Abstract
The inactive full-length form of GLP-1(1-37) stimulates conversion of both rat and human intestinal epithelial cells into insulin-secreting cells. We investigated whether oral administration of human commensal bacteria engineered to secrete GLP-1(1-37) could ameliorate hyperglycemia in a rat model of diabetes by reprogramming intestinal cells into glucose-responsive insulin-secreting cells. Diabetic rats were fed daily with human lactobacilli engineered to secrete GLP-1(1-37). Diabetic rats fed GLP-1–secreting bacteria showed significant increases in insulin levels and, additionally, were significantly more glucose tolerant than those fed the parent bacterial strain. These rats developed insulin-producing cells within the upper intestine in numbers sufficient to replace ∼25–33% of the insulin capacity of nondiabetic healthy rats. Intestinal tissues in rats with reprogrammed cells expressed MafA, PDX-1, and FoxA2. HNF-6 expression was observed only in crypt epithelia expressing insulin and not in epithelia located higher on the villous axis. Staining for other cell markers in rats treated with GLP-1(1-37)–secreting bacteria suggested that normal function was not inhibited by the close physical proximity of reprogrammed cells. These results provide evidence of the potential for a safe and effective nonabsorbed oral treatment for diabetes and support the concept of engineered commensal bacterial signaling to mediate enteric cell function in vivo.
Introduction
Reprogramming non–β-cells into β-cells or cells with insulin-secreting potential has been the subject of several studies over the past decade (1–9). Research has focused on a number of areas, including in vitro generation of β-cells from pancreatic (e.g., acinar cells) and liver cell lineages for transplantation as well as causing either pancreatic or other tissue-specific cells to convert to β-cells in vivo (10). The potential of this latter approach became evident with the discovery by Suzuki et al. (11) that the full-length form of GLP-1(1-37), previously thought to be inactive, could stimulate rat intestinal epithelial cells to become glucose-responsive insulin-secreting cells, ostensibly through the Notch signaling pathway. The results from Suzuki et al. (11) suggested that undifferentiated intestinal epithelia in rats (differentiation occurring after E15) can develop into β-like cells. The study also demonstrated the reversal of streptozotocin (STZ)-induced type 1 diabetes in adult rats after surgical implantation with embryonic jejunum (E14.5) incubated with GLP-1(1-37) in vitro. The authors concluded that adult enterocyte differentiation, which occurs from the intestinal crypts, would not give rise to significant numbers of insulin-producing cells and that the proliferating and pseudostratified cells of the developing fetus (pre-E17) would likely be required for significant differentiation into cells with β-like functionality.
While the study by Suzuki et al. (11) reported positive results with GLP-1(1-37) as an agent to reprogram intestinal cells, their study also highlighted the difficulty in delivering this bioactive compound by injection and surgery. The circulating active form of GLP-1 is GLP-1(7-37), which has a very short biological half-life of the order of just a few minutes in blood (12). This short half-life may be a reason for the lower reprogramming rates with GLP-1(1-37) observed in adult rats, as it would be necessary for GLP-1(1-37) to be present in systemic circulation for a longer period of time in order to reach the intestinal crypts.
Other means of delivering bioactive compounds to the luminal (villous) side of the upper intestine, avoiding the potential pitfalls of surgery or degradation in the bloodstream, have been published using intestinal commensal bacteria that populate the gut with the ability to secrete specific signals (13–24). In this approach, signals (small molecules, peptides) can be delivered directly to the luminal side of the intestine by bacteria that already have an established line of communication with intestinal epithelia.
In a previous in vitro study, we demonstrated that engineered commensal bacteria can deliver GLP-1(1-37) to human intestinal carcinomas and stimulate glucose-responsive insulin secretion (23). In that work, Escherichia coli Nissle 1917 was transformed to secrete GLP-1(1-37) from a plasmid in response to an exogenous inducer. We also confirmed that GLP-1(1-37) and not the active form [GLP-1(7-37)] reprograms enterocytes as part of the work being reported here (Supplementary Fig. 1).
Further, for this investigation, we tested the hypothesis that a chromosomally modified human gram-positive bacterial strain that constitutively secretes GLP-1(1-37) could reduce hyperglycemia in a rat model of diabetes. Our goal was to reprogram rat intestinal cells into glucose-responsive insulin-secreting cells through daily oral administration of GLP-1(1-37)–secreting bacteria. We measured coexpression of β-cell and enteroendocrine markers to determine the extent and possible mechanism of reprogramming.
Research Design and Methods
Strain Construction
To transform Lactobacillus gasseri ATCC 33323 (L) into a strain that secretes GLP-1(1-37), constitutively standard techniques were used. Details are in the Supplementary Data. We called the positive integrants LG.
Rat Experiments
All rats used in this study were purchased from The Jackson Laboratory and housed at the East Campus Research Facility at Cornell University. Studies were conducted in accordance with protocols approved by the Cornell University Institutional Animal Care and Use Committee. All rat experiments were repeated twice with six rats per treatment group.
STZ Model
Rats were fasted for 6 h before being injected with STZ at a dose of 70 mg/kg of body weight (BW; in cold 0.1 mol/L sodium citrate buffer pH 4.2) via intraperitoneal route. Blood glucose levels were monitored every 3 days until diabetic glucose levels (>350 mg/dL) were reached. Once rats had sustained blood glucose levels over 350 mg/dL, they were enrolled in the study.
Bacterial Feeding
After enrollment in the study, rats were given ampicillin-treated (1 g/L) water for 18 h. Lactobacillus strains LG (2 µg/mL erythromycin) or L were grown in MRS media. The resulting pellet was redissolved in sterile MRS with 1% sucrose. The rats were fed 1.6 mL/kg BW MRS with 1% sucrose containing 1010 CFU/mL of MRS-grown Lactobacillus strains separately (L or LG). All bacterial strain–fed rats were fed 2 times per day with bacteria for 90 days.
