Abstract
Objectives
Over 40% of individuals with Parkinson’s disease (PD) have rapid eye movement sleep behavior disorder (RBD). This is associated with excessive sustained (tonic) or intermittent (phasic) muscle activity instead of the muscle atonia normally seen during REM sleep. We examined characteristics of manually-quantitated surface EMG activity in PD to ascertain whether the extent of muscle activity during REM sleep is associated with specific clinical features and measures of disease severity.
Methods
In a convenience sample of outpatients with idiopathic PD, REM sleep behavior disorder was diagnosed based on clinical history and polysomnogram, and severity was measured using the RBD sleep questionnaire. Surface EMG activity in the mentalis, extensor muscle group of the forearms, and anterior tibialis was manually quantitated. Percentage of REM time with excessive tonic or phasic muscle activity was calculated and compared across PD and RBD characteristics.
Results
Among 65 patients, 31 had confirmed RBD. In univariate analyses, higher amounts of surface EMG activity were associated with longer PD disease duration (srho = 0.34; p = 0.006) and greater disease severity (p < 0.001). In a multivariate regression model, surface EMG activity was significantly associated with RBD severity (p < 0.001) after adjustment for age, PD disease duration, PD severity and co-morbid sleep abnormalities.
Conclusion
Surface EMG activity during REM sleep was associated with severity of both PD and RBD. This measure may be useful as a PD biomarker and, if confirmed, may aid in determining which PD patients warrant treatment for their dream enactment to reduce risk of injury.
Keywords: Parkinson’s disease, REM sleep behavior disorder, REM sleep without atonia, Surface EMG activity
1. Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by loss of normal muscle atonia during REM sleep leading to dream-enactment. Idiopathic RBD is a harbinger of neurodegenerative disorder in over half of cases [1–4]. However, RBD is also an important disorder among individuals with already-established neurodegenerative disorders. It affects more than 40% of Parkinson’s Disease (PD) patients [5], and may portend more severe motor and non-motor PD manifestations [5–10].
The severity of REM sleep without atonia (RSWA) among individuals with idiopathic RBD has prognostic implications since it may predict the emergence of PD [11]. However, little is known about the clinical correlates of RSWA in established PD. One study suggests that RSWA severity is associated with more severe motor manifestations [12]. The implications of RSWA in PD patients without a history of dream enactment are also not well understood. To that end, our main objectives were to examine characteristics of manually-quantitated RSWA in mentalis and limbs in PD and to determine whether the extent of RSWA is associated with measures of PD and RBD. We hypothesized that higher amounts of RSWA would be associated with greater motor and dream enactment severity.
2. Methods
The University of Pennsylvania (UPenn) Institutional Review Board approved the study, and written informed consent was obtained from participants.
2.1. Sample
As part of a study examining RBD in PD, a convenience sample of PD patients was consecutively recruited from UPenn’s PD and Movement Disorders Center.
Included patients had a diagnosis of idiopathic PD based on established criteria [13], an available bed-partner, and were English-speaking. Patients with deep brain stimulation, previously diagnosed but untreated obstructive sleep apnea, dementia, or significant mobility problems precluding participation in overnight polysomnography (PSG) were excluded. We also excluded individuals taking benzodiazepines or melatonin on >2 nights a week since these medications can confound clinical history of RBD. No alcohol consumption was reported prior to sleep on the night of PSG. Seventy-five PD patients underwent PSG. Data on this cohort have been reported previously [14]. Sixty-five achieved REM sleep and were included in this analysis.
Demographics, disease duration (from diagnosis), and Hoehn &Yahr (H&Y) stage [15] were collected. Total intake of dopaminergic medications over a 24 h period was converted to levodopa equivalents daily dose (LEDD) according to standard equivalencies [16]. Medication dosing of >4 times per day was considered a proxy measure of motor fluctuations. Body side predominantly affected by motor symptoms was determined by review of the treating neurologist’s documentation and confirmed by the patient.
2.2. Polysomnographic analysis
All participants underwent one-night standard PSG. Two blinded, board-certified sleep specialists independently reviewed the PSGs. Standard American Academy of Sleep Medicine (AASM) guidelines to score and stage sleep were followed, and periodic limb movement (PLM) index and apnea-hypopnea (AHI) index calculated [17]. However, REM sleep stage was scored on the basis of EEG and electro-oculograms only, without requiring atonia. PLM indices were calculated separately for REM (PLMI-REM) and non-REM (PLMI-NREM) sleep. PLMs were distinguished from RBD-related phasic activity of RSWA based on their regular periodicity [17].
