Abstract
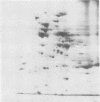
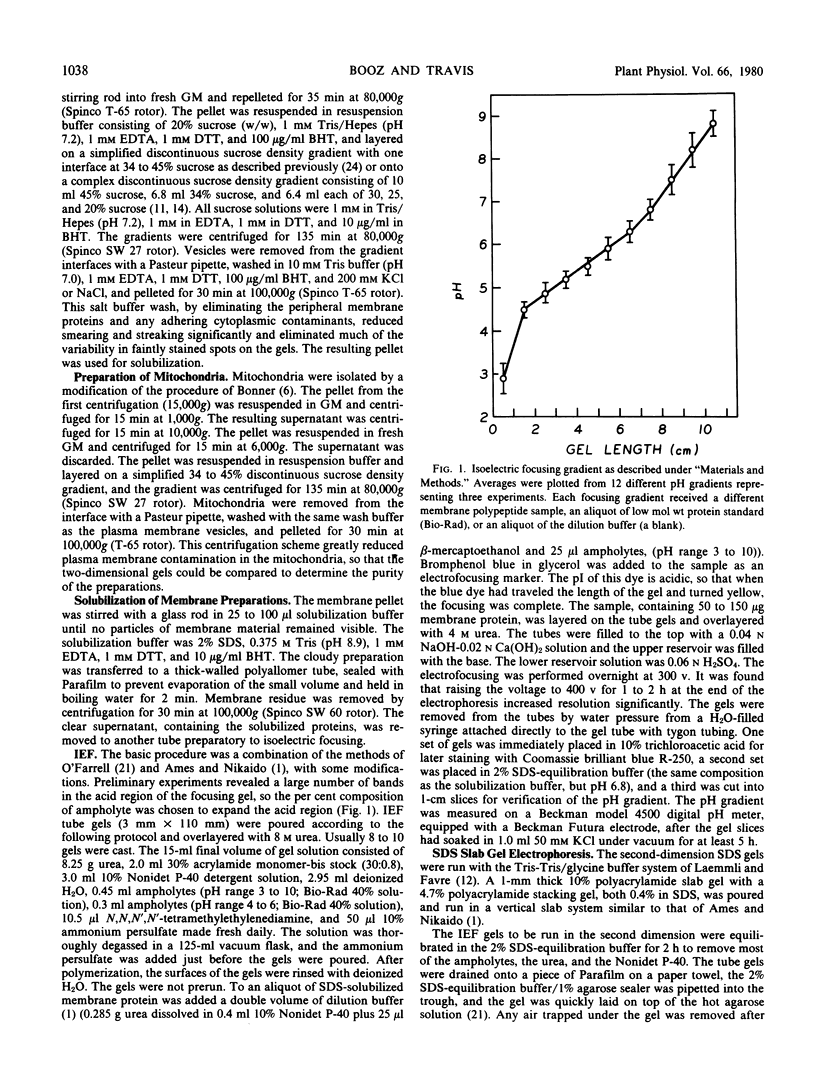
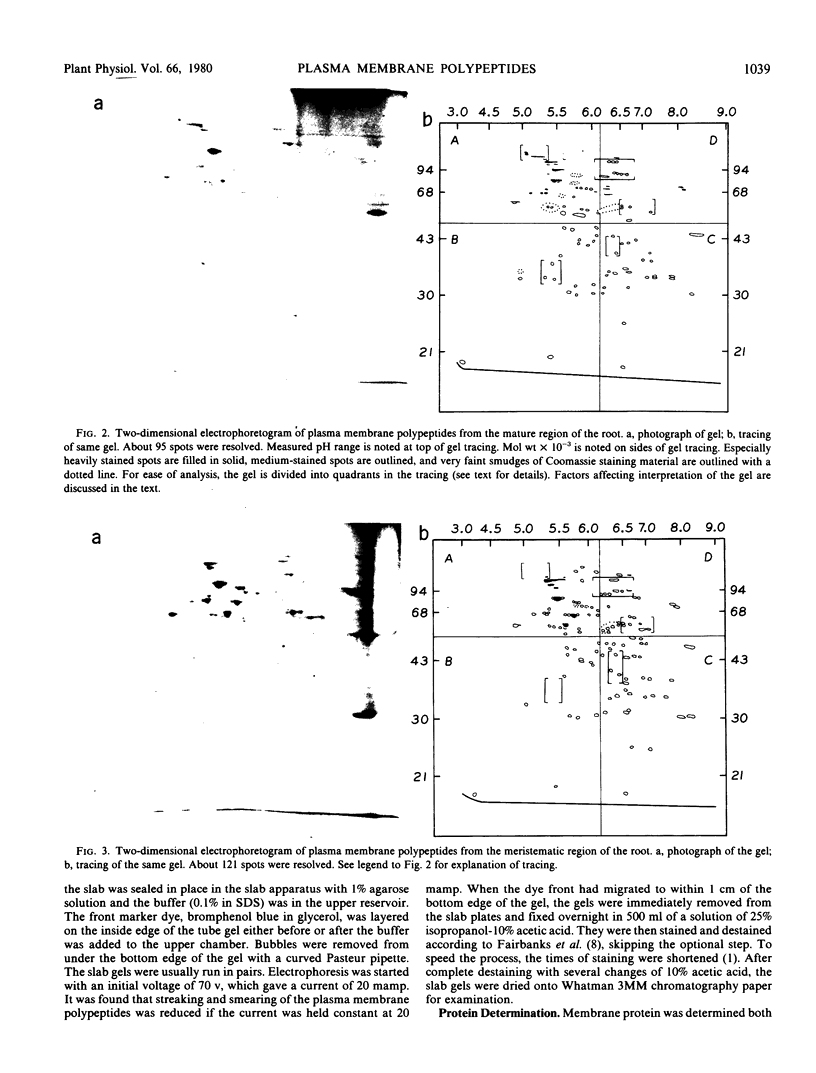
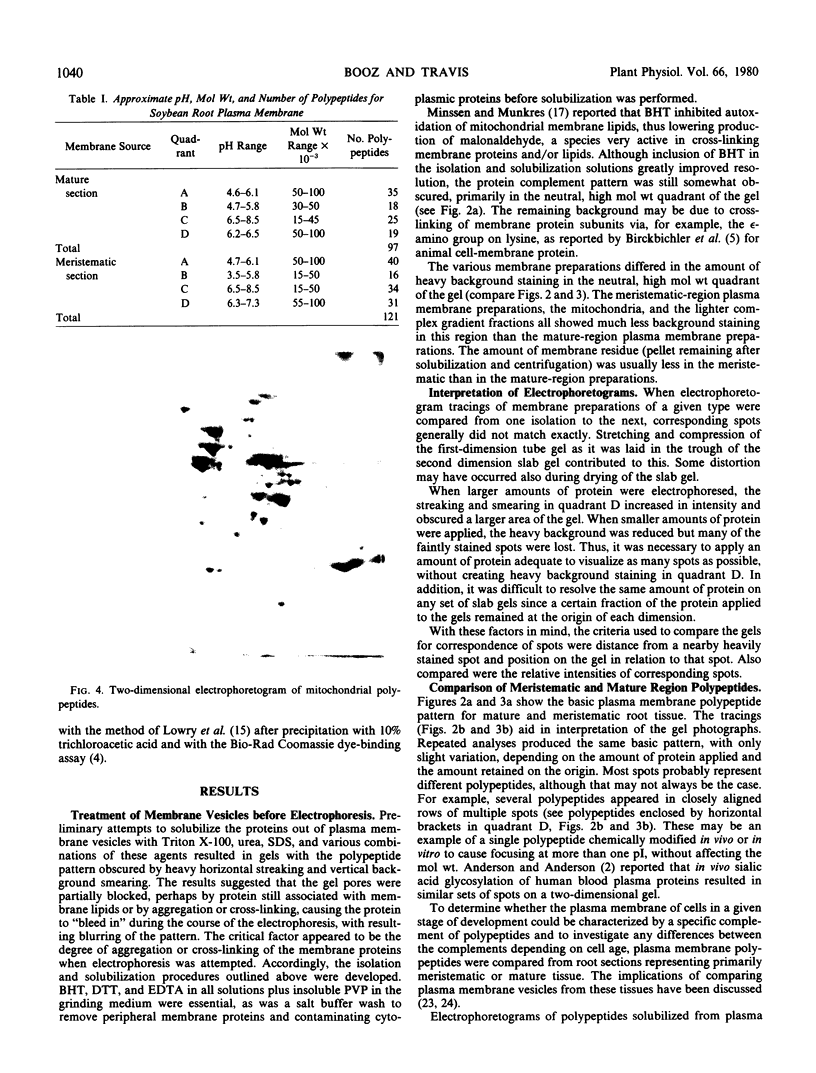
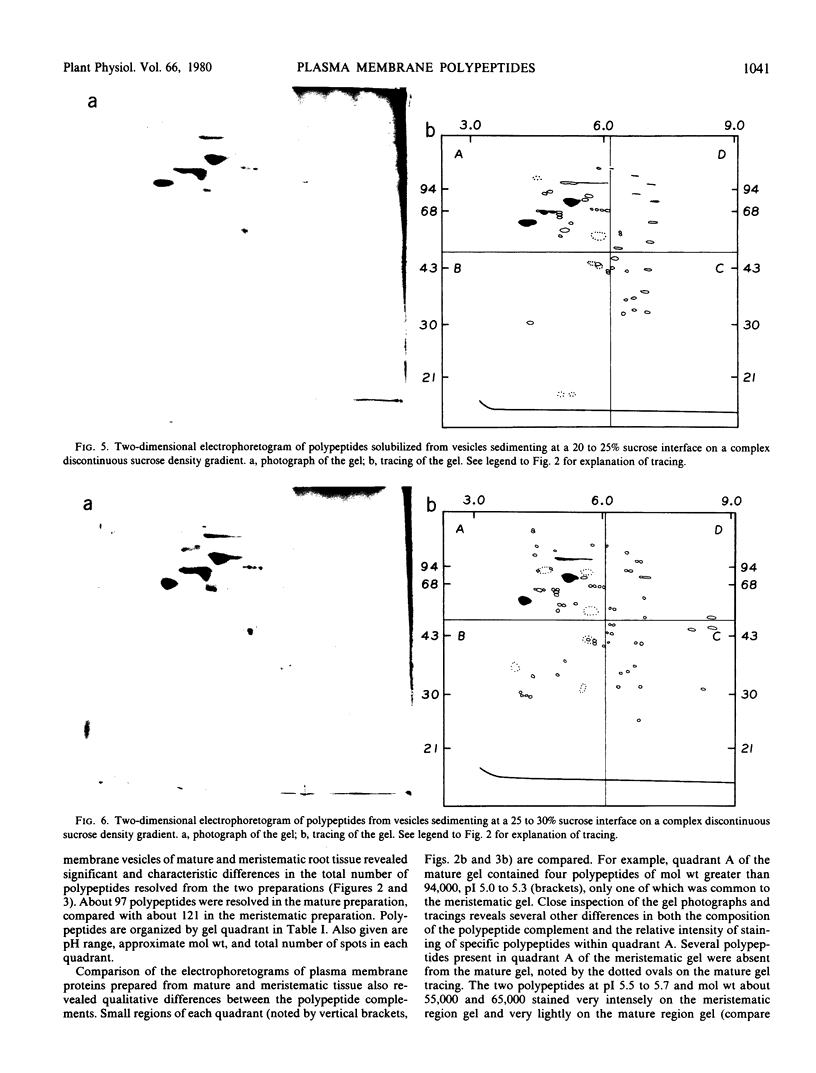
The polypeptide complement of enriched soybean (Glycine max [L.] Merr. cult. wells) root plasma membrane fractions was studied by two-dimensional gel electrophoresis. Good resolution was obtained when polypeptides were solubilized in sodium dodecyl sulfate and when butylated hydroxytoluene was included in the vesicle isolation and solubilization media. The pattern obtained on the two-dimensional slab gel for root plasma membrane was characteristic for that membrane. The polypeptide complements from mitochondrial membranes and from enriched fractions of three other endomembrane components were solubilized and electrophoresed for comparison. Each membrane preparation was identifiable on the basis of its characteristic electrophoretogram. Electrophoresis of protein solubilized from plasma membrane fractions isolated from meristematic and mature root tissue revealed both qualitative and quantitative differences in the respective protein complements.