Glucose Tolerance Test and ELISA
After bacterial feeding for 51 days, STZ-treated rats were fasted 10 h and weighed, and a blood sample was collected from the tail vein using heparinized Microhematocrit Capillary Tubes (Fisher, PA). They were then orally administered 1 g glucose/kg BW, and blood samples were taken at 0.5, 1, 1.5, and 2 h. Plasma glucose was measured using the Breeze2 blood glucose monitoring system. Plasma insulin was measured using Rat/Mouse Insulin ELISA Kit (Millipore, MA) according to the manufacturer’s instructions.
Tissue Homogenization of Pancreas and Upper Intestine, Blood Sampling, and ELISA
After 90 days of bacterial feeding, rats were killed and their pancreata and intestines were removed and frozen at −80°C until analysis. For each rat, the pancreas and upper intestine were weighed and independently homogenized using a chilled mortar with 1× PBS. Insulin was measured for both pancreata and upper intestines using Rat/Mouse Insulin ELISA Kit (Millipore, MA) according to the manufacturer’s instructions.
Bacterial Colonization Counts
After feeding for 90 days, rats were transferred to new cages for 3 days. Feces were collected from the new cages, and rats were killed. Three rats from each treatment were dissected, and their upper gastrointestinal (GI) tracts were removed. The upper GI tracts were each weighed and homogenized in 2 mL of fresh MRS medium. Homogenized tissue was plated onto MRS with erythromycin (2 µg/mL) by serial dilution. Plates were incubated overnight at 37°C and their colonies counted.
Immunofluorescence
Upper intestinal, pancreatic, and liver tissues of rats killed after feeding with L or LG for 90 days and treated IEC-6 cells were fixed, and tissues were dissected. After deparaffinization, fixed tissue slides were steamed in 0.01 mol/L citrate buffer, pH 6.0. After washing in PBS, 10% normal blocking serum (Santa Cruz Biotechnology, CA) was applied for 1 h at room temperature. Two hundred microliters of 4% formaldehyde diluted in warm PBS for 15 min in a fume hood was added to treated IEC-6 cells in six-well plates. The cells were washed three times in 1× PBS for 5 min each, and 10% normal blocking serum (Santa Cruz Biotechnology, CA) was applied for 1 h at room temperature. For all tissues and IEC-6 cells, 1:500 diluted goat anti–PDX-1, 1:50 diluted genuine pig anti-insulin, 1:50 mouse anti–GLP-1 (ab23447; Abcam, Cambridge, MA), 1:50 diluted rabbit anti-insulin, 1:500 rabbit anti–PDX-1, 1:50 rabbit anti–HNF-6, 1:50 goat anti-chromogranin A (ChrA), 1:50 goat anti-lysozyme, 1:50 goat anti–SOX-9, 1:50 goat antisucrase isomaltase (anti-SI), 1:50 goat anti-glucagon, or 1:50 goat anti-FoxA2 (Santa Cruz Biotechnology, CA) was applied to blocked samples that were then incubated overnight at 4°C. After washing four times in PBS, a fluorochrome-conjugated secondary antibody Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 555 donkey anti-goat IgG or Alexa Fluor 488 donkey anti-goat IgG or Alexa Fluor 488 goat anti-guinea pig IgG and Alexa Fluor 555 donkey anti-rabbit IgG or Alexa Fluor 488 donkey anti-mouse IgG (Invitrogen) diluted 1:200 in PBS was applied to samples for 1.5 h at room temperature in a humid chamber. After washing three times in PBS, samples were then mounted with Vectashield mounting medium with DAPI (Vector, CA). Specimens were examined immediately using the appropriate excitation wavelength for each fluorophore. Images were taken with a Zeiss 710 Confocal Microscope (Zeiss, Jena, Germany).
RT-PCR
Total RNA was isolated from the intestines of rats fed with LG or L and also from the pancreata and livers of healthy control rats using the RNAqueous Kit (Life Technologies, NY). cDNA was synthesized by SuperScript III First-Strand Kit (Invitrogen, CA). The primers used for amplifying different genes are listed in the Supplementary Data.
IEC-6 Cell Culture With GLP-1(1-37) and Small Interfering RNA and ELISA
Sixty percent confluent monolayers of IEC-6 cells in four-chamber culture slides were covered with 1 mL DMEM with 10% FBS and 10 µg/mL insulin incubated at 37°C with 5% CO2. Small interfering RNA (siRNA) transfection was processed according to manufacturer’s manual. After 24 h, 200 nmol/L, 400 nmol/L, or 2 μmol/L GLP-1(1-37) (Bachem, King of Prussia, PA) or 1× PBS was added separately into different wells. Following a 16-h incubation, cells were washed with DMEM with 10% FBS three times, and 1 mL DMEM with 10% FBS plus 200 nmol/L, 400 nmol/L, or 2 μmol/L GLP-1(1-37) or GLP-1(7-37) was added to the cells, supplemented with 0.4% glucose for an additional 2 h. The media were removed from the cells, supplemented with leupeptin (10 ng/mL), 0.2 mmol/L phenylmethylsulfonyl fluoride, and aprotinin (10 ng/mL); centrifuged (12,000× rpm; Eppendorf 5804 R, Westbury, NY); and kept briefly at 4°C prior to ELISA analysis for insulin expression. Insulin was measured using Rat/Mouse ELISA Kit (Millipore, MA) according to the manufacturer’s instructions. The treated IEC-6 cells were used for RT-PCR or immunofluorescence (see Immunofluorescence and RT-PCR in the research design and methods section) for specific gene expression.
Statistical Analysis
Oral glucose tolerance test (OGTT) and nonfasting glucose data were compared using area under the curve (AUC) estimates over the course of the time period specified. Specifically, AUC data for rats under different conditions were grouped (LG or L) and compared with control rats using one-way ANOVA, with Dunnett test used to determine significant differences. Differences were considered significant at α < 0.05 (n = 6).