2.3. RBD determination
We determined clinical history of dream-enactment behavior through semi-structured interview with the patient and bed-partner. Patients were determined to meet International Classification of Sleep Disorders-II (ICSDII) criteria for RBD if there was polysomnographic evidence of RSWA along with a clinical history of dream enactment.
Patients were also administered the RBD screening questionnaire (RBDSQ) [18], a 10-item questionnaire with scores ranging from 0 to 13. Bed-partners were administered question 1 of the Mayo questionnaire (MQ1) [19] which asks “Have you ever seen the patient appear to “act out his/her dreams” while sleeping? (punched or flailed arms in the air, shouted or screamed).” A response of “yes” on MQ1 was considered consistent with RBD. Subquestions 1a–e that assess dream enactment duration and manifestations were also administered.
2.4. Designation of RSWA and quantification of surface EMG activity
Bipolar surface electrodes were placed on the mentalis, extensor surface of the forearms bilaterally, and anterior tibialis bilaterally. Although automated methods for detection of RSWA and quantification of surface EMG activity have been developed [20,21], manual scoring is considered the gold standard [22], and was utilized in this study. Epochs were classified as having “excessive tonic activity” (ETA) when the amplitude of the EMG activity reached ≥2 times the baseline for >50% of the epoch duration. Percent of time during which ETA was present during REM sleep was calculated (number of epochs with ETA/total number of REM epochs). Excessive phasic activity (EPA) was assessed in each 3-s mini-epoch of REM sleep, independently for the mentalis and each limb lead, as previously described [23]. The percentage of 3-s mini-epochs of REM sleep having EPA in ≥1 lead (mentalis or any limb) was calculated (RSWAmentalis+limbs), as was percentage of mini-epochs having EPA in just mentalis (RSWAmentalis, excluding limbs) or just limbs (RSWAlimbs, excluding mentalis).
In the application of AASM criteria [17], EMG activity was considered consistent with RSWA if ≥1 epoch from mentalis recording showed excessive tonic activity or ≥1 epoch from mentalis or limb recording contained excessive phasic activity in >50% of mini-epochs.
2.5. Analysis
Clinical (age, sex, disease duration, motor severity, medication use, RBD features) and PSG characteristics (total sleep time, REM sleep duration and number of episodes, RSWA, PLMs) were compared between patients with and without a clinical history of dream enactment. Between group differences were assessed using t-test or Wilcoxon rank sum test for non-parametric continuous variables and chi-square test or Fisher’s exact test for categorical variables. Spearman correlation coefficients (srho) were used to assess for univariate associations among the EMG determinations and other continuous variables.
To analyze clinical associations with RSWA, three groups were defined: those who met ICSDII criteria for RBD (group 1, n = 31), those without history of dream enactment but with RSWA on PSG (group 2, n = 12), and those without history of dream enactment and without RSWA on PSG (group 3, n = 15). We excluded the 7 patients with history of dream enactment who did not have RSWA from this analysis since all those patients had insufficient time in REM sleep (<10 min). To compare continuous variables among the three groups, ANOVA or Kruskal Wallis tests were used, with Bonferroni adjustment for multiple comparisons, and to compare categorical variables, chi-square test or Fisher’s exact test were used.
To examine whether the amount of phasic muscle activity during REM sleep is associated with RBDSQ scores, RSWA measures were square-root transformed and entered as the main independent variable in a multivariate regression model with RBDSQ score as the dependent variable. Any variables identified as being significantly associated with RBDSQ total score in univariate analyses were entered as covariates. The impact of PLMI-REM, SSRI use, and AHI as potential contributors to phasic activity during REM sleep was also assessed by adding them in as covariates.
3. Results
Clinical characteristics of the entire cohort and a comparison of those with and without a clinical history of RBD are shown in Table 1. Polysomnographic findings are listed in Table 2. Of the 7 subjects with motor fluctuations, 4 were H&Y stage II and 3 were H&Y stage III.
Table 1.
