Abstract
Some report that adult human β-cells do not replicate, but we postulate this assumption is erroneous due a postmortem decline in replication markers such as Ki67. Our earlier report showed that Ki67-marked β-cells were rarely found in human cadaveric pancreases but were in the range of 0.2–0.5% in human islets transplanted into mice. This study subjected 4-week-old mice to autopsy conditions that typically occur with humans. Mice were killed, left at room temperature for 3 h, and then placed at 4°C for 3, 9, or 21 h. There was a rapid marked fall in Ki67 staining of β-cells compared with those fixed immediately. Values at death were 6.9 ± 0.9% (n = 6) after a 24-h fast, 4.1 ± 0.9% (n = 6) at 3 h room temperature, 2.7 ± 0.7% (n = 5) at 6 h, 1.6 ± 0.6% (n = 5) at 12 h, and 2.9 ± 0.8% (n = 5) at 24 h. Similar postmortem conditions in newborn pigs resulted in very similar declines in Ki67 staining of their β-cells. These data support the hypothesis that conclusions on the lack of replication of adult human β-cells are incorrect and suggest that adult human β-cells replicate at a low but quantitatively meaningful rate.
Introduction
Because a reduction in β-cell mass is fundamental to the pathogenesis of diabetes, there has been great interest in the replicative capacity of human β-cells (1). A marked expansion of β-cell number occurs in childhood such that much of the β-cell mass found in adults is achieved by the age of 10 years (2). Many have argued that there is virtually no replication of β-cells in the adult pancreas (2–4), and pathologists have rarely found mitotic figures in β-cells of autopsied pancreases (5).
To examine β-cell replication, one study used autopsy samples from individuals who had received thymidine analogs in clinical trials before death; tissue sections were immunostained for these analogs to search for replication. The same study used isolated islets from organ donors to estimate the genomic DNA integration of atmospheric C14. It concluded that no new β-cells were born after the age of 30 years (6). Another approach examined the accumulation of intracellular lipofucsin bodies in β-cells, concluding that human β-cells live for many years and have very little, if any, replication in adult life (7). Other studies used samples taken from pancreases at autopsy or after cadaveric organ donation. Studies that used Ki67, a nuclear protein expressed by cells in the active cell cycle, found that the β-cells of adult pancreases usually had no Ki67 positivity (2,4,8). However, evidence of active replication was found in many of the pancreases of children younger than the age of 10 years (2,4). A puzzling aspect of these studies is that the β-cells of a number of pancreases from young children had no Ki67 positivity.
Others suggest that adult human β-cells are able to replicate. β-Cells in cultured human islets replicate when stimulated by lactogenic hormones, albeit considerably less so than rodent β-cells (9). More recent studies have also found a low rate of replication, which can be considerably enhanced by genetic manipulation of cell cycle genes (10). In our study of islets obtained from adult organ donors and transplanted into immunocompromised mice, there was clear Ki67 positivity of β-cells in the range of 0.2–0.7% (11). Others have reported similar results (10). Also, samples taken at the time of surgery have been reported to have Ki67 staining, ranging from 0.1% to 0.5%, of their β-cells (11,12).
Because of these discrepancies, we postulated that pancreases subjected to warm and cold ischemia during the process of autopsy and cadaveric donation would have a reduction in the appearance of replication markers. Therefore, we subjected young mice and newborn pigs to autopsy conditions of warm ischemia, followed by cold ischemia, and evaluated their pancreatic tissue using immunostaining for the replication markers Ki67 and proliferating cell nuclear antigen (PCNA).
Research Design and Methods
The Joslin Institutional Animal Care and Use Committee approved all animal experiments.