Results
Bacterial Secretion of GLP-1(1-37) in Rat Upper Intestinal Tracts
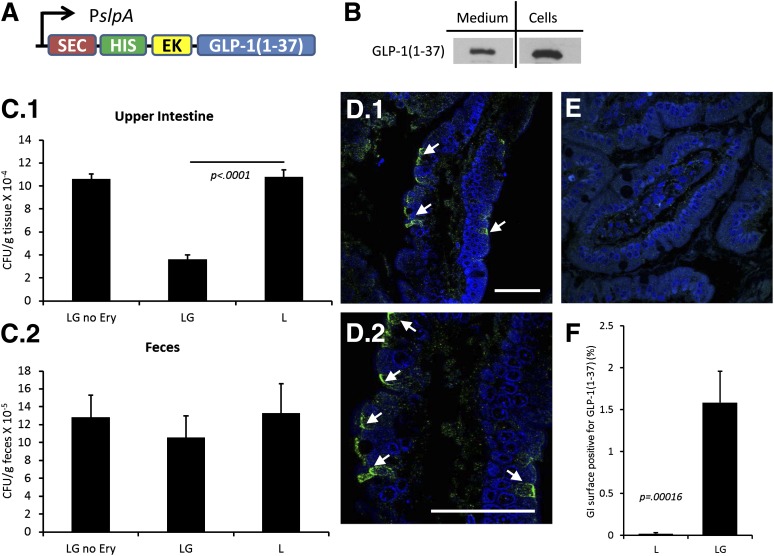
L was engineered to secrete GLP-1(1-37) using the SlpA promoter and USP45-LEISS secretion tag (SEC) (19) (LG) (Fig. 1A). A polyhistidine (HIS) tag was added to the N-terminus (for Western blotting) separated by an enterokinase site so that the protein would be separated from the tag once secreted in the intestine. Secretion of GLP-1(1-37) was verified in culture (Fig. 1B). The secreted fraction of GLP-1(1-37) was recovered from the culture medium (medium) and compared with that which was still in the cell pellet (cells).
Figure 1.
Bacterially secreted GLP-1 in the rat upper intestine. GLP-1(1-37) was secreted from L that were administered twice daily to rats over the course of 90 days. A: A schematic of the cassette inserted into the bacterial chromosome of L to make LG is shown. B: Western blots were used to confirm bacterial secretion of recombinant GLP-1(1-37). Supernatants (medium) and pellets (cells) from LG and parent strain L are shown. C: Homogenized upper intestinal and fecal bacterial counts. Homogenized intestines (C.1) and feces (C.2) were serially plated with no antibiotic (LG no erythromycin and L) or with erythromycin (LG). Counts were performed to obtain CFU/g tissue. Fecal counts are per gram of feces. D.1: Immunofluorescence images were obtained for GLP-1 expression (green staining) in the upper GI tract of LG-fed rats. Arrows point to GLP-1 along the luminal surface. Nucleic acid is stained blue (DAPI). D.2: A higher magnification view of the intestinal section in D.1. E: Images from upper intestines of L-fed rats showed no clear GLP-1 staining. F: Morphometric analysis of intestinal sections stained for GLP-1(1-37) is shown. Values are averages of images taken from three rats, and error bars represent 1 SD. P value is from a Student t test (n = 3). Scale bars in D.1 and D.2 are 50 µm. EK, enterokinase site; Ery, erythromycin; HIS, polyhistidine tag; PslpA, S-layer protein gene (slpA) promoter; SEC, strain-specific secretion tag.
Rats were fed two times per day with L, LG, or sterile media for 90 days. At the end of the experiment, individual rat intestines were homogenized and bacterial counts were performed in order to establish the level of colonization in the upper GI tract and to compare that to counts in the feces. From these data, it is clear that bacterial strains colonized the intestines (Fig. 1C.1). There was no difference between L and LG in colonizing the feces (Fig. 1C.2). It should be noted that L did not carry antibiotic resistance, hence the counts are much higher than LG due to the lack of selective pressure. To count total amounts of Lactobacillus present in the rat upper intestines, homogenates were plated on MRS plates which select (without antibiotics) predominately for Lactobacillus strains. For rats fed LG or L, the counts were almost identical, indicating that both strains did little to change the total number of lactobacilli in the homogenates. To select only for LG, erythromycin was used as a selectable marker (Fig. 1C). The lower counts on erythromycin plates, as compared with the MRS plates, indicated the difference in colonization between culturable native rat lactobacilli and LG.
Diabetic rats that were fed LG stained positively for GLP-1(1-37) in their upper intestines as revealed by immunofluorescence using antibodies that react specifically with the first six amino acids of GLP-1 (Fig. 1D, LG results are shown), while rats fed either L or buffer did not exhibit similar staining (Fig. 1E, L shown). Quantification of coverage was carried out by scanning images of intestinal sections and estimating the percentage of the intestinal surface that stained positive for GLP-1(1-37) (Fig. 1F). The amount of background staining can be seen with the L control (Fig. 1F).
Whether LG can partially restore euglycemia in a drug-induced diabetes rat model was tested. Representative results from these experiments are presented below.