Clinical characteristic of the cohort with a comparison of subjects with vs. without a clinical history of RBD.
| Entire sample (n = 65) |
RBD by history (n = 38) |
No RBD by history (n = 27) |
p-value | |
|---|---|---|---|---|
| Median age in years (range) | 63.6 (38.3–86.7) | 64.6 (38.3–86.7) | 62.7 (52.6–70.1) | 0.19 |
| M:F | 44:21 | 27:11 | 17:10 | 0.50 |
| Hoehn and Yahr, N(%) | 0.40 | |||
| 1 | 7 (10.8) | 3 (7.9) | 4 (14.8) | |
| 2 | 53 (81.5) | 31 (81.) | 22 (81.5) | |
| 3 | 5 (7.7) | 4 (10.5) | 1 (3.7) | |
| Predominantly affected side at onset, N (%) | ||||
| Right | 33 (50.8) | 21 (55.2) | 12 (44.4) | 0.20 |
| Left | 29 (44.6) | 14 (36.8) | 15 (55.6) | |
| No clear asymmetry | 3 (5%) | 3 (8.0) | 0 (0) | |
| Median disease duration from diagnosis in years (range) | 3 (<1–18) | 4 (<1–18) | 2 (<1–14) | 0.018 |
| Motor fluctuations, N (%) | 7 (10.8) | 6 (15.8) | 1 (3.7) | 0.12 |
| Anti-depressant (SSRI) use, N (%) | 12 (18.5) | 9 (23.7) | 3 (11.1) | 0.20 |
| Median levodopa equivalent daily dose (range) | 460 (0–1908) | 524 0 (0–1908) | 400.0 (0–1050) | 0.30 |
| “Yes” to MQ1, n (%) | 39 (60) | 35 (92.1) | 4 (14.8) | <0.001 |
| Mean RBDSQ total score (SD) | 4.9 (3.4) | 6.6 (3.2) | 2.5 (1.6) | <0.001 |
Table 2.
Polysomnographic findings in the cohort with a comparison of subjects with vs. without a clinical history of RBD.
| Entire sample (n = 65) |
RBD by history (n = 38) |
No RBD by history (n = 27) |
p-value | |
|---|---|---|---|---|
| Mean total sleep time in minutes (SD) | 316.3 (70.2) | 311.7 (58.6) | 322.8 (84.4) | 0.40 |
| Median AHI (range)a | 8 (0–89) | 8.85 (0–89) | 7.6 (0.9–48.8) | 0.59 |
| Median REM time (range) | 36.0 (2.5–145.5) | 32.8 (2.5–125) | 37.5 (10–145.5) | 0.66 |
| Median REM as percentage of total sleep time (range) | 10.4 (1.2–34.5) | 7.9 (1.2–21.8) | 14.6 (3.9–34.5) | 0.21 |
| Number of REM periods, N (%) | ||||
| 1 | 22 (33.8) | 16 (42.1) | 6 (22.2) | 0.10 |
| 2 | 22 (33.8) | 12 (31.6) | 10 (37.0) | |
| 3 | 13 (20.0) | 6 (15.8) | 7 (25.9) | |
| 4 or 5 | 8 (12.3) | 4 (10.5) | 4 (14.8) | |
| Median REM latency in minutes (range) | 175.5 (52.5–419.0) | 184.5 (52.5–419) | 162.5 (54.5–379.5) | 0.15 |
| Median apnea-hypopnea index (SD; range) | 8 (0–89) | 8.85 (0–89.0) | 7.6 (0.9–48.8) | 0.60 |
| Median periodic limb movement index in NREM (range) | 0 (0–79.9) | 0 (0–45.8) | 0 (0–79.9) | 0.50 |
| Median periodic limb movement index in REM (range) | 0 (0–3.1) | 0 (0–3.1) | 0 (0–0.5) | 0.40 |
| Presence of RSWA by AASM criteria, N (%) | 43 (66.2) | 31 (81.6) | 12 (44.4) | 0.002 |
| Mean number of mini-epochs with RSWA (phasic activity) in (SD; range): | ||||
| mentalis | 7.6 (8.5; 0–42.5) | 10.2 (0.3; 0–42.5) | 4.1 (5.8; 0–27.5) | 0.003 |
| right arm | 7.0 (8.3; 0–45.2) | 9.8 (9.2; 0–45.2) | 3.2 (4.7; 0–21.4) | <0.001 |
| left arm | 7.1 (7.3; 0–34.9) | 9.9 (7.6; 0–34.9) | 3.2 (4.6; 0–21.7) | <0.001 |
| right leg | 7.9 (9.2; 0–51.8) | 9.14 (8.5; 0–35.4) | 6.1 (10.0; 0–51.8) | 0.030 |
| left leg | 9.0 (9.41; 0–41.0) | 10.7 (9.6; 0.2–39.5) | 6.5 (8.7; 0–41.0) | 0.011 |
| mentalis + all limbs | 25.4 (18.2; 1.3–88.0) | 31.8 (15.6; 5.3–68.3) | 16.3 (18.1; 1.3–88.0) | <0.001 |
| all limbs (excluding mentalis) | 20.7 (16.0; 0.9–82.0) | 25.7 (14.0; 4.7–65.2) | 13.6 (16.3; 0.9–82.9) | <0.001 |
Two patients with known, treated obstructive sleep apnea were included. Polysomnogram was performed while the patients were receiving continuous positive airway pressure. One patient had a clinical history of dream enactment and had evidence of RSWA on polysomnogram. The other had no clinical history of dream enactment and did not have evidence of RSWA on polysomnogram.
