex. Since both spatial and visual discrimination reversals are disrupted by gestational exposure, but the spatial-to-visual discrimination transition, an extradimensional shift, is not, it can even be speculated that the orbitofrontal cortex is especially sensitive. This particular speculation requires further confirmation, however.
The deficits in spatial and visual discrimination reversals appear to be linked to the impact of the reinforcing consequences on behavior. MeHg exposed rats tolerated higher fixed-ratio schedules of reinforcement and advanced further on progressive ratios. In addition, MeHg exposed rats showed prolonged extinction as compared with controls. It is noteworthy that dopamine neurotransmission, especially in pathways that include the prefrontal cortex, play key roles in reinforcement processes and choice (Montague et al., 2004; Schultz, 2001; Spanagel and Weiss, 1999; Wise, 2004) and MeHg-exposed rats show a two-fold sensitivity to dopamine reuptake inhibitors. If these data had derived from a study of a drug, then the conclusion would be that the drug has abuse potential, and abuse of such drugs is correlated with behavioral rigidity (Crews and Boettiger, 2009; Izquierdo and Jentsch, 2012). Here, the conclusion is not about abuse potential per se, but rather about a distortion in the manner by which consequences mold behavior and the ability of behavior to change in response to changes in environmental demands.
Numerous hypotheses, not necessarily incompatible, have been advanced about the mechanisms underlying MeHg’s neurotoxicity, including oxidative damage, sequestration of bioactive selenium, and various biochemical mechanisms (Aschner and Syversen, 2005; Berry and Ralston, 2008; Castoldi et al., 2003; Kauret al., 2011; Limke et al., 2003; Polunas et al., 2011). The hypothesis advanced here is not inconsistent with many of these hypotheses since they specify how damage might occur, but not necessary which systems are particularly vulnerable. For example, specific biochemical cascades appearing at exposure levels similar to the ones reported here impairs neurite outgrowth early in development in the rodent cortex.
Another public health implication can be noted. Attempts to model the economic impact of exposure to MeHg, and other environmental contaminants, focus on scores on IQ tests (Bellanger et al., 2013; Bellinger, 2011; Bellinger, 2012). This is because correlations between IQ and such important measures as income, education, and productivity can be used to estimate the economic burden of exposure (Bellinger, 2011; Trasande et al., 2005). However, there is little relation between score on IQ tests and reversal-type procedures or some other executive functions (Boone et al., 1993; Fried, 2002). IQ may be related to performance on memory tasks but disrupting memory tasks by MeHg, at least in animal models, requires higher exposure levels than those reviewed here (reviewed in Newland et al., 2008) so attempts at estimating the economic costs of exposure may underestimate these costs.
A brief comment about modeling brain and behavior development can also be added. There is considerable interest in the impact of early life experience on behavior and its neural correlates in adulthood and aging. Much of this work involves lesions or drug exposure at different life stages. The impact of environmental contaminants on development can play an important role in addressing these issues. For many compounds, including MeHg but also lead or other contaminants, there is a sizeable literature to draw upon and the effects noted are highly pertinent to human behavior.
Highlights.
Methylmercury disrupts brain development.
Pregnant women are advised to avoid certain fish because of methylmercury.
Developmental exposure causes behavioral deficits in adulthood and aging.
Effects includes behavioral rigidity, disrupted reinforcement processing, and irreversible changes in sensitivity to GABAergic and dopaminergic drugs.
It is hypothesized that enhanced impacts of reinforcers caused by early methylmercury exposure results in disrupted choice and perseveration.
