Abstract
Most adults with Major Depressive Disorder (MDD) will not experience a remission with the first antidepressant trial. No practical biomarkers presently exist to predict responsiveness to antidepressants. Herein we report pilot data for a rest-activity biomarker of antidepressant response.
Fifty-eight medication-free adults with MDD underwent a week-long collection of actigraphic data before beginning a 9 week open label trial of fluoxetine, coupled with blinded randomized assignment to eszopiclone/placebo. Depression severity was repeatedly measured with the Hamilton Rating Scale for Depression (HRSD). Baseline actigraphic data was analyzed with functional data analysis to create smoothed 24-hour curves of activity. The time of the lowest point of activity (the bathyphase) was calculated for each patient, as well the mean difference between bedtime and the bathyphase (BBD). At the end of treatment, patients were characterized as treatment responders (50% reduction in HRSD) or non-responders, and receiver operating curves were calculated to find the optimal cut point of the BBD for prediction of treatment response.
The best cut point for BBD was at 260.2 minutes, resulting in an effect size of 1.45, and with a positive predictive value of 0.75 and a negative predictive value of 0.88.
We conclude that actigraphically-determined measures of rest-activity patterns show promise as potential biomarker predictors of antidepressant response. However, this conclusion is based upon a small number of patients who received only one choice of antidepressant, for a single trial. Replication with a larger sample is needed.
Keywords: depression, circadian, predictor, fluoxetine, actigraphy, bathyphase
INTRODUCTION
Selective serotonin reuptake inhibitors (SSRIs) are the mainstay pharmacotherapy for major depressive disorder (MDD).(Kubitz et al., 2013) Yet the enthusiasm for SSRIs was severely tested by the Star*D effectiveness trial, which showed that the antidepressant remission rate for an initial trial of citalopram (a SSRI) monotherapy was only 36.8%. Non-remitters to citalopram in Star*D were then offered a series of sequenced antidepressant alternatives and augmentation strategies, with the second, third, and fourth sequential treatment trials associated with remission rates of 30.6%, 13.7%, and 13%, respectively. The overall cumulative remission rate was 67% at the end of about a year.(Rush et al., 2006)
The low cumulative rate of remission with antidepressant medications has highlighted the public health importance of treatment resistant depression (TRD). While definitions of TRD vary somewhat, the emerging consensus definition is the failure to remit from an episode of MDD after “at least two courses of evidenced based antidepressant treatment approaches of adequate duration and dose.”(Bennabi et al., 2015;Kubitz et al., 2013) Under conditions of ‘care as usual’, an average of 479 days elapse before a patient in the USA meets the definition of TRD.(Kubitz et al., 2013)
A recent review of potential biomarkers of TRD noted some evidence to support the measurement of brain neurotransmitters with proton magnetic resonance spectroscopy, or peripheral blood brain derived neurotrophic factor, or genetic polymorphisms.(Bennabi et al., 2015) However, these approaches are logistically or financially difficult for many psychiatric providers and their patients. As a result, present-day practitioners and patients are left with a long and painful trial-and-error approach to the treatment of MDD.