The percent of epochs with EPA in the mentalis correlated with percent of epochs with ETA (srho = 0.422, p = 0.005), and with EPA in the limbs combined (Spearman's rho (srho) = 0.52, p < 0.0001), right arm (srho = 0.36, p = 0.003), left arm (srho = 0.49, p < 0.0001), right leg (srho = 0.52, p < 0.0001), and left leg (srho = 0.42, p = 0.0005). The percent of epochs with EPA correlated between the arms (srho = 0.82, p < 0.0001) and legs (srho = 0.59, p < 0.0001). Phasic activity in ipsilateral arm and leg correlated (srho = 0.40, p = 0.0009 on right and srho = 0.57, p < 0.0001 on left).
When group 1 (those who met ICSDII criteria for RBD (n = 31)), group 2 (those without history of dream enactment but with RSWA on PSG (n = 12)), and group 3 (those without history of dream enactment and no RSWA (n = 15)) were compared there were no significant differences in age, sex, or LEDD. PD disease duration was significantly longer in those who met ICSDII criteria compared to those with no history of dream enactment and no RSWA (median 4 vs 2 years respectively, p = 0.005), but was not otherwise different in pairwise comparisons among the 3 groups.
Of the 38 patients with a clinical history of RBD, 31 had RSWA based on AASM criteria. In 24 cases, AASM criteria would not have been met when taking only mentalis phasic activity into account, but were met when considering mentalis plus limbs.
3.1. Correlates of muscle activity with patient characteristics and PD disease features
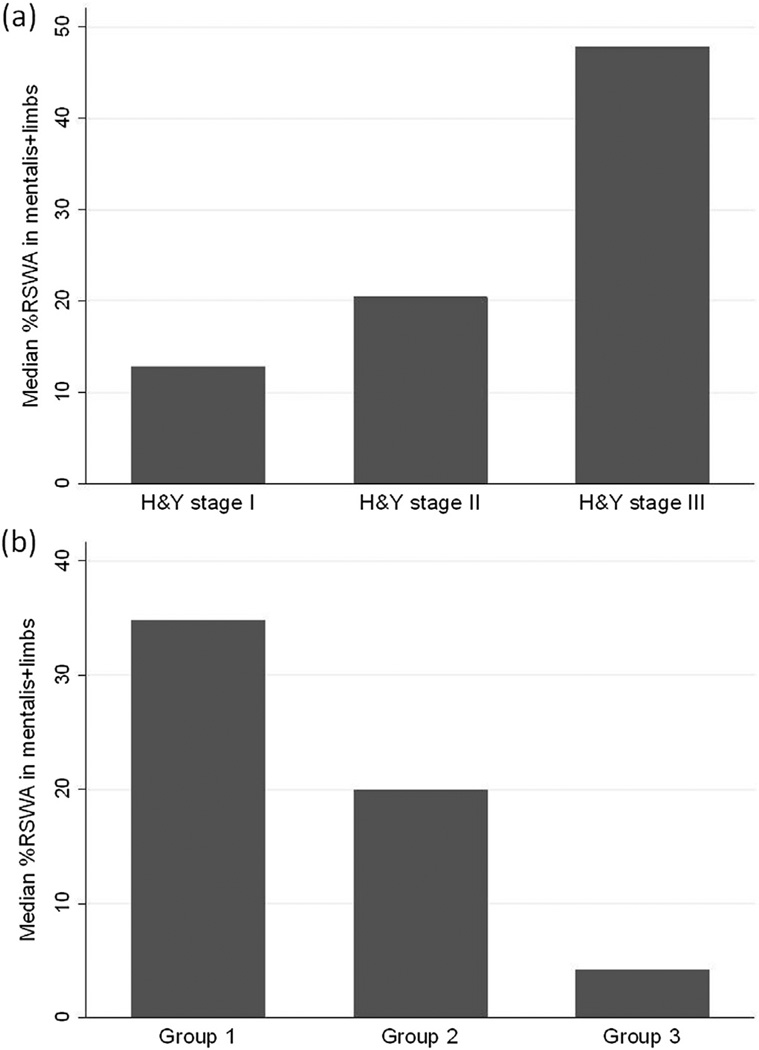
Univariate correlations between all measures of surface EMG activity and clinical factors are shown in Table 3 (Supplement). In multivariate analysis, greater RSWAmentalis+limbs correlated with longer PD disease duration (β = 0.12, 95% CI 0.0081–0.24, p = 0.036), but not age (β = 0.05, 95% CI −0.012–0.12, p = 0.109). Median RSWAmentalis+limbs was significantly greater in those with motor fluctuations compared to those without (median 35.12% vs. 20.36 respectively, p = 0.030) and in those with H&Y stage 3 compared to H&Y stage 1 (median 47.86% vs. 12.84% respectively, p = 0.003) or H&Y stage 2 (median 20.4%; p = 0.005; Fig. 1a).