Reduction of Hyperglycemia in an STZ-Mediated Model of Type 1 Diabetes
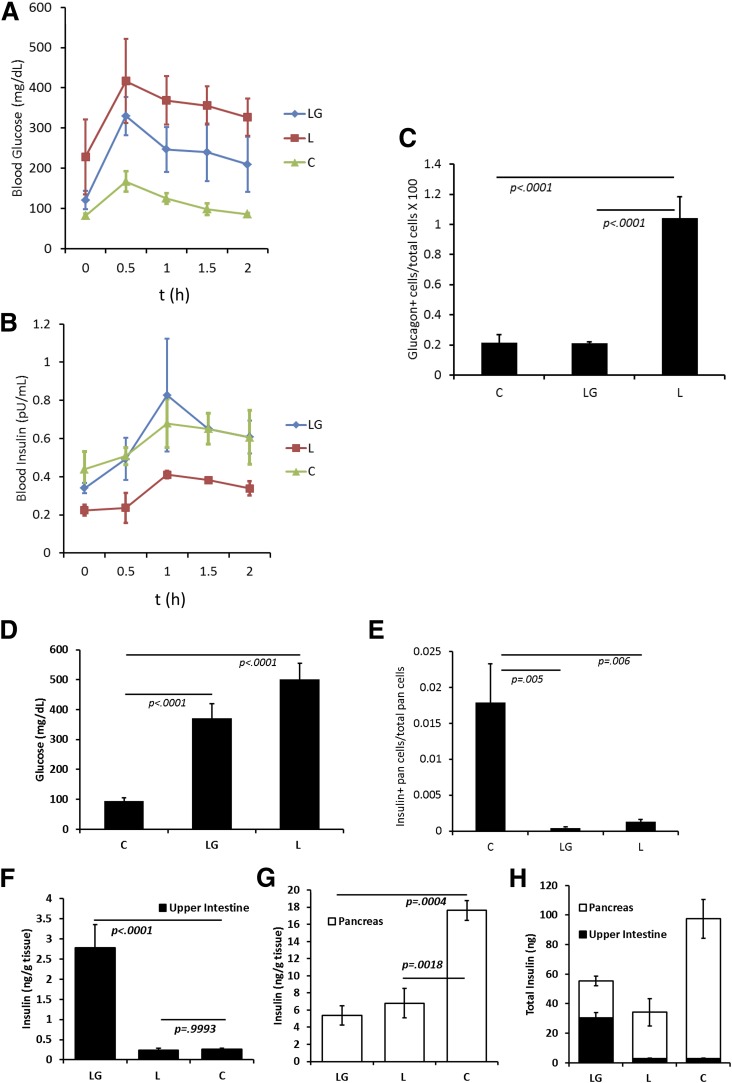
Six- to eight-week-old female Wistar rats were injected with STZ to specifically reduce their β-cell mass. With the onset of hyperglycemia, groups of rats were fed either LG or L (all bacteria were fed two times daily). As an euglycemic control, one group of rats received no STZ treatment and was only fed sterile media rather than bacteria (control). Rat weights and nonfasting glucose levels were monitored over 90 days. Nonfasting glucose levels were compared using AUC calculations. As an example, nonfasting glucose levels taken from the last day of the study are presented in Fig. 2D.
Figure 2.
Reducing hyperglycemia in diabetic rats. Hyperglycemia was induced in Wistar rats via STZ depletion of pancreatic β-cells. After treatment with L. gasseri (L) and L. gasseri-secreting GLP-1(1-37) (LG) rats were subjected to an OGTT. One group of rats was not treated with STZ and fed sterile media rather than a bacterial suspension (control). Blood glucose (A) and insulin (B) levels were measured for the OGTT. Presented are averages for each treatment group in a single experiment (n = 6). Statistical analysis was performed using one-way ANOVA for AUC measurements, and results are discussed in the text. C: Pancreatic sections from each treatment group were morphometrically analyzed for the ratio of glucagon-positive cells to total pancreatic cells. D: Random (nonfasting) blood glucose levels were obtained for all rats in the study two times per week. Shown are representative average blood glucose levels from one day of an experiment for each group (n = 6). E: Fraction of insulin-positive cells in the pancreas. Values are averages. Intestinal (F) and pancreatic (G) tissues were homogenized and assayed for insulin content. Shown are representative average insulin levels. Error bars represent 1 SD, and P values are from a Dunnett test for significance (n = 6). H: Estimates for average total insulin in combined intestinal and pancreatic tissues were obtained from weight measurements of harvested tissues. Error bars represent 1 SD (n = 6). C, control; pan, pancreas.
After 50 days of commensal bacterial treatment, rats were subjected to an OGTT (Fig. 2A and B). The data were compared using AUC and one-way ANOVA with Dunnett test for significance. When compared with the nondiabetic control rats, L-fed diabetic rats exhibited both higher blood glucose and lower plasma insulin (P = 0.0037 and 0.0059, respectively). Blood glucose and plasma insulin were not significantly different between the LG-fed rats and the nondiabetic control rats (P = 0.0903 and 0.9284, respectively). At the end of the 90-day treatment period, pancreata and intestines were harvested from rats in the study and pancreata were fixed and immunostained for glucagon. There were significant differences in the ratios of glucagon-positive cells to total pancreatic cells between both LG-fed rats and control rats when compared with L-fed rats (Fig. 2C). Pancreata were also scanned for β-cell numbers (Fig. 2E). L-fed rats had slightly more remaining β-cells than LG-fed rats (Fig. 2E). It was estimated that control rats had more than 17-fold the remaining β-cells than the other treatments. Rat intestines and pancreata were homogenized to determine the level of insulin in each (Fig. 2F–H). Upper intestinal measurements of insulin per gram of tissue indicated that only rats fed LG expressed more insulin in their intestines than the normal control rats. On average, LG-fed rats exhibited more than fivefold the levels of insulin in their upper intestinal tissues than either L-fed rats or control rats (Fig. 2F). In pancreatic tissue, there was no difference in insulin levels per gram of tissue between LG- and L-fed rats but as much as threefold more insulin per gram of tissue in control rats compared with the diabetic rats (LG and L in Fig. 2G). When the upper intestinal and pancreatic tissues were weighed, estimates of total insulin indicated that LG-fed rats had on average 60% more total insulin (intestines and pancreata combined) than L-fed rats. Control rats had almost twice as much total insulin in these tissues as LG-fed rats (Fig. 2H).