The NIMH has stated a desire to support development of predictive tests in psychiatry, with the expectation that such tests have acceptable effect sizes and positive and negative predictive values ‘assuming realistic contexts,’(Kapur & Phillips, 2012) thus advancing development of ‘precision medicine’.(Insel, 2014)
Investigations into the role of rest-activity patterns as biomarker predictors of SSRI-response meet these expectations. While the relationship between insomnia, MDD, and polysomnography (PSG, “sleep studies”), have garnered a lot of interest, the same has not been true for circadian rhythms and MDD. Short rapid eye movement (REM) sleep latencies are established as a specific finding in severe MDD, (Kupfer & Foster, 1972) and are related to MDD treatment outcomes. (Steiger & Kimura, 2010) However, less is known about how circadian rhythms may affect MDD and its treatment. The arousal/modulatory system of the brain, including the study of biological rhythms, has been stipulated as a biological domain of interest in the understanding of mental illness and mental health treatments. (Cuthbert & Insel, 2013) The master governor for circadian rhythms resides within the suprachiasmatic nucleus (SCN) of the hypothalamus.(Moore, 2013) Enhanced serotonin activity in the SCN during light exposure phase advances circadian rhythms. (De Berardis et al., 2013) Light and melatonin influence circadian rhythms through modulation of the activity of a select group of genes, including CLOCK and PER. Studies of the contribution of single nucleotide polymorphisms (SNPs) in circadian genes to the susceptibility for mood disorders have been mostly disappointing , especially with unipolar depression.(Kripke et al., 2009) However, at least one study found that response to SSRIs (fluvoxamine) in depressed patients is related to SNPs of the CLOCK gene.(Kishi et al., 2009) The SSRI fluoxetine has been reported to produce a phase advance in SCN neuronal firing, (Courtet & Olie, 2012) and a shortening of rhythms of the Per1 gene.(Nomura et al., 2008) Complimentarily, the melatonin-1 receptor agonist, agomelatine, advances circadian rhythms and has antidepressant properties.(Castanho et al., 2014;Srinivasan et al., 2012) Similarly, early evidence points towards a phase-advancing effect of electroconvulsive therapy (ECT) in depressed patients.(Winkler et al., 2014) In sum, pre-clinical data suggest linkage between circadian systems, serotonergic systems, and MDD.
In cross-sectional samples, a delay in circadian patterns of the rest-activity cycle, as determined by wrist-worn actigraphy, is associated with prevalent depression in older adults.(Maglione et al., 2014) Among depressed patients, a tendency toward a preference for ‘eveningness’ (late bedtimes and late rising times) is associated with greater severity of MDD. (Courtet & Olie, 2012) This ‘morningness-eveningness’ dimension shows about 50% heritability.(Kripke et al., 2009) A recent cross-sectional study found that a self-report of ‘eveningness’ was associated with poorer antidepressant response in a naturalistic study. (Chan et al., 2014) The cross-sectional nature of these studies and the absence of systematic treatment makes it impossible to know whether variability in circadian rhythms play a causative role in MDD or play a role in the effectiveness of antidepressant medications.
We have completed a secondary analysis of rest-activity patterns in a sample of medication-free outpatient with MDD, for the purpose of collecting pilot data to develop a rest-activity biomarker of antidepressant response, and ultimately serve as a predictive test of TRD. Actigraphy is a practical, noninvasive measure of activity, continuously records changes in velocity in any direction for weeks at a time, providing ‘ecologically-valid’ measurement of a patient’s activity in their home environment. The activity counts are locked to the time of day and allow the data to be analyzed in relation to the clock-time. We recently reported a new analytic approach to actigraphy data that calculates averaged and smoothed 24-hour restactivity patterns.(Xian et al., 2014) As we detail, below, this novel approach has revealed a candidate biomarker for SSRI antidepressant effect.
MATERIALS AND METHODS
Overview
Fifty-eight adult non-psychotic MDD patients with insomnia, free of psychotropics, completed a weeklong collection of actigraphy data prior to beginning treatment with fluoxetine, and after signing informed consent.(McCall et al., 2010) The study was approved by the IRB. After collection of actigraphy, participants received a one-week run-in of open-label FLX monotherapy, followed by 8 more weeks of open label FLX combined with randomization 1:1 to the hypnotic eszopiclone 3 mg versus placebo at bedtime. FLX was initiated at 20 mg with the option to increase to 40 mg after 4 weeks if the 24-item Hamilton Rating Scale for Depression (HRSD) score was > 15 at the end of 4 weeks. The HRSD was administered at baseline and every 1–2 weeks until study completion.(Hamilton, 1960) Insomnia was measured with the Insomnia Severity Index(Bastien et al., 2001) and with daily sleep diaries.