Fig. 1.
(a) Percentage of mini-epochs containing excessive phasic activity in the mentalis and limbs in patients who were Hoehn and Yahr (H&Y) stages I, II, and III (b) Percentage of mini-epochs containing excessive phasic activity in the mentalis and limbs in group 1 (n = 31, met ICSDII criteria for RBD), group 2 (n = 12, RSWA but no dream enactment), and group 3 (n = 15, no RSWA, no dream enactment).
There were no differences in any measures of surface EMG by sex, LEDD, or SSRI intake. Individuals with asymmetric motor symptoms (n = 63) did not have different amounts of phasic activity when comparing the ipsilateral or contralateral side to the more motorically affected side. In those with disease predominantly on the right or left body, the median percentage of mini-epochs containing RSWA in the right arm and leg was 10.88 and 8.04 respectively (p = 0.40), and the median percentage of mini-epochs containing RSWA in the left arm and leg was 13.82 and 8.98 respectively (p = 0.20).
PLMI-REM correlated with RSWAmentalis+limbs (srho = 0.27, p = 0.028) and RSWAlimbs (srho = 0.3162, p = 0.010) but not RSWAmentalis (srho = 0.14, p = 0.26). The PLMI-NREM did not correlate with RSWAmentalis+limbs, RSWAlimbs, or RSWAmentalis
3.2. Correlates of muscle activity with RBD features
Median RSWAmentalis+limbs was 34.83% in group 1, 19.92% in group 2, and 4.29% in group 3 (Fig. 1b). After adjustment for multiple comparisons, median differences were significantly different between group 1 and group 3 (p = 0.004) and group 2 and group 3 (p < 0.001). This was also true for RSWAlimbs.
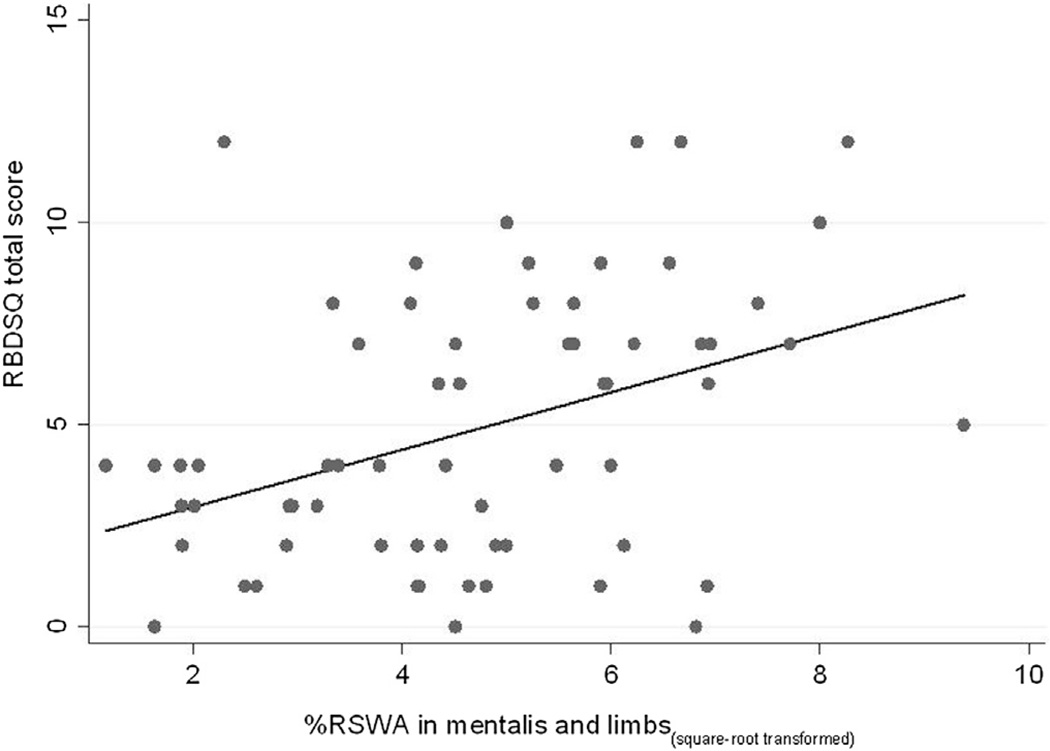
Median RSWAmentalis+limbs was 30% (range 17.1–46.4%) and 13% (range 4.2–20.4%; p = 0.0001) in those whose bed-partners responded yes or no to MQ1 respectively; RSWAlimbs was higher (median 26.11 vs. 12.07 respectively, p = 0.006) as was RSWAmentalis (median 8.85 vs 2.22 respectively, p = 0.005), but percent of epochs with ETA was not (p = 0.127). RSWAmentalis+limbs correlated with RBDSQ total scores (srho = 0.37, p = 0.003; Fig. 2), as did RSWAlimbs and percent of epochs with ETA.
Fig. 2.
Scatterplot of RBDSQ score (Y) against the square-root transformed percentage of mini-epochs containing excessive phasic activity in the mentalis and limbs (X).