Methylmercury neurotoxicity may offer a model of abnormal brain development.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (Reed - U54GM104942), the Alzheimer’s Association (Reed - NIRG-12-242187), a WVU Faculty Research Senate Grant, and a WVU PSCOR grant and NIH ES003299 and ES 10865 (Newland).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Alzheimer’s Association.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Wakabayashi M, Sakimura K, Kushiya E, Ozawa H, Kumamoto T, Takahashi Y. Decreased uptake of GABA by dorsal ganglia in methylmercury-treated rats. Neurotoxicology (Little Rock, AR) 1981;2(3):557–566. [PubMed] [Google Scholar]
- Aschner M, Syversen T. Methylmercury: recent advances in the understanding of its neurotoxicity. Ther Drug Monit. 2005;27(3):278–283. doi: 10.1097/01.ftd.0000160275.85450.32. 00007691-200506000-00004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barone S, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Developmental Brain Research () 1998;109(1):13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Xhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology (Little Rock, AR) 1993;14(1):83–144. [PubMed] [Google Scholar]
- Bellanger M, Pichery C, Aerts D, Berglund M, Castaño A, Čejchanová M, Rudnai P. Economic benefits of methylmercury exposure control in Europe: Monetary value of neurotoxicity prevention. Environmental Health: A Global Access Science Source. 2013;12(1):1–10. doi: 10.1186/1476-069x-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. A Strategy for Comparing the Contributions of Environmental Chemicals and Other Risk Factors to Neurodevelopment of Children. Environ Health Perspect. 2011;120(4) doi: 10.1289/ehp.1104170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Comparing the population neurodevelopmental burdens associated with children’s exposures to environmental chemicals and other risk factors. Neurotoxicology. 2012;33(4):641–643. doi: 10.1016/j.neuro.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Ralston NVC. Mercury Toxicity and the Mitigating Role of Selenium. EcoHealth. 2008;5:456–459. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- Boone KB, Ghaffarian S, Lesser IM, Hill-Gutierrez E, Berman NG. Wisconsin Card Sorting Test performance in healthy, older adults: relationship to age, sex, education, and IQ. Journal of Clinical Psychology (Brandon, VT) 1993;49(1):54–60. doi: 10.1002/1097-4679(199301)49:1<54::aid-jclp2270490108>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bourdineaud JP, Bellance N, Benard G, Brethes D, Fujimura M, Gonzalez P, Laclau M. Feeding mice with diets containing mercury-contaminated fish flesh from French Guiana: a model for the mercurial intoxication of the Wayana Amerindians. Environmental Health. 2008;7(1):53. doi: 10.1186/1476-069X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdineaud JP, Fujimura M, Laclau M, Sawada M, Yasutake A. Deleterious effects in mice of fish-associated methylmercury contained in a diet mimicking the Western populations’ average fish consumption. Environment International. 2011;37(2):303–313. doi: 10.1016/j.envint.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environmental Health Perspectives (Research Triangle Park, NC) 2007;115(3):323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi A, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Reviews on Environmental Health (Tel Aviv) 2003;18(1):19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, Manzo L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regulatory Toxicology and Pharmacology (New York, NY) 2008;51(2):215–229. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B. Assessment of Behavioral Toxicity. In: Hayes AW, Kruger CL, editors. Hayes’ Principles and Methods of Toxicology. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2014. pp. 1831–1890. [Google Scholar]
- Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry and Behavior. 2009;93(3):237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews (New York, NY) 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Daré E, Fetissov S, Hökfelt T, Hall H, Ögren S, Ceccatelli S. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2003;367(5):500–508. doi: 10.1007/s00210-003-0716-5. [DOI] [PubMed] [Google Scholar]
- Dasari S, Yuan Y. In vivo methylmercury exposure induced long-lasting epileptiform activity in layer II/III neurons in cortical slices from the rat. Toxicology Letters. 2010;193(2):138–143. doi: 10.1016/j.toxlet.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Elias RW, Grant LD. Current issues in human lead exposure and regulation of lead. Neurotoxicology (Little Rock, AR) 1993;14:15–28. [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology (Lisse) 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Dezfouli A, Balleine B. Habits, action sequences, and reinforcement learning. The European Journal of Neuroscience. 2012;35(7):1036–1051. doi: 10.1111/j.1460-9568.2012.08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable Forms of Inhibitory Control within Prefrontal Cortex with an Analog of the Wisconsin Card Sort Test: Restriction to Novel Situations and Independence from On-Line Processing. Journal of Neuroscience (New York, NY) 1997;17(23):9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domestic Public Health Achievements Team C. Ten Great Public Health Achievements–-United States, 2001–2010. Morbidity and Mortality Weekly Report (MMWR) 2011;60(19):619–623. [PubMed] [Google Scholar]