Expression of Pancreatic and Intestinal Markers
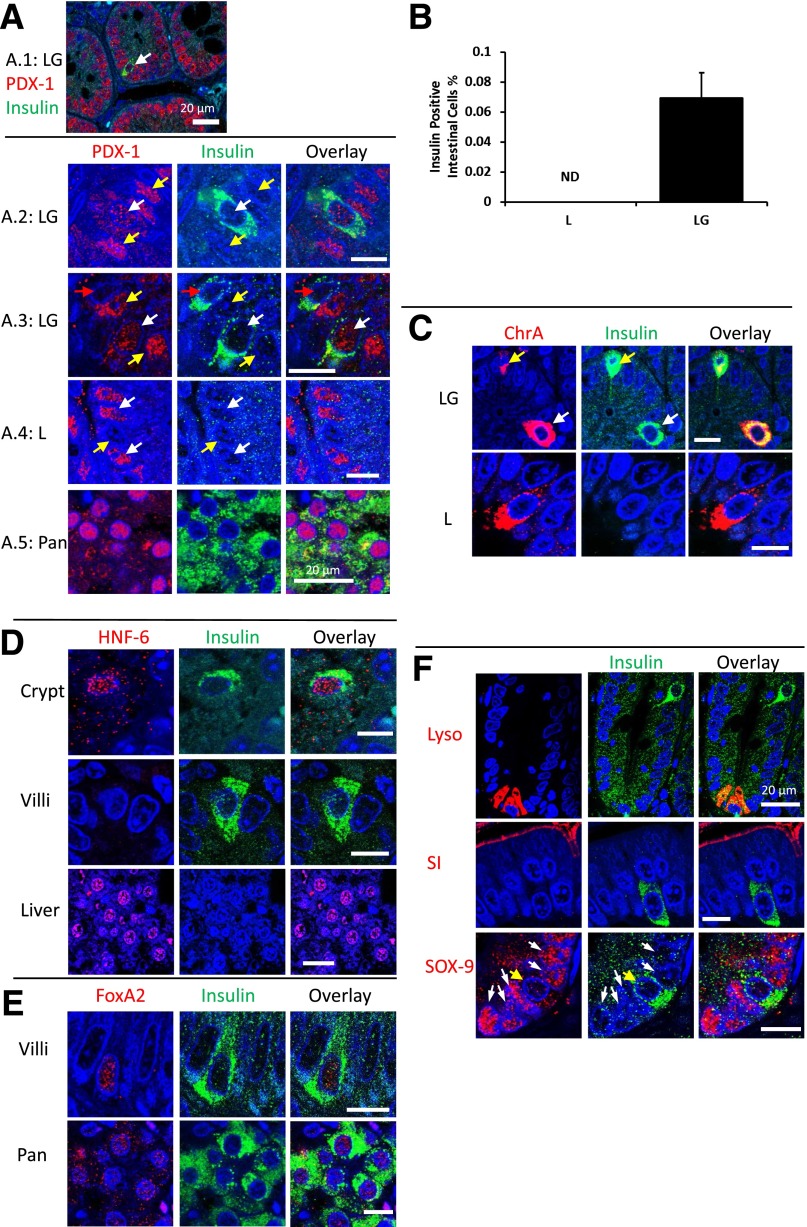
Sections of rat small intestines were fixed and immunofluorescently probed for various markers. Insulin-containing cells were found in the small intestines of rats treated with LG but not in L or control groups (Fig. 3, LG and L results shown). The relative frequency of insulin-producing cells was estimated from image analysis to be ∼0.06% (±0.016%) of the average small intestinal cell count (or ∼1 in 1,600 epithelial cells) (Fig. 3B). PDX-1 production was observed in the upper intestine and pancreas as expected for all rats in the study (red staining in Fig. 3A). Epithelial cells in intestinal sections of rats had various levels of PDX-1 (Fig. 3A.2, yellow arrows) that were frequently lower for insulin-positive cells than for surrounding epithelia (Fig. 3A.3, red arrows); however, insulin-positive cells also stained for PDX-1 at levels comparable to surrounding epithelia (Fig. 3A.2 and A.3, white arrows).
Figure 3.
Immunofluorescence for β-cell and intestinal epithelia markers. A: Sections from the different groups were stained for PDX-1 (red) and insulin (green). B: Intestinal sections were analyzed by image analysis for the number of insulin-positive cells as a percentage of total epithelial cells. Average values are shown (n = 6). Error bars represent 1 SD. C: ChrA (red) and insulin (green). D: Sections from the LG-fed group for HNF-6 (red) and insulin (green). Shown are cells from intestinal crypts and intestinal villi from LG-fed rats and liver cells from healthy rats. E: Sections from the LG-fed group for FoxA2 (red) and insulin (green) in intestinal villi and from healthy rat pancreata. F: Sections from the LG-fed group for lysozyme, SI, SOX-9 (red), and insulin (green). Scale bars are 10 µm unless otherwise noted. A.1: White arrow indicates reprogrammed cell. A.2: White arrows indicate reprogrammed cells. Yellow arrows indicate normal PDX-1 expression in epithelial cells. A.3: White arrows indicate reprogrammed cells making PDX-1. Red arrows indicate reprogrammed cells not expressing PDX-1. Yellow arrows indicate normal PDX-1 expression in epithelial cells. A.4: White arrows indicate normal PDX-1 expression in epithelial cells. Yellow arrows indicate lack of PDX-1 expression in epithelial cells. C: White arrows indicate reprogrammed cells making less insulin than ChrA. Yellow arrows indicate reprogrammed cells making less ChrA than insulin. F: White arrows indicate cells normally expressing SOX-9. Yellow arrows indicate reprogrammed cells. Lyso, lysozyme; ND, none detected; Pan, pancreata.
ChrA is normally expressed by neuroendocrine cells, enteroendocrine cells, and islet β-cells in secretory granules. To better understand the physiology of the reprogrammed cells, intestinal sections were costained for ChrA and insulin. In some instances, insulin staining (green) overlapped with ChrA staining (red) (Fig. 3C), and in ∼50% of observed insulin-producing cells, counterstaining did not detect significant amounts of ChrA (Fig. 3C). In Fig. 3C, yellow arrows denote an enteroendocrine cell expressing more insulin than ChrA, while white arrows point to a cell expressing more ChrA than insulin. In L-fed rats, only ChrA was detected with no visible costaining for insulin (Fig. 3C).
HNF-6, previously shown to be transcriptionally upregulated by GLP-1(1-37) in vitro (11), was also assayed. HNF-6 mediates transition from endodermal cell lineages to PDX-1–expressing cells in the pancreata of developing embryos (25). HNF-6 and insulin colocalization was observed in the epithelia of intestinal crypts of rats fed LG (Fig. 3D). Colocalization of HNF-6 and insulin in cells that had migrated up the villous axis and were present on the villi (Fig. 3D) was not observed. Liver tissue from healthy rats was used as a positive control for HNF-6 (Fig. 3D). In addition to HNF-6, cells staining positive for insulin in the upper GI tracts of rats fed bacteria secreting GLP-1(1-37) expressed FoxA2, a transcription factor required for PDX-1–mediated insulin synthesis in pancreatic cells (26) (Fig. 3E).