Mouse Autopsy Conditions
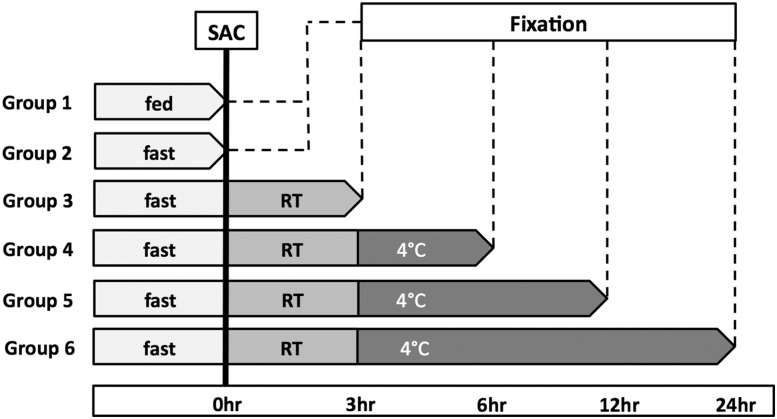
Autopsy conditions that typically occur in humans were replicated in 4-week-old C57BL/6 mice. Mice were killed by CO2 in a fed state or after a 24-h fast. The cadavers were left at room temperature (RT) for 3 h and then moved to 4°C. Pancreases were removed and fixed in 4% paraformaldehyde (PFA) immediately after death, after 3 h at RT, or after 3, 9, or 21 h at 4°C (Fig. 1).
Figure 1.
Study plan for mice. Six groups of killed mice were studied at five different times —0, 3, 6, 12, and 24 h—with varying lengths of exposure to warm ischemia (RT) and cold ischemia (4°C). SAC, sacrificed.
Pig Autopsy Conditions
Piglets (6-day-old Yorkshire; Parsons Farm, Hadley, MA) were killed by anesthetic overdose (ketamine and xylazine) and exsanguination of the carotid and jugular arteries. The cadavers were left at RT for 3 h, at which point the first samples were taken, and then moved to 4°C for 21 h, after which the second sample was taken from each pig.
Tissue Processing and Immunohistochemistry
Samples of mouse and pig pancreas were fixed in 4% PFA for 6 h and then stored in PBS at 4°C until processing and paraffin embedding. Immunostaining was performed on adjacent sections for 1) Ki67 and insulin and 2) PCNA and insulin. Sections were washed in 0.3% Triton X for 15 min, followed by antigen retrieval in citrate buffer (pH 6.0) for 15 min in the microwave. After blocking with an avidin-biotin blocking kit (Vector Laboratories, Burlingame, CA) and normal donkey serum at 1:50 (Jackson ImmunoResearch, West Grove, PA), rabbit anti-Ki67 (Abcam, Cambridge, MA) and rabbit anti-PCNA at 1:200 (Dako, Carpenteria, CA), primary antibodies were added. Sections were incubated overnight at 4°C. To identify the β-cells, sections were costained with guinea pig anti-insulin (1:500, Dako) for 1 h at RT. Incubation with secondary antibodies (biotinylated anti-rabbit IgG, Alexa Fluor 488 streptavidin; Alexa Fluor 594 anti-guinea pig IgG, 1:200; all Jackson ImmunoResearch) was for 1 h at RT. Slides were mounted with Vectashield with DAPI (Vector Laboratories).
Microscopy and Analysis
Photographs of insulin-positive cells were systematically taken across the whole section using a LSM 710 confocal microscope (×25 objective, Zeiss). Zen software was used to quantify all cells stained for insulin or costained for insulin and Ki67 or PCNA on blinded images; at least 500 β-cells were counted per sample. To assess the peri-insular acinar cells under mouse autopsy conditions, the same images containing islets in at least 20 cells in cross section were blindly counted for Ki67 positivity and total acinar cells (determined by nuclear morphology visualized with DAPI); more than 1,000 peri-insular acinar cells were counted per sample. Glucagon-rich areas of the pancreas were counted as confirmed by immunostaining for glucagon and pancreatic polypeptide.
Statistics
Data are presented as mean ± SEM. Statistical significance was tested with unpaired two-tailed Student t tests. For comparison of multiple time points, ANOVA, followed by the Bonferroni test, was used.