In univariate analysis, RBDSQ total was not associated with age (srho = −0.14, p = 0.28), PD disease duration (srho = −0.035, p = 0.78), LEDD (srho = −0.020, p = 0.88), or AHI (srho = 0.06, 0.61). Median RBDSQ total scores were higher in the 12 patients taking an SSRI vs. the 53 who were not (7 vs. 4 respectively; p = 0.044) (9 of the 12 on an SSRI had a history of dream enactment). Median RBDSQ total scores were 4, 4, and 6 in those who were H&Y stage I, II, and III respectively (p = 0.80). Median RBDSQ total scores were 4 and 6 (p = 0.09) respectively, in those without vs. with motor fluctuations.
In terms of responses on individual items of the RBDSQ, mean RSWAmentalis+limbs was significantly greater in those that reported aggressive or action-packed dream content (p = 0.018), awareness of arms and legs moving during sleep (p < 0.0001), sudden limb movements (p = 0.002), and awakenings due to movements (p = 0.004). Mean RSWAmentalis+limbs was higher in those whose bed-partners reported injury to self or patient but this was not significant (30% compared to 23% respectively, p = 0.11).
In multivariate analyses with age, PD disease duration, and H&Y as covariates, (square-root transformed) RSWAmentalis+limbs was associated with RBDSQ (β = 0.23, p < 0.001, CI 0.11–0.35; r2 = 0.323 for the model). Substituting H&Y with fluctuator status or adding AHI, PLMI-REM, or SSRI intake into the model did not significantly impact the relationship between RSWA and RBDSQ (Table 4, Supplement). A significant relationship between (square-root transformed) RBDSQ and RSWAmentalis (β = 0.13, p < 0.016, CI 0.025–0.23) or RSWAlimbs (β = 0.22, p < 0.001, CI 0.10–0.35) was also demonstrated in multivariate regression analysis adjusting for covariates (Supplemental Tables 5 and 6).
4. Discussion
Our findings show that the amount of surface EMG activity during REM sleep in PD correlates with PD disease duration and PD disease severity. Further, we found that the amount of muscle activity correlates with clinical features of RBD as ascertained by the RBDSQ. This lends support to the concept that RBD is a marker of more severe disease manifestations, and identifies RSWA as a marker of both PD disease severity and RBD severity.