Participants
Participants were 18–70 years old, reporting baseline sleep latency > 30 minutes and sleep efficiency < 85% at least 4 nights per week. Participants were excluded if their reported habitual bedtime was earlier than 9 PM or later than 1 AM more than 2 times per week per baseline sleep diaries, or if their reported habitual rising time was later than 9 AM more than twice per week. All participants met criteria for MDD episode per the Structured Clinical Interview for DSM-IV, (American Psychiatric Association, 1994) with a Mini Mental State Exam score > 24,(Folstein et al., 1975) and a baseline HRSD score > 20.(Hamilton, 1960) Further, all participants completed one night of in-lab polysomnography and were free of moderate-severe sleep apnea or periodic limb movement disorder.
Measures of 24-hour rest-activity
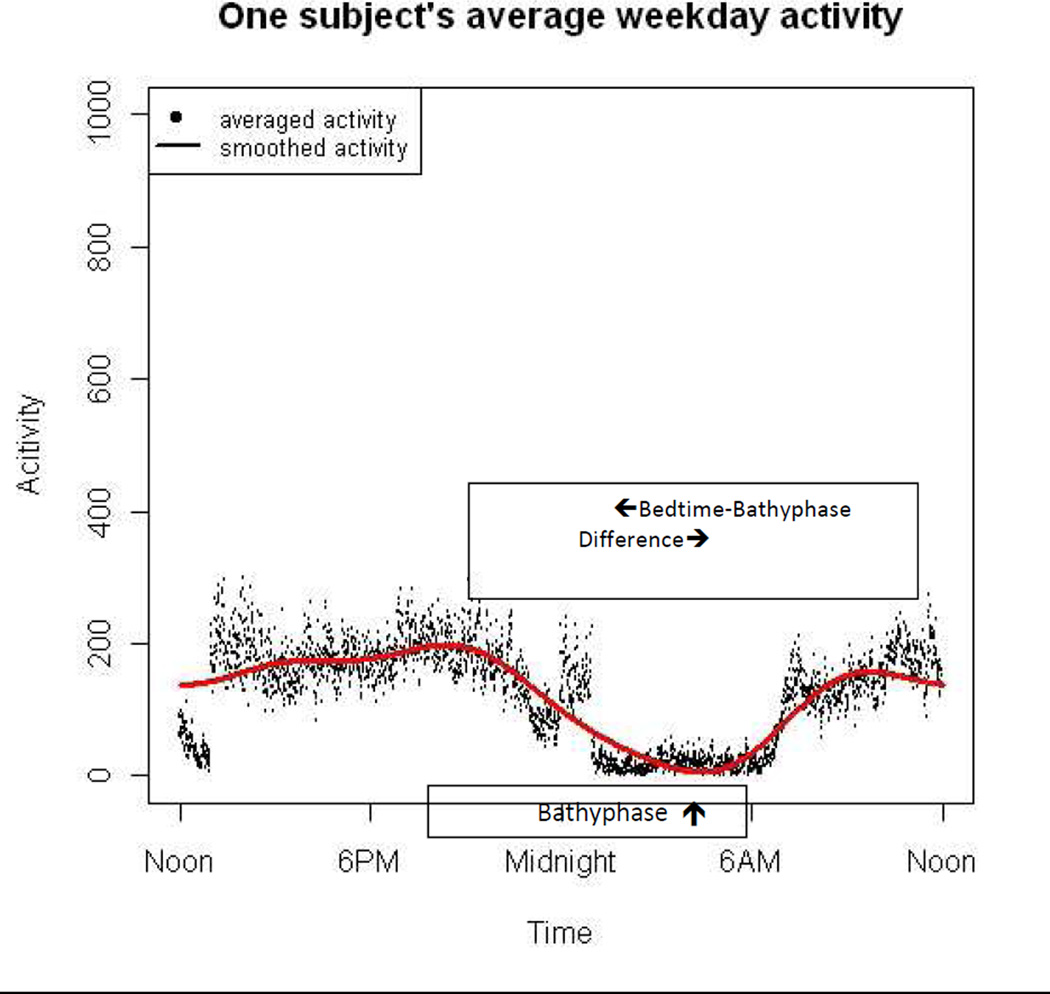
Sleep diaries were completed each morning, noting the time of bedtime. Actigraphic data were collected with a Mini Mitter actigraphy monitor (AW64 Actiwatch, Mini Mitter, Bend, OR, USA) on the non-dominant wrist, set at medium sensitivity setting at 30-seconds epochs, and then analyzed with functional data analysis (FDA). FDA extends classical statistical methods for analyzing sets of numbers (scalars for univariate analyses, and vectors for multivariate analyses) to analyzing sets of functions. (Symanzik & Shannon, 2008;Ding et al., 2012;Ramsey & Silverman, 2005;Xian et al., 2014) For example, actigraphic data can be naturally viewed as random functions over time. The goal of FDA is to analyze functional data or curves in their natural form and study functional features by assuming a smooth underlying process. The actigraphy data were averaged into a single 24 hour profile and a smooth Fourier expansion function was fitted using 24 hour periodicity. This produced a single summarized 24 hour circadian activity pattern for each subject