To determine if normal function could be seen juxtaposed next to transformed cells, intestinal sections from LG-fed rats were costained for lysozyme, SI, and SOX-9. All of these markers appeared in close proximity to insulin-producing cells (Fig. 3F).
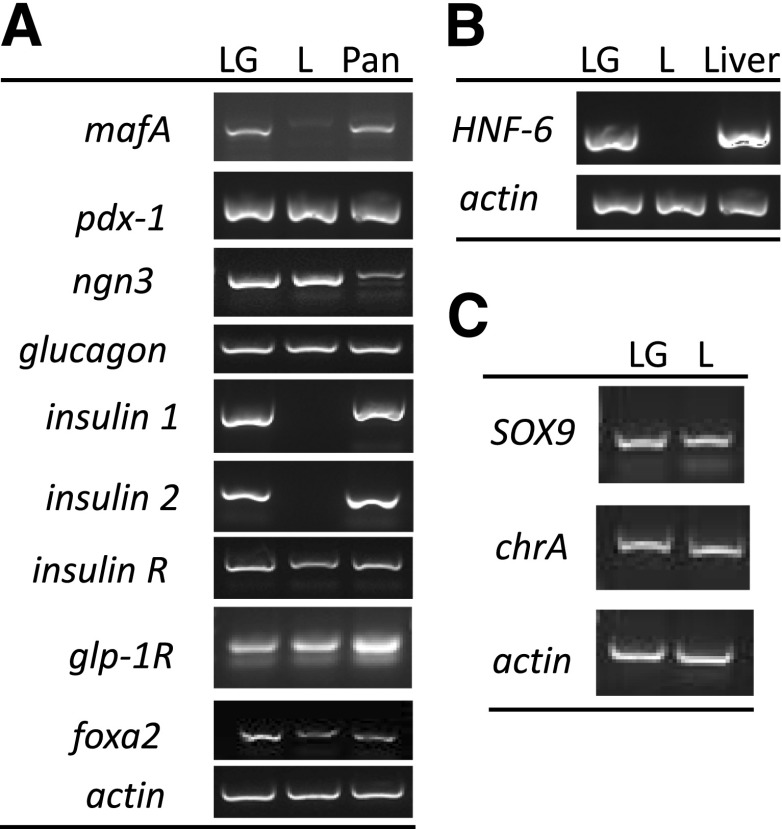
Transcriptional analysis via RT-PCR was in agreement with immunofluorescence data for expression of insulin, pdx-1, and foxa2 (Fig. 4A); HNF-6 (Fig. 4B); and SOX9 and chrA (Fig. 4C). In addition, mafA, neurogenin 3 (ngn3), glucagon, insulin receptor (insulin R) and GLP-1 receptor (glp-1R) (Fig. 4A) transcripts were assayed in both intestinal and pancreatic tissues. Differential expression between rats fed LG and L was seen for mafA (Fig. 4A).
Figure 4.
RT-PCR for β-cell and intestinal epithelia markers. A: LG- and L-fed rat intestinal sections were homogenized, and their mRNA transcripts were assayed for expression of the genes shown. Healthy rat pancreatic sections were used as a positive control. B: LG- and L-fed rat intestinal mRNA was assayed for HNF-6 expression. Healthy rat liver mRNA was used as a positive control. C: SOX9 and chrA mRNA were measured from intestinal tissues of LG- and L-fed rats. Actin was used as a loading control for A, B, and C. Pan, pancreas.
Stimulation of IEC-6 Cells With GLP-1(1-37) in the Presence and Knockdown of GLP-1R
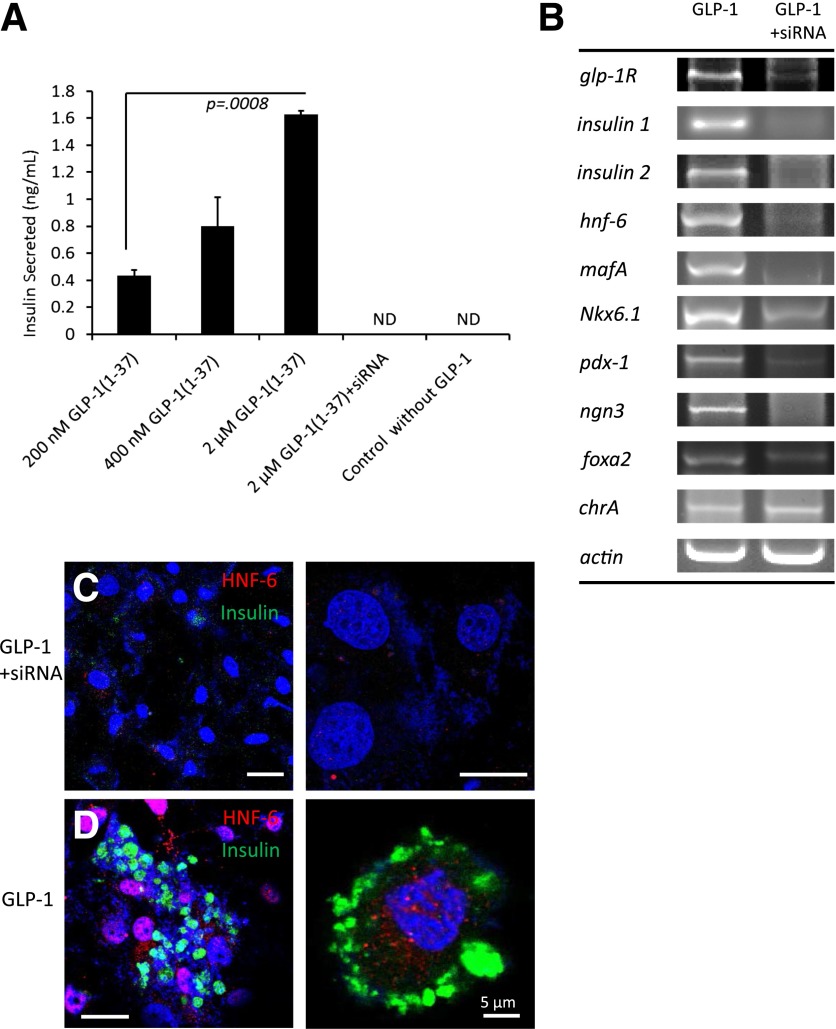
Rat intestinal stem cells (IEC-6) were used to understand the importance of the GLP-1R in mediating reprogramming. IEC-6 cells were stimulated with GLP-1(1-37) and showed dose-dependent secretion of insulin in response to glucose (Fig. 5A). siRNA was used to knock down expression of GLP-1R in IEC-6 cell culture. siRNA-treated cultures (siRNAi+) did not exhibit reprogramming when stimulated with GLP-1(1-37) as per RT-PCR of various β-cell markers (Fig. 5B). HNF-6 was immunodetected in GLP-1(1-37)–treated IEC-6 cells (Fig. 5D) but not in cells pretreated with siRNA against GLP-1R (Fig. 5C).