- Eto K. Review Article: Pathology of Minamata Disease. Toxicologic Pathology. 1997;25(6):614–623. doi: 10.1177/019262339702500612. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicology and Teratology (New York, NY) 1996;18(4):499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Fried PA. Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. Journal of Child Psychology and Psychiatry. 2002;43(1):81–102. doi: 10.1111/1469-7610.00005. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Maurissen JPJ. Assessment of the effects of acrylamide, methylmercury, an 2,5-hexanedione on motor functions in mice. Journal of Toxicology and Environmental Health (New York, NY) 1982;10:31–41. doi: 10.1080/15287398209530228. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Rice DC. Low-level lifetime lead exposure produces behavioral toxicity (spatial discrimination reversal) in adult monkeys. Toxicology and Applied Pharmacology (New York, NY) 1987;91:484–490. doi: 10.1016/0041-008x(87)90070-6. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neurotoxicology (Little Rock, AR) 2006;27(5):693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Llort L, Ahlbom E, Daré E, Vahter M, Ögren SO, Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environmental Toxicology and Pharmacology () 2001;9(3):61–70. doi: 10.1016/s1382-6689(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology (Boca Raton, FL) 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harada Y. Study Group of Minamata Disease, editor. Minamata Disease. Kumamato, Japan: Kumamato University; 1968. Congential (or fetal) Minamata disease. [Google Scholar]
- Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, Newland MC. Dietary selenium protects against selected signs of methylmercury exposure and aging. Neurotoxicology (Little Rock, AR) 2010;31:169–179. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Okouchi H, Matsumoto A, Lattal KA. Some determinants of remote behavioral history effects in humans. Journal of the Experimental Analysis of Behavior. 2011;96(3):387–415. doi: 10.1901/jeab.2011.96-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch J. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219(2):607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Bailey JM, Newland MC. Using pentobarbital to assess the sensitivity and independence of response-bout parameters in two mouse strains. Pharmacology, Biochemistry and Behavior (New York, NY) 2011;97(3):470–478. doi: 10.1016/j.pbb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Pesek EF, Newland MC. High-rate operant behavior in two mouse strains: A response-bout analysis. Behavioural Processes. 2009;81(2):309–315. doi: 10.1016/j.beproc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Biochemical factors modulating cellular neurotoxicity of methylmercury. J Toxicol. 2011;2011:721, 987. doi: 10.1155/2011/721987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MH, Mahaffey KR, Schoeny R, Rice GE, Bullock OR. Mercury study report to congress. Volume 1: Executive summary. (NT EPA/452/R-97/003) Research Triangle Park, N.C: U. S. EPA; 1997. [Google Scholar]
- Kinjo Y, Higashi H, Nakano A, Sakamoto M, Sakai R. Profile of subjective complaints and activities of daily living among current patients with Minamata disease after 3 decades. Environmental Research (New York, NY) 1993;63(2):241–251. doi: 10.1006/enrs.1993.1144. [DOI] [PubMed] [Google Scholar]
- Kulig BM, Jaspers RMA. Assessment techniques for detecting neurobehavioral toxicity. In: Niesink RJM, Jaspers RMA, Kornet LMW, van JM, Tilson HA, editors. Introduction to Neurobehavioral Toxicology. Boca Raton, Florida: CRC Press; 1999. pp. 70–113. [Google Scholar]
- LeFrancois JR, Metzger B. Low-response-rate conditioning history and fixed-interval responding in rats. Journal of the Experimental Analysis of Behavior. 1993;59 (3):543–549. doi: 10.1901/jeab.1993.59-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Inskip M, Newhook D, Messier C. Neurobehavioral effect of chronic and bolus doses of methylmercury following prenatal exposure in C57BL/6 weanling mice. Neurotoxicology and Teratology (New York, NY) 2009;31(6):372–381. doi: 10.1016/j.ntt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Limke TL, Otero-Montanez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. Journal of Pharmacology and Experimental Therapeutics (Baltimore, MD) 2003;304(3):949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the [omega]-3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: risks and benefits. Environmental Research (New York, NY) 2004;95(3):414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Marien K, Yasutake A. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutrition Reviews. 2011;69(9):493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurissen J. Practical considerations on the design, execution and analysis of developmental neurotoxicity studies to be published in Neurotoxicology and Teratology. Neurotoxicology and Teratology. 2010;32(2):121–123. doi: 10.1016/j.ntt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature (London) 2004;431(7010):760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behavioural Brain Research (Amsterdam) 2008;191(1):55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals. Washington, D.C: National Academy Press; 1995. [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behavioral and Brain Sciences. 2000;23:73–130. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Nevin R. Trends in preschool lead exposure, mental retardation, and scholastic achievement: Association or causation? Environmental Research (New York, NY) 2009;109 (3):301–310. doi: 10.1016/j.envres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Newland MC. Motor function and the physical properties of the operant: applications to screening and advanced techniques. In: Chang LW, Slikker W, editors. Neurotoxicology: Approaches and Methods. San Diego: Academic Press; 1995. pp. 265–299. [Google Scholar]