Results
Markers of β-Cell Replication in Mouse Pancreas After Warm and Then Cold Ischemia
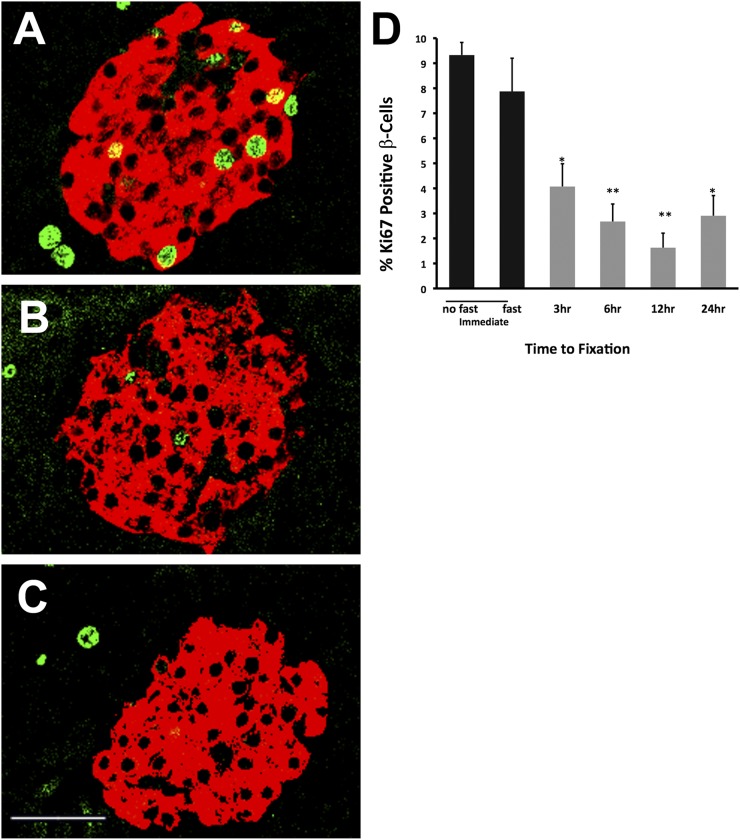
To replicate human autopsy conditions, we followed the schema shown in Fig. 1. With delayed fixation of the pancreas, there was a rapid marked fall in the frequency of Ki67 staining in β-cells compared with those fixed immediately (Fig. 2). At the time of death in the fed state, frequency was 9.3 ± 0.5% (n = 3) and was 6.9 ± 0.9% after a 24-h fast (n = 6, no significant difference). However, the frequency of Ki67-positive β-cells fell to 4.1 ± 0.9% (n = 6) after just 3 h at RT, to 2.7 ± 0.7% at 6 h (n = 5), to 1.6 ± 0.6% at 12 h (n = 5), and to 2.9 ± 0.8% (n = 5) at 24 h (ANOVA P < 0.001).
Figure 2.
Autopsy conditions show a rapid marked reduction of Ki67 staining in mouse β-cells. Confocal images show insulin (red) and Ki67 (green) staining of representative islets. Compared with immediate fixation (A), there is a marked loss of Ki67 already by 3 h of warm ischemia (B); with an additional 21 h of cold ischemia (C), even less Ki67 can be seen in the β-cells. D: With quantification of Ki67-positive insulin-positive cells, the fed and fasted controls did not differ, but reduction of Ki67 staining in β-cells was found after warm and cold ischemia (n = 5–9). Scale bar, 40 μm. *P < 0.005, **P < 0.001.
The replication marker PCNA was also evaluated on the adjacent sections of nonfasted mice after immediate fixation and after 24 h of warm/cold ischemia. For the immediate fixation group, values were 7.9 ± 1.4% (n = 8), very similar to the Ki67 values. At 24 h the PCNA was 5.2 ± 1.3% (n = 5), which failed to be statistically lower than after immediate fixation (P = 0.176).
Markers of Acinar Cell Replication in Mouse Pancreas After Warm and Then Cold Ischemia
To determine if the declines in Ki67 positivity were restricted to β-cells, acinar cells were evaluated on the same images. Peri-insular acinar cells were considered to be the acinar cells surrounding islets of at least 20 cells in cross section and no more than 5 acinar cells in any direction from islets of this size; this selection of acinar cells was done to provide consistency. Similar to what we saw in β-cells, Ki67 positivity in the peri-insular acinar cells fell from 8.26 ± 1.5 after immediate fixation to 2.69 ± 0.78 after 24 h of warm/cold ischemia (P = 0.006).