Figure 5.
In vitro stimulation of IEC-6 cells with GLP-1(1-37). A: IEC-6 cell cultures were incubated with either siRNA against the GLP-1R (siRNA+) or sterile media and then incubated with GLP-1(1-37) at various concentrations before challenge with glucose. Values are averages of three wells, and error bars represent 1 SD. P value is from a Student t test. B: Various cell markers were assayed using RT-PCR for cells either treated with siRNA (siRNA+) or not prior to GLP-1(1-37) addition. siRNA+ cells (C) and normal cells (D) were stimulated with GLP-1(1-37) and immunostained for HNF-6 and insulin. All scale bars are 20 µm unless otherwise indicated. ND, none detected.
In Vitro Analysis of Human Epithelial Insulin Stimulation and GLP-1 in Rat Serum
To rule out any potential stimulation for insulin production in intestinal cells by the active form of GLP-1 [GLP-1(7-37)], an in vitro assay of human epithelia was developed to compare the relative levels of glucose-mediated insulin production following stimulation by either GLP-1(7-37) or GLP-1(1-37) (Supplementary Fig. 1). GLP-1(1-37) was observed to stimulate Caco-2 cells to secrete insulin in response to glucose in a dose-dependent manner (although differences were not significant, averages trended according to dose of GLP-1). GLP-1(7-37) stimulated no significant secretion of insulin (Supplementary Fig. 1).
Blood from representative rats from all groups in this study were assayed for the presence of GLP-1 and any changes that may have arisen from feeding bacteria secreting GLP-1(1-37). ELISA assays indicated that there was no significant difference in blood levels of either GLP-1(7-37) or GLP-1(1-37) (by conversion curve) in rats from the LG, L, or control groups (Supplementary Figs. 2–4).
Discussion
The use of bacterial strains to deliver compounds to the upper intestinal tract has been the subject of several studies and review articles (27,28). While there has been limited success in this area, thus far the use of engineered commensal bacteria for changing intestinal cell differentiation has not been demonstrated. The work described herein demonstrates the potential of bacterial delivery of a compound to the luminal side of the upper intestine for the purpose of controlling epithelial cell fate. The objective for this investigation was to test the hypothesis that delivering the inactive form of GLP-1 [GLP-1(1-37)] to the luminal side of the upper intestine using engineered commensal bacterial strains could serve as a treatment for hyperglycemia.
In rats given a high dose of STZ to ablate their pancreatic β-cells, hyperglycemia was significantly reduced by ingestion of GLP-1(1-37)–secreting bacteria. The mechanism of blood glucose reduction appears to be from intestinal cells reprogrammed to secrete insulin in response to glucose as is evidenced by the increase in intestinal insulin (Fig. 2F and H), the presence of insulin-secreting endocrine cells in the intestine (Fig. 3), the presence of insulin mRNA in intestinal tissue (Fig. 4A), and the in vitro result that GLP-1(1-37) converts rat cells to insulin-secreting cells. Further, insulin secretion in rats fed LG followed the same kinetics and was not significantly different from insulin secretion in healthy control rats, indicating that their increased insulin-making capacity is glucose responsive (Fig. 2B), as has been demonstrated in vitro with human intestinal cells (23).
Rat intestinal epithelia reprogrammed to secrete insulin expressed important β-cell markers PDX-1, MafA, and FoxA2. These proteins are all essential for normal β-cell function and are differentially expressed in pancreatic tissue in response to glucose and insulin (29,30). Similarly, in this work, reprogrammed cells were shown to express various levels of PDX-1 and, as is the case with both pancreatic (31) and intestinal (32) cells normally, in some instances, failed to translocate PDX-1 from the cytosol to the nuclear compartment, pointing to an active control of insulin levels rather than a constitutive one. This phenomenon could also be observed with ChrA production. With ChrA, as with PDX-1, levels varied between individual cells expressing insulin. FoxA2 regulation of PDX-1 is an important part of pancreatic development in rodents (33) and adds to the complexity of PDX-1 expression.
These data suggest that at least some reprogrammed cells maintain enteroendocrine function and have the capacity to act as multifunctional endocrine cells with β-cell functionality. This change is possibly mediated through expression of HNF-6, which is essential in normal development for the early transition between intestinal endoderm and PDX-1–expressing pancreatic cells (34,35). However, continued expression of HNF-6 in mature β-cells leads to loss of pancreatic function and apoptosis (36,37). Our investigations in vivo suggest that HNF-6 expression, which was only observed in insulin-secreting cells in the crypts of the intestine, not in more well-differentiated insulin-positive cells along the villous axis, transforms potential enteroendocrine cells into β-like cells that then migrate up the axis normally while ceasing to express HNF-6 at detectable levels. That the loss of GLP-1R function via RNAi eliminates reprogramming indicates that the GLP-1R is essential for this effect, although the exact mechanism for activation of HNF-6 remains to be determined.