Many studies demonstrate that PD patients with RBD have more severe motor and non-motor manifestations [5–10]. More recently, RSWA alone (independent of history of dream enactment) has been associated with more severe PD manifestations such as freezing of gait [9]. Our finding that EMG surface activity correlated with PD disease duration, without an association between disease duration and RBDSQ scores, supports the suggestion that the severity of RSWA may be a marker of PD disease severity independent of history of dream enactment [9]. The pathophysiology of RSWA is unknown but is postulated to result from dysfunction of glutamatergic neurons in the locus subcoeruleus and cholinergic neurons in the pedunculopontine nucleus [9,10,24]. Thus, RSWA has been proposed as a measure of non-dopaminergic pathway dysfunction [9,25]. The more severe motor manifestations seen in those with greater surface EMG activity in our study may too be a reflection of involvement of non-dopaminergic pathways which are implicated in PD motor manifestations [26].
In contrast to our findings, a prior study of manually-quantitated mentalis EMG activity during REM sleep in PD found that phasic mentalis activity did not correlate with PD disease duration [12], It is likely these disparate findings are related to differences in methods of quantification of the surface EMG including their evaluation of only phasic mentalis activity. As is evident from our data, the correlation between mentalis and limb EMG activity is modest. Differences in clinical correlates of mentalis vs. limb activity are also observed in idiopathic RBD [11] and may relate to different hypothesized neuroanatomical sources of mentalis vs. limb atonia during REM. However, similar to Bliwise and colleagues [12], we also found greater phasic EMG activity in those with more severe PD motor manifestations using H&Y stage and levodopa dosing frequency as measures of disease severity. This suggests that the amount of phasic activity in REM sleep in PD can be an objective marker of motor disease severity. Future prospective studies are needed to confirm these findings since biomarkers of motor disease severity in PD are currently lacking.
While the motor manifestations of PD are often asymmetric, we did not find that phasic muscle activity differed on the side more affected by PD. Neurodegeneration in PD is often bilateral even in those with asymmetric clinical findings, and absence of asymmetry in the phasic muscle activity may be secondary to bilateral degeneration of the brainstem nuclei that mediate atonia during REM sleep. Greater amounts of muscle activity in mentalis and limbs during REM sleep in PD patients with symmetric motor manifestations have been reported [12,27]. Unfortunately, our sample was predominantly early and mild PD with clear asymmetry of motor symptoms, and only 3 patients had symmetric motor symptoms. Thus, we could not examine this relationship.
We also found that greater amounts of phasic activity are associated with higher RBDSQ scores. The RBDSQ has not been previously used as a measure of RBD severity, but our data suggest that it could be. Our findings are supported by the literature. Consens et al. [28] applied Lapierre and Montplaisir’s manual quantification criteria of surface EMG activity during REM sleep [29] in 17 patients with a neurodegenerative disorder. RBD symptoms were rated by bed-partners using a Likert scale. They also found an association between the RBD symptom score and amount of muscle activity. Variance in mentalis EMG activity during REM sleep using a computerized algorithm was also assessed [20] and a correlation was found between RBD symptom severity and percentage of REM mini-epochs with variance above a defined threshold.
At this time, data regarding indications for treatment of RBD are limited, but RBD is generally treated if the history suggests risk of injury to the patient and bed-partner [30]. We propose that the amount of surface muscle activity in REM sleep be studied as an ancillary measure of this risk. If our findings are confirmed, use of surface EMG to guide treatment decisions would be particularly useful in cases when collateral information from a bed-partner (regarding risk of injury) is not available. In confirming our findings, night-to-night variability in surface EMG activity as a marker of RBD disease severity will be important to examine. However, until the utility of surface EMG as a measure of RBD severity is better characterized, a clinical history suggesting risk of injury would indicate a need to treat RBD even if there is minimal surface EMG activity present during PSG.
5. Limitations
For logistic reasons including patient comfort, PSG was limited to one night, and it is possible that first-night effects confounded our results. In addition, we ascertained information related to motor manifestations (such as asymmetry of features) through clinical chart review and patient interview rather than direct patient assessment, which may have impacted the accuracy of this data. Unfortunately, the Unified Parkinson’s Disease Rating Scale (UPDRS) score was not uniformly available, but the correlation between H&Y and UPDRS is strong and we feel the former, along with dosing frequency as a proxy measure of motor fluctuations, adequately captured motor disease severity. Finally, while we included patients who had more advanced PD, the majority of our cohort had mild PD, and thus our findings may not be generalizable to the entire PD population.
