Obesity and heart failure (HF) are twin public health problems. Moreover, obesity can contribute to HF (1). Each contributes to increased mortality. Conversely, weight loss in obese patients without HF improves diastolic function and decreases oxygen requirements and left ventricular (LV) mass (2,3). In two large studies, surgery-induced weight loss reduced cardiac death and improved survival in humans (4,5), but it is unknown how many suffered from HF. So physicians should recommend weight loss, right?
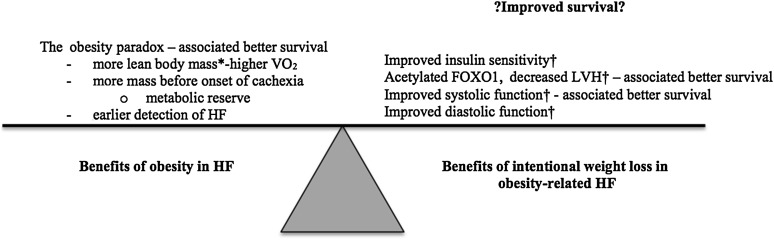
The problem is—there is a paradox. That is “the rub.” The “obesity paradox” is based on outcomes data that show that patients who are obese and have HF live longer than patients who are not obese (Fig. 1). There are multiple possible reasons for the paradox (recently reviewed by Lavie et al. [6]). Patients with obesity-related HF may be diagnosed earlier; they do not suffer from cachexia; they may also have an advantage because they are starting from a higher body weight before the cachexia of chronic disease (HF) begins; and/or there may be other benefits from adipose and lean muscle tissue. Regardless of the mechanism, the paradox leaves us with a conundrum: to recommend weight loss or not for patients with obesity-related HF.
Figure 1.
A summary of the competing beneficial effects of obesity and intentional weight loss. *The murine model in the study by Sankaralingam et al. (7) did not show a change in lean body mass with intentional weight loss. Most human studies do show higher lean body mass in obesity and a decrease with weight loss. †Changes seen in the study by Sankaralingam et al. (7).
The study by Sankaralingam et al. (7) in this issue of Diabetes gives us more information on both the effects of diet-induced obesity and weight loss on the failing heart. In this study, obesity and HF were induced in a murine model by high-fat feeding and abdominal aortic constriction. These led to increased left ventricular hypertrophy (LVH), diastolic dysfunction, and myocardial insulin resistance. Whether the heart can become insulin resistant in a nongenetically modified animal was not clear. One study in human type 1 diabetes suggested that only the skeletal muscle—not the heart—could become insulin resistant (8). However, on the basis of the data from the study by Sankaralingam et al., the heart can become insulin resistant (7). The authors demonstrate this by measuring changes in myocardial glucose oxidation and by demonstrating appropriate changes in insulin-signaling pathways, such as increased SOCS3 expression. Interestingly, the study goes on to show that neither pyruvate dehydrogenase kinase 4 nor phosphorylated pyruvate dehydrogenase changed with increasing obesity. Thus, the authors suggest that obesity-related decrease in myocardial glucose oxidation resulted from the increase in myocardial fatty acid oxidation via the Randle cycle.
Weight loss induced by a low-fat, low-calorie diet (7) reversed many of these obesity-related HF changes. LVH regressed after weight loss and was accompanied by increased acetylation of FOXO1 (a key mediator of hypertrophy) and increased atrogin-1 expression (7). Diastolic function improved and insulin sensitivity increased (7), the latter accompanied by STAT3 activation, decreased SOCS3 expression, and increased GLUT4 expression.
This elegant and well-controlled study by Sankaralingam et al. has several strengths. First, the authors used a diet-induced obesity model rather than an extreme model of obesity based on genetically modified strain. Thus, the authors used a model that is more akin to most human obesity. Another strength of this study is that because it was in a murine model, it was easier to enforce strict and specific diet adherence, with resultant predictable weight gain and loss. Results in human diet studies are often not quite so predictable. The current study also had several control groups, including a sham surgery group. This is also not usually possible in humans. Last, Sankaralingam et al. were able to sample myocardial tissue to evaluate myocardial expression and acetylation of key components of the hypertrophic and metabolic pathways. Thus, these findings extend our understanding of the changes in these pathways with weight gain and loss in the setting of HF. Previously, Leichman et al. (2) demonstrated in humans without HF that weight loss is associated with improved whole-body insulin sensitivity and skeletal muscle expression of key metabolic enzymes (e.g., peroxisome proliferator–activated receptor α and medium-chain acetyl-CoA dehydrogenase). Sampling myocardial tissue is also typically not done in human studies and is potentially more prone to sampling error than in studies of rodent heart tissue.
One difficulty with extending the current study's findings to humans is that the animals’ ejection fractions decreased to ∼45–50%. This range of ejection fraction is difficult to categorize as either “HF with preserved ejection fraction” or “HF with reduced ejection fraction”—the two main categories of human HF. These two types of HF often have different etiologies and responses (or lack of responses) to treatment. In addition, there are no survival data in the current study. When trying to apply the study findings to our understanding of human obesity-related HF and how to treat it, it would be best to try to rigorously phenotype the subjects (as was done by Sankaralingam et al.), determine the degree of obesity, and ascertain as best as possible how much the obesity caused or contributed to each particular patient's HF and symptoms. We cannot ignore the mortality data supporting the obesity paradox simply because it is logical to do so. Although there are multiple society guidelines supporting intentional weight loss in obese patients with HF (6), it would be best to build from the solid data from Sankaralingam et al. (7) and perform outcomes studies on obese mice and humans with HF that undergo intentional weight loss. Randomized weight-loss studies in well-phenotyped subjects with HF would also help us answer the simple question: whether ‘tis nobler to lose weight or not to lose weight in obesity-related HF.
Acknowledgments
Funding. The research of L.R.P. is supported by funding from the National Institutes of Health (P20 HL113444-01 and R01 HL107406-01A1).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1643.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313 [DOI] [PubMed] [Google Scholar]
- 2.Leichman JG, Wilson EB, Scarborough T, et al. Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med 2008;121:966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin CH, Kurup S, Herrero P, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring) 2011;19:1804–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöström L, Narbro K, Sjöström CD, et al.; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 5.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 6.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93–102 [DOI] [PubMed] [Google Scholar]
- 7.Sankaralingam S, Abo Alrob O, Zhang L, et al. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the obesity paradox. Diabetes 2015;64:1643–1657 [DOI] [PubMed] [Google Scholar]
- 8.Nuutila P, Knuuti J, Ruotsalainen U, et al. Insulin resistance is localized to skeletal but not heart muscle in type 1 diabetes. Am J Physiol 1993;264:E756–E762 [DOI] [PubMed] [Google Scholar]