that can be used to estimate a patient’s activity level at any time point throughout the day. By fitting a cosine function with 24 hour cycle, we calculated the time of lowest activity (i.e., the bathyphase) for the week-long average of the activity curves for each patient. (for an example, see arrow on Figure 1).(Marler et al., 2006) FDA and bathyphase analysis were conducted using R.(2008) We chose the bathyphase as our actigraphic variable of interest as we suspect it is most closely tied to the patient’s underlying circadian clock.
Figure 1.
Smoothed curve for one-week of actigraphy data in a single subject. The location of the bathyphase is indicated by the upward arrow. The bedtime-bathyphase difference is indicated by the opposite-direction arrows.
Statistics
Averages are presented as means with standard deviations. Univariate associations between continuous variables are tested with Pearson’s r. Logistic regression was used for modeling of dichotomous outcomes. ANOVA was used to test the multi-sample means.
RESULTS
The participants’ mean age was 42.1 ± 12.4, with 67% women. The mean of the individual median bedtimes was 11:10 PM ± 55.5 minutes. The mean bathyphase was at 2:58 AM ± 117 minutes, and the mean difference between bedtime and the bathyphase was 227.8 ± 98.5 minutes. Of note, the bathyphase did not significantly change after 8 weeks of FLX. Bathyphase was negatively correlated with final HRSD score (Pearson’s r= −0.38, p< 0.05), meaning that a later bathyphase was correlated with lower final HRSD scores. The strength of the correlation was improved by testing the correlation of ‘bathyphase minus bedtime difference’ (BBD) with final HRSD score (r= −0.45, p=0.01), meaning that a greater BBD was associated with a lower final HRSD. Of note, BBD was not related to baseline insomnia severity as measured by the ISI. By implication, BBD is a separate construct from insomnia symptoms.