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. L., Travis R. L. An electron microscope comparison of plasma membrane vesicles from meristematic and mature soybean root tissue. Plant Physiol. 1979 Jun;63(6):1191–1197. doi: 10.1104/pp.63.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birckbichler P. J., Dowben R. M., Matacic S., Loewy A. G. Isopeptide bonds in membrane proteins from eukaryotic cells. Biochim Biophys Acta. 1973 Jan 2;291(1):149–155. doi: 10.1016/0005-2736(73)90070-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews R. A., Johnson T. C., Hudson J. E. Synthesis and turnover of plasma-membrane proteins and glycoproteins in a neuroblastoma cell line. Biochem J. 1976 Jan 15;154(1):57–64. doi: 10.1042/bj1540057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minssen M., Munkres K. D. Preparation of mitochondrial membrane proteins from Neurospora crassa: prevention of lipid autoxidation damage by an antioxidant. Biochim Biophys Acta. 1973 Jan 26;291(2):398–410. doi: 10.1016/0005-2736(73)90492-6. [DOI] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Plasma membrane protein subunit composition. A comparative study by discontinuous electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1971 Oct 25;246(20):6335–6338. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Siu C. H., Lerner R. A., Loomis W. F., Jr Rapid accumulation and disappearance of plasma membrane proteins during development of wild-type and mutant strains of Dictyostelium discoideum. J Mol Biol. 1977 Nov 5;116(3):469–488. doi: 10.1016/0022-2836(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Berkowitz R. L. Characterization of Soybean Plasma Membrane during Development: FREE STEROL COMPOSITION AND CONCANAVALIN A BINDING STUDIES. Plant Physiol. 1980 May;65(5):871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweto J., Doyle D. Turnover of the plasma membrane proteins of hepatoma tissue culture cells. J Biol Chem. 1976 Feb 10;251(3):872–882. [PubMed] [Google Scholar]