- Newland MC. Neural, Behavioral, and Measurement Considerations in the Detection of Motor Impairment. In: Philbert M, editor. Comprehensive Toxicology: Vol. 13. Nervous System and Behavioral Toxicology. 2. Vol. 13. New York: Academic Press; 2010. pp. 289–315. [Google Scholar]
- Newland MC. Methylmercury and Fish Nutrients in Experimental Models. In: Ceccatelli S, Aschner M, editors. Methylmercury and Neurotoxicity (Current Topics in Neurotoxicity) Springer; 2012. [Google Scholar]
- Newland MC, Hoffman DJ, Heath JC, Donlin WD. Response inhibition is impaired by developmental methylmercury exposure: Acquisition of low-rate lever-pressing. Behavioural Brain Research. 2013;253(0):196–205. doi: 10.1016/j.bbr.2013.05.038. http://dx.doi.org/10.1016/j.bbr.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Miller RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology (New York, NY) 1986;34:231–242. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM. Animal studies of methylmercury and PCBs: what do they tell us about expected effects in humans? Neurotoxicology (Little Rock, AR) 2000;21(6):1003–1027. [PubMed] [Google Scholar]
- Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition: Adult effects of fetal exposure in experimental models. Neurotoxicology (Little Rock, AR) 2008;29(5):783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicology and Teratology (New York, NY) 2000;22(6):819–828. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology and Teratology (New York, NY) 2004;26(2):179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: Reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology and Applied Pharmacology (New York, NY) 1994;126(1):6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- Norton S. Principles and Methods of Toxicology. New York: Raven Press; 1989. Methods for behavioral toxicology. [Google Scholar]
- O’Kusky JR, McGeer EG. Methylmercury poisoning of the developing nervous system in the rat: decreased activity of glutamic acid decarboxylase in cerebral cortex and neostriatum. Brain Research (Amsterdam) 1985;353(2):299–306. doi: 10.1016/0165-3806(85)90219-6. [DOI] [PubMed] [Google Scholar]
- Oken E, Choi AL, Karagas MR, Mariën K, Rheinberger CM, Schoeny R, Korrick S. Which Fish Should I Eat? Perspectives Influencing Fish Consumption Choices. Environ Health Perspect. 2012;120(6) doi: 10.1289/ehp.1104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castrén E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. Journal of Neurochemistry. 2008;106(3):1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicology and Teratology (New York, NY) 2006;28(1):59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n - 3 polyunsaturated fatty acids. Neurotoxicology (Little Rock, AR) 2007;28(4):707–719. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, Peter S, Olivier B. Impulsive-like behavior in differential-reinforcement-of-low-rate 36 s responding in mice depends on training history. Neuroscience Letters (Limerick) 2004;354(2):169–171. doi: 10.1016/j.neulet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Perone M, Courtney K. Fixed-ratio pausing: Joint effects of past reinforcer magnitude and stimuli correlated with upcoming magnitude. Journal of the Experimental Analysis of Behavior (Bloomington, IN) 1992;57:33–46. doi: 10.1901/jeab.1992.57-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polunas M, Halladay A, Tjalkens RB, Philbert MA, Lowndes H, Reuhl K. Role of oxidative stress and the mitochondrial permeability transition in methylmercury cytotoxicity. Neurotoxicology. 2011;32(5):526–534. doi: 10.1016/j.neuro.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to d amphetamine and pentobarbital in adult rats. Neurotoxicology and Teratology (New York, NY) 2001;23:45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement efficacy in adulthood. Neurotoxicology and Teratology (New York, NY) 2008;30(1):29–37. doi: 10.1016/j.ntt.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked fixed interval schedules of reinforcement. Neurotoxicology and Teratology (New York, NY) 2007;29(4):492–502. doi: 10.1016/j.ntt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. Behavioral Neuroscience (Washington, DC) 2009;123(2):408–417. doi: 10.1037/a0014595. [DOI] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. Neurotoxicology (Little Rock, AR) 2006;27(5):721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JW. Environmental policy as social policy: The Impact of childhood lead exposure on crime. The B E Journal of Economic Analysis and Policy. 2007;7(1):1–41. [Google Scholar]