Next, we developed models for HRSD based upon BBD. Logistic regression of a treatment response (decrease in HRSD ≥ 50%) in those assigned to FLX monotherapy showed that BBD was a significant predictor of HRSD response (Χ2 =6.3, df=1, p<0.02), and with the corresponding receiver operating curve showing that the best cut point for BBD was at 260.2 minutes, where the best cut point was defined as maximizing “sensitivity – (1-specificity)”. This cut point resulted in an effect size of 1.45, and with a positive predictive value of 0.75 and a negative predictive value of 0.88. We subsequently tested 2X2 ANOVAS for HRSD scores, with medication assignment and BBD status as the predictive variables, and adjusting for age because of unexpected imbalances in age between groups. These models revealed that an unfavorable (shorter) BBD was associated with a higher final HRSD score and a smaller reduction in HRSD score in those participants who received placebo at bedtime (see highlighted data in Table 1). Of note, the poor SSRI antidepressant effect among those with unfavorable (shorter) BBD status included equally poor response to both the sleep (14.9% decrease) and the non-sleep items (25.4% decrease) of the HRSD, as compared with the sleep (43.6% decrease) and the non-sleep (66.1% decrease) for favorable BBD status, indicating that ‘unfavorable BBD’ has treatment implications beyond sleep symptoms. We also tested gender and baseline HRSD score as potential predictors in the ANOVA models for the HRSD change score, and neither was significant. The bathyphase and the BBD status were not related to antidepressant outcome in those patients who were randomized to eszopiclone, and the degree of improvement in those assigned to eszopiclone was numerically superior to the improvement seen in those assigned to placebo with an unfavorable BBD status (Table 1). Further, those assigned to eszopiclone had better overall response than those assigned to placebo.
Table 1.
Impact of a rest-activity biomarker on antidepressant response
| Fluoxetine + Placebo | Fluoxetine + Eszopiclone |
P for ANOVA whole model |
P for age |
P for Drug |
P for BBD |
P for interaction of Drug X BBD |
|||
|---|---|---|---|---|---|---|---|---|---|
| Favorable BBD (>260.2 minutes) |
Yes | No | Yes | No | |||||
| N= | 12 | 17 | 10 | 19 | |||||
| Bathyphase time ± min |
4:59 AM ± 85 | 2:23 AM ± 76 | 5:00 AM ± 65 |
1:30 AM ± 90 |
|||||
| Age | 34.3 ± 9.2 | 41.8 ± 13.3 | 40.5 ± 12.9 | 48.2 ± 10.0 |
00.2 | ----- | 0.04 | 0.02 | 0.98 |
| HRSD baseline | 27.5 ± 3.8 | 26.2 ± 4.8 | 26.9 ± 2.4 | 27.3 ± 3.8 | 0.84 | 0.57 | 0.93 | 0.56 | 0.42 |
| HRSD final | 9.8 ± 7.3 | 19.4 ± 6.6 | 13.5 ± 6.9 | 12.0 ± 7.9 | 0.009 | 0.6 | 0.30 | 0.08 | 0.007 |
| HRSD change score | −17.7 ± 8.5 | −6.8 ± 6.8 | −13.4 ± 7.0 | −15.3 ± 8.8 |
0.007 | 0.84 | 0.32 | 0.06 | 0.005 |
| HRSD change score, age-adjusted |
−17.5 | −6.8 | −13.4 | −15.4 | |||||
All variance expressed as standard deviation.
BBD=`bathyphase minus bedtime difference’
DISCUSSION
This preliminary data from a secondary analysis of a clinical trial suggests that rest-activity patterns could be developed as a biomarker of antidepressant response to FLX monotherapy. The exact relationship between the bathyphase and the underlying phase of the circadian clock remains to be elucidated. Dim light melatonin onset (DLMO) is the gold standard for determining the position of the underlying circadian clock. (Lewy, 2007) It seems likely that the timing of the bathyphase is related to the timing of DLMO, although restactivity cycles are also influence by external factors such as social interaction and hence rest-activity cycles likely represent a “masking” of the underlying circadian clock. The estimated effects sizes, positive predictive value and negative predictive values were large and acceptable. Considering ‘BBD’ as a biomarker, it is a single variable that incorporates both a constituent element of a neural process (bathyphase: biological rhythms) and a patient behavior (choice of bedtime). By implication, BBD suggests that poor alignment between a patient bedtime and circadian rhythm lead to poor response to FLX. The data then go further to show that the vulnerability to poor antidepressant effect to FLX is mitigated with the addition of a second psychotropic (in this case, hypnotic treatment).