From the data presented here, the authors conclude that feeding GLP-1(1-37)–secreting bacteria to diabetic rats can cause glucose-responsive insulin production, reducing blood glucose levels significantly. Although additional characterization is necessary, the mechanism of insulin secretion appears to be from transformed intestinal endocrine cells. Given that rats were fed an inactive form of GLP-1 that does not stimulate β-cell insulin secretion, it is unlikely that this approach led to increased insulin production from remaining β-cells. Pancreatic morphometric analysis indicated that β-cell counts were not improved in rats fed GLP-1–secreting bacteria. Tissue homogenization revealed that insulin in rats fed GLP-1–secreting bacteria originated from the intestine. More work is needed to further understand how the GLP-1R stimulates HNF-6 activation.
Supplementary Material
Article Information
Acknowledgments. The authors thank David Brillon of Weill Cornell Medical College and Andrea Quaroni of Cornell University for helpful discussions of this work. L, NCK334, EC1000, NCK1391, and NCK1609 were kind gifts from Todd R. Klaenhammer at North Carolina State University.
Funding. This work was supported by grants to J.C.M. from the National Institutes of Health (DP2 New Innovator Award), the NIH Small Business Innovation Research (SBIR) grant (1R41DK094638-01A1), and from the Hartwell Foundation (Hartwell Investigator Award).
Duality of Interest. J.C.M. has a financial conflict of interest with a company that has licensed this technology from Cornell University. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.F.D. designed and performed experiments and wrote the manuscript. J.H.L. edited the manuscript and performed experiments. J.C.M. designed experiments and wrote the manuscript. J.C.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0635/-/DC1.
References
- 1.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 2001;50:1691–1697 [DOI] [PubMed] [Google Scholar]
- 2.Alipio Z, Liao W, Roemer EJ, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A 2010;107:13426–13431 [DOI] [PMC free article] [PubMed]
- 3.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000;6:568–572 [DOI] [PubMed] [Google Scholar]
- 4.Zalzman M, Gupta S, Giri RK, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A 2003;100:7253–7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A 2003;100:998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kania G, Blyszczuk P, Czyz J, Navarrete-Santos A, Wobus AM. Differentiation of mouse embryonic stem cells into pancreatic and hepatic cells. Methods Enzymol 2003;365:287–303 [DOI] [PubMed] [Google Scholar]
- 7.Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A 2002;99:16105–16110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001;292:1389–1394 [DOI] [PubMed] [Google Scholar]
- 9.Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 2000;49:157–162 [DOI] [PubMed] [Google Scholar]
- 10.Noguchi H. Stem cells for the treatment of diabetes. Endocr J 2007;54:7–16 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A 2003;100:5034–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 13.Farrar MD, Whitehead TR, Lan J, et al. Engineering of the gut commensal bacterium Bacteroides ovatus to produce and secrete biologically active murine interleukin-2 in response to xylan. J Appl Microbiol 2005;98:1191–1197 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed FE. Genetically modified probiotics in foods. Trends Biotechnol 2003;21:491–497 [DOI] [PubMed] [Google Scholar]
- 15.Westendorf AM, Gunzer F, Deppenmeier S, et al. Intestinal immunity of Escherichia coli NISSLE 1917: a safe carrier for therapeutic molecules. FEMS Immunol Med Microbiol 2005;43:373–384 [DOI] [PubMed] [Google Scholar]
- 16.Duan F, March JC. Interrupting Vibrio cholerae infection of human epithelial cells with engineered commensal bacterial signaling. Biotechnol Bioeng 2008;101:128–134 [DOI] [PubMed] [Google Scholar]
- 17.Corthésy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr 2007;137(Suppl. 2):781S–790S [DOI] [PubMed] [Google Scholar]
- 18.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol 2004;4:953–964 [DOI] [PubMed] [Google Scholar]
- 19.Hazebrouck S, Pothelune L, Azevedo V, Corthier G, Wal JM, Langella P. Efficient production and secretion of bovine beta-lactoglobulin by Lactobacillus casei. Microb Cell Fact 2007;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel C, Repa A, Wild C, et al. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 2006;61:812–819 [DOI] [PubMed] [Google Scholar]
- 21.Kuehn MJ. Genetically engineered probiotic competition. Gastroenterology 2006;130:1915–1916 [DOI] [PubMed] [Google Scholar]
- 22.Ait-Belgnaoui A, Han W, Lamine F, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006;55:1090–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan F, Curtis KL, March JC. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol 2008;74:7437–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil 2007;19:166–172 [DOI] [PubMed] [Google Scholar]
- 25.Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol 2003;259:109–122 [DOI] [PubMed] [Google Scholar]
- 26.Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH. Foxa2 controls Pdx1 gene expression in pancreatic beta-cells in vivo. Diabetes 2002;51:2546–2551 [DOI] [PubMed] [Google Scholar]
- 27.Aurand TC, Russell MS, March JC. Synthetic signaling networks for therapeutic applications. Curr Opin Biotechnol 2012;23:773–779 [DOI] [PubMed] [Google Scholar]
- 28.Goh YL, He H, March JC. Engineering commensal bacteria for prophylaxis against infection. Curr Opin Biotechnol 2012;23:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology 2009;150:1627–1635 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kebede M, Ferdaoussi M, Mancini A, et al. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc Natl Acad Sci U S A 2012;109:2376–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang R, Olds LC, Sibley E. Spatio-temporal patterns of intestine-specific transcription factor expression during postnatal mouse gut development. Gene Expr Patterns 2006;6:426–432 [DOI] [PubMed] [Google Scholar]
- 33.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev 2008;22:3435–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol 2003;258:105–116 [DOI] [PubMed] [Google Scholar]
- 35.Poll AV, Pierreux CE, Lokmane L, et al. A vHNF1/TCF2-HNF6 cascade regulates the transcription factor network that controls generation of pancreatic precursor cells. Diabetes 2006;55:61–69 [PubMed] [Google Scholar]
- 36.Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development 2000;127:2883–2895 [DOI] [PubMed] [Google Scholar]
- 37.Hara M, Shen J, Pugh W, Polonsky KS, Le Beau MM, Bell GI. Sustained expression of hepatocyte nuclear factor-6 leads to loss of pancreatic beta-cells by apoptosis. Exp Clin Endocrinol Diabetes 2007;115:654–661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.