- Rice DC. Chronic low-lead exposure from birth produces deficits in discrimination reversal in monkeys. Toxicology and Applied Pharmacology (New York, NY) 1985;77:201–210. doi: 10.1016/0041-008x(85)90319-9. [DOI] [PubMed] [Google Scholar]
- Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology (Little Rock, AR) 1996;17(3–4):583–596. [PubMed] [Google Scholar]
- Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology (Little Rock, AR) 1996;17(1):139–154. [PubMed] [Google Scholar]
- Rice DC. Overview of modifiers of methylmercury neurotoxicity: Chemicals, nutrients, and the social environment. Neurotoxicology (Little Rock, AR) 2008;29(5):761–766. doi: 10.1016/j.neuro.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Rice DC, Barone S. Critical periods of vulnerability for the developing nervous system: Evidence from human and animal models. Environmental Health Perspectives (Research Triangle Park, NC) 2000;108(3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Levin ED, Bowman RE, Heironimus MP, Laughlin NK. Effects of perinatal PCB exposure on discrimination-reversal learning in monkeys. Neurotoxicology and Teratology (New York, NY) 1989;11(3):243–250. doi: 10.1016/0892-0362(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Societal Benefits of Reducing Lead Exposure. Environmental Research (New York, NY) 1994;66(1):105–124. doi: 10.1006/enrs.1994.1048. [DOI] [PubMed] [Google Scholar]
- Shull RL. The postreinforcement pause: Some implicatoins for the correlational law of effect. In: Zeiler MD, Harzem P, editors. Advances in the Analysis of Behavior: Vol. 1. Reinforcement and the Organization of Behavior. New York: Wiley; 1979. pp. 193–221. [Google Scholar]
- Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: Effects of relative reinforcement and schedule type. Journal of the Experimental Analysis of Behavior (Bloomington, IN) 2001;75(3):247–274. doi: 10.1901/jeab.2001.75-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM. Investigating habits: Strategies, technologies and models. Frontiers in Behavioral Neuroscience. 2014;8:39. doi: 10.3389/fnbeh.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WE, Smith AM. Minamata. New York: Holt, Rinehart, and Winston; 1975. [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neurosciences (Barking) 1999;22(11):521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spyker DA, Spyker JM. Response model analysis for cross-fostering studies: prenatal versus postnatal effects on offspring exposed to methylmercury dicyandiamide. Toxicology and Applied Pharmacology (New York, NY) 1977;40(3):511–527. doi: 10.1016/0041-008x(77)90077-1. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berlin) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Ström S, Helmfrid I, Glynn A, Berglund M. Nutritional and toxicological aspects of seafood consumption–An integrated exposure and risk assessment of methylmercury and polyunsaturated fatty acids. Environmental Research (New York, NY) 2011;111(2):274–280. doi: 10.1016/j.envres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Su MQ, Okita GT. Effects of methylmercury on hypnotic action of hexobarbital, liver hydroxylase and cytochrome P-450 in mice. Toxicology (Limerick) 1986;39(3):233–245. doi: 10.1016/0300-483x(86)90025-9. [DOI] [PubMed] [Google Scholar]
- Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW, 3rd, DeLuca SC, Miller NE. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. Journal of the Experimental Analysis of Behavior. 1994;61(2):281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA, MacPhail RC. Neurobehavioral Toxicity: Analysis and Interpretation. New York: Raven; 1994. Interpretation of neurobehavioral in toxicologic studies; pp. 345–356. [Google Scholar]
- Trasande L, Landrigan PJ, Schecter C. Public health and economic consequences of methylmercury toxicity to the developing brain. Environmental Health Perspectives (Research Triangle Park, NC) 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex.[see comment] Nature (London) 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Urbain C, Poling A, Millam J, Thompson T. d-amphetamine and fixed-interval performance: effects of operant history. Journal of the Experimental Analysis of Behavior. 1978;29(3):385–392. doi: 10.1901/jeab.1978.29-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. Controlling human fixed-interval performance. Journal of the Experimental Analysis of Behavior. 1969;12(3):349–373. doi: 10.1901/jeab.1969.12-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environmental Health Perspectives (Research Triangle Park, NC) 2002;110(Suppl 5):851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, O’Donoghue JL. Neurobehavioral Toxicity: Analysis and Interpretation. New York: Raven Press; 1994. [Google Scholar]
- Weiss B, Reuhl K. Delayed neurotoxicity: A silent toxicity. In: Chang LW, editor. Principles of Neurotoxicology. New York: Marcel Dekker; 1994. pp. 765–784. [Google Scholar]
- Weiss B, Stern S, Cox C, Balys M. Perinatal and lifetime exposure to methylmercury in the mouse: behavioral effects. Neurotoxicology (Little Rock, AR) 2005;26 (4):675–690. doi: 10.1016/j.neuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicology and Applied Pharmacology (New York, NY) 2001;174(2):188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicology and Teratology (New York, NY) 2003;25 (4):459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]