Limitations
Our preliminary data is consistent with the literature showing that circadian variables influence the expression of depressive symptoms, serotonin function, and antidepressant effect. Still, this preliminary data is based upon small numbers and bears replication. We also did not directly measure dim light melatonin onset, so the precise relationship of our findings to the underlying circadian clock is unknown. We only examined the response to one drug, FLX, but this choice is defended by the fact that SSRIs are the most commonly used class of antidepressants for both TRD MDD and non-TRD MDD.(Kubitz et al., 2013) The study was limited by our examination of response to only a single course of SSRI, and the emerging definition of TRD requires failure to two or more sequential courses of antidepressant treatments. Our statistical analysis of dichotomous outcomes only considered ‘treatment response’ and not ‘remission’, as there were only 13 patients who met a common definition of remission (HRSD ≤ 7) among the entire sample of 58 patients in this report. This was an insufficient number of remitted patients to allow for a meaningful analysis of remission. In retrospect, it would have been ideal to parse insomnia and depression into various subtypes, such as early morning awakening, or vegetative versus non-vegetative depression items, as these distinctions might be expected to explain some of the poor antidepressant results seen with an unfavorable BDD. However, we did not have the data needed to measure insomnia subtypes. While we noted that the timing of the bathyphase did not change with treatment, it would have been interesting to examine whether BDD might change with treatment; however we did have the bedtime data available at the end of treatment which would be needed to calculate a change in BDD.
Highlights.
Initial treatment of major depressive disorder (MDD) with selective serotonin reuptake inhibitor (SSRI) monotherapy is associated with low rates of remission, resulting in lost time, prolonged suffering, and subsequent trial-and-error approaches to treatment
A relative delay in rest-activity patterns is associated with more severe depression, while antidepressant treatments including SSRIs and ECT produce a compensatory advance in rest-activity patterns
Our pilot data shows that 24-hour measurement of rest-activity patterns with actigraphy, and the calculation of the average time of the lowest point of activity (the bathyphase), can be used to predict the likelihood of antidepressant response to an SSRI.
Patients with more delayed bathyphase values had the best antidepressant effect with SSRIs
Acknowledgements
The calculation of the bathyphase was conducted by Drs. William Shannon and Hong Xian. The author is grateful for their assistance.
Role of the funding source
The original data collection was supported by NIMH award MH70821, and by unrestricted financial support from Sunovion, and financial and material support from Mini Mitter. The funding sources has no role in the study design, collection of data, interpretation of the data, or drafting or approval of the manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
Reference List
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bennabi D, Aouizerate B, El-Hage W, Doumy O, Moliere F, Courtet P, Nieto I, Bellivier F, Bubrovsky M, Vaiva G, Holtmann T, Bougerol T, Richieri R, Lancon C, Camus V, Saba G, Haesbaert F, d'Amato T, Charpeaud T, Llorca P, Leboyer M, Haffen E. Risk factors for treatment resistance in unipolar depression: a systematic review. Journal of Affective Disorders. 2015;171:137–141. doi: 10.1016/j.jad.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Castanho A, Bothorel B, Seguin L, Mocaer E, Pevet P. Like melatonin, agomelatine (S20098) increases the amplitude of oscillations of two clock outputs: melatonin and temperature rhythms. Chronobiol Int. 2014;31:371–381. doi: 10.3109/07420528.2013.860457. [DOI] [PubMed] [Google Scholar]