6. Conclusion
In PD, the amount of surface EMG activity during REM sleep in the mentalis and limbs is associated with longer PD disease duration and greater PD disease severity. Quantification of RSWA could potentially be used as a marker of motor disease severity in PD. The amount of phasic muscle activity also correlates with clinical features of RBD as ascertained by the RBDSQ. If confirmed, this may also help guide medical management of RBD in PD.
Acknowledgments
We thank the patients whose participation made this work possible. We also thank the staff at the Hospital of the University of Pennsylvania Clinical Research Center for Sleep. This work was supported through the generosity of the Vandrevala family. Dr. Dahodwala was supported by K23 AG034236 during this project.
Appendix A. Supplementary data
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.parkreldis.2014.04.011.
References
- 1.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): 16year update on a previously reported series. Sleep Med. 2013 Jan 21;14(8):744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000 Feb;123(Pt 2):331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Lomena F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010 Nov;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009 Dec;13(6):385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011 Sep 13;77(11):1048–1054. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 6.Sommerauer M, Valko PO, Werth E, Poryazova R, Hauser S, Baumann CR. Revisiting the impact of REM sleep behavior disorder on motor progression in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(4):460–462. doi: 10.1016/j.parkreldis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord. 2012 May;27(6):720–726. doi: 10.1002/mds.24939. [DOI] [PubMed] [Google Scholar]
- 8.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008 Oct;79(10):1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 9.Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, Mackinnon CD. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology. 2013;81(12):1030–1035. doi: 10.1212/WNL.0b013e3182a4a408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2007 Nov 6;69(19):1843–1849. doi: 10.1212/01.wnl.0000278114.14096.74. [DOI] [PubMed] [Google Scholar]
- 11.Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010 Jan 19;74(3):239–244. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Ann Neurol. 2010 Sep;68(3):353–359. doi: 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992 Mar;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahine LM, Daley J, Horn S, Colcher A, Hurtig H, Cantor C, et al. Questionnaire-based diagnosis of REM sleep behavior disorder in Parkinson’s disease. Mov Disord. 2013 Mar 20;28(8):1146–1149. doi: 10.1002/mds.25438. [DOI] [PubMed] [Google Scholar]
- 15.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010 Nov 15;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 17.for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. In: Iber C, Ancoli-Israel S, Chesson A, Quan SF, editors. Westchester: American Academy of Sleep Medicine Ed. 2007. [Google Scholar]
- 18.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord. 2007 Dec;22(16):2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 19.Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011 May;12(5):445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns JW, Consens FB, Little RJ, Angell KJ, Gilman S, Chervin RD. EMG variance during polysomnography as an assessment for REM sleep behavior disorder. Sleep. 2007 Dec 1;30(12):1771–1778. doi: 10.1093/sleep/30.12.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferri R, Fulda S, Cosentino FI, Pizza F, Plazzi G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson’s disease with or without REM sleep behavior disorder. Sleep Med. 2012 Jun;13(6):707–713. doi: 10.1016/j.sleep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Frauscher B, Ehrmann L, Hogl B. Defining muscle activities for assessment of REM sleep behavior disorder: from a qualitative to a quantitative diagnostic level. Sleep Med. 2012;14(8):729–733. doi: 10.1016/j.sleep.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Montplaisir J, Gagnon JF, Fantini ML, Postuma RB, Dauvilliers Y, Desautels A, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010 Oct 15;25(13):2044–2051. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 24.de la Fuente-Fernandez R. A predictive model of neurodegeneration in idiopathic REM-sleep behavior disorder. Parkinsonism Relat Disord. 2013;19(11):1009–1012. doi: 10.1016/j.parkreldis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Yoon IY, Kim JM, Jeong SH, Kim KW, Shin YK, et al. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur J Neurol. 2010 Mar;17(3):487–492. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 26.Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013 Feb;28(2):131–144. doi: 10.1002/mds.25273. [DOI] [PubMed] [Google Scholar]
- 27.Postuma RB, Gagnon JF. Symmetry of Parkinson’s disease and REM sleep: one piece of the puzzle. Ann Neurol. 2011 May;69(5):905. doi: 10.1002/ana.22259. author reply 906. [DOI] [PubMed] [Google Scholar]
- 28.Consens FB, Chervin RD, Koeppe RA, Little R, Liu S, Junck L, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005 Aug 1;28(8):993–997. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 29.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992 Jul;42(7):1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 30.Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010 Feb 15;6(1):85–95. [PMC free article] [PubMed] [Google Scholar]