- Chan JWY, Lam SP, Li SX, Yu MWM, Chan NY, Zhang Jihui, Wing Y-K. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37:911–917. doi: 10.5665/sleep.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtet P, Olie E. Circadian dimension and severity of depression. European Neuropsychopharmacology. 2012;22:S476–S481. doi: 10.1016/j.euroneuro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Marini S, Fornaro M, Srinivasan V, Iasevoli F, Tomasetti C, Valchera A, Perna G, Quera-Salva M-A, Martinotti G, di Giannantonio M. The melatonergic system in mood and anxiety disorders and the role of agomelatine: implications for clinical practice. Int J Mol Sci. 2013;14:12458–12483. doi: 10.3390/ijms140612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Symanzik J, Sharif A, Wang P, Duntley S, Shannon W. Powerful actigraphy data through functional representation. Chance. 2012 in press. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J.Psychiatr.Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. American Journal of Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AITR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kitajima T, Ikeda M, Yamanouchi Y, Kinoshita Y, Kawashima K, Okumura T, Tsunoka T, Ozaki N, Iwata N. CLOCK may predict the response to fluvoxamine treatment in Japanese Major Depressive Disorder patients. Neuromol Med. 2009;11:53–57. doi: 10.1007/s12017-009-8060-7. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. Journal of Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitz N, Mehra M, Potluri RC, Garg N, Cossrow N. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial cliams database. PLoS One. 2013;8:e76882. doi: 10.1371/journal.pone.0076882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972 Sep 30;:684–686. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- Lewy A. Melatonin and human chonobiology. Cold Spring Harbor Symposium on Quantitative Biology. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, Tranab GJ, Stone KL. Depressive symptoms and circadian activity rhythm disturbances in community-dwelling older women. American Journal of Geriatric Psychiatry. 2014;22:349–361. doi: 10.1016/j.jagp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler M, Gehrman P, Martin J, Ancoli-Israel S. The sigmoidaly transformed cosine curve: a mathematical model for circadian rhythms with a symmetric non-sinusoidal shapes. Statistics Medicine. 2006;25:3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- McCall WV, Blocker JN, D'Agostino RB, Jr, Kimball J, Boggs N, Lasater B, Haskett R, Krystal A, McDonald WM, Rosenquist PB. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. Journal of Clinical Sleep Medicine. 2010;6:322–329. [PMC free article] [PubMed] [Google Scholar]
- Moore R. The suprachiasmatic nucleus and the circadian timing system. Progress Molecular and Biological Translational Science. 2013;119:1–28. doi: 10.1016/B978-0-12-396971-2.00001-4. [DOI] [PubMed] [Google Scholar]
- Nomura K, Castanon-Cervantes O, Davidson A, Fukuhara C. Selective serotonin reuptake inhibitors and raft inhibitors shorten the period of Period-1-driven circadian bioluminescence rhythms in rat-1 fibroblasts. Life Science. 2008;82:1169–1174. doi: 10.1016/j.lfs.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2008 http://cran.r-project.org/doc/manuals/fullrefman.pdf.
- Ramsey J, Silverman B. Functional Data Analysis. New York: Springer; 2005. [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart J, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath P, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther M, Fava M. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Zakaria R, Othman Z, Lauterbach E, Acuna-Castroviejo D. Agomelatine in depressive disorders: its novel mechanism of action. Journal Neuropsychiatry Clin Neuroscience. 2012;24:290–308. doi: 10.1176/appi.neuropsych.11090216. [DOI] [PubMed] [Google Scholar]
- Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. Journal of Psychiatric Research. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Symanzik J, Shannon W. Exploratory graphics for functional actigraphy data. Proceedings, American Statistical Association. 2008 [Google Scholar]
- Winkler D, Pjrek E, Lanzenberger R, Baldinger P, Eitel D, Kasper S, Frey R. Actigraphy in patients with treatment-resistant depression undergoing electroconvulsive therapy. Journal of Psychiatric Research. 2014;57:96–100. doi: 10.1016/j.jpsychires.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Xian H, Gonzales C, Deych E, Shannon W, Farris S, Ding J, McCall W. Age-related effects on circadian phase in the sleep of patients with depression and insomnia. Behavioral Sleep Medicine. 2014 doi: 10.1080/15402002.2013.855213. in press. [DOI] [PubMed] [Google Scholar]