Markers of β-Cell Replication in Neonatal Porcine Pancreas After Warm and Then Cold Ischemia
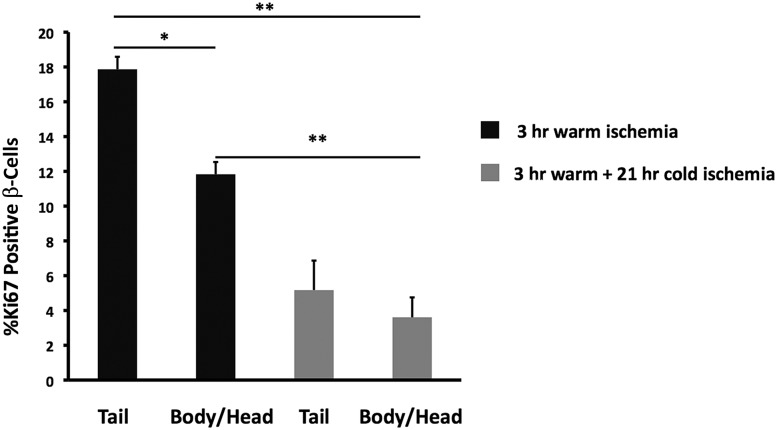
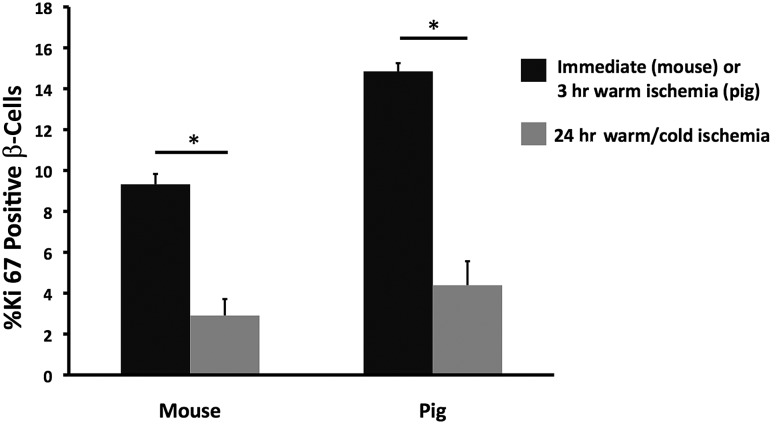
Pancreases taken from 6-day-old neonatal pigs were subjected to 3 h of warm ischemia at RT, followed by 21 h of cold ischemia at 4°C. Pancreatic tissue was not taken for immediate fixation, but only after 3 h of warm ischemia, at which point the β-cell Ki67 positivity was higher in the tail of the pancreas than in the body/head portion (17.9 ± 0.7 vs. 11.8 ± 0.7, n = 4 each; P = 0.001) (Fig. 3). We do not have an explanation for the difference between the tail and body/head regions, but because of glucagon staining of the islets in the body/head region, the difference cannot be attributed to sampling from the ventral lobe. Comparing 3 h of warm ischemia with the following 21 h of cold ischemia, the Ki67 positivity of β-cells fell markedly from 17.9 ± 0.7 to 5.2 ± 1.7 in the tail region (P = 0.002) and from 11.8 ± 0.7 to 3.6 ± 1.1 for the body/head region (P = 0.002). As shown in Fig. 4, the marked decline in Ki67-positive β-cells was similar in mice and newborn pigs. When the replication marker PCNA was evaluated on adjacent sections, the positivity of β-cells fell from 23.4 ± 1.9 at the end of the 3 h warm ischemia period to 3.7 ± 1.8 after 21 h of cold ischemia (P = 0.0003). The difference between the PCNA positivity of the β-cells in the tail and body/head portions for either condition was not significant.
Figure 3.
Pig pancreas under autopsy conditions show marked reduction of Ki67 in their β-cells. Porcine β-cells subjected to 3 h of warm ischemia at RT, followed by 21 h of cold ischemia at 4°C, showed a significant loss of Ki67 staining compared with 3 h of warm ischemia only. There was also a significant difference between the percentage of Ki67-positive β-cells in the tail and the body/head part of the pancreas after 3 h of warm ischemia. This difference is not due to overrepresentation of ventral lobe tissue in the body/head portion (n = 4). *P < 0.001, **P < 0.005.
Figure 4.
Decline of Ki67 staining in β-cells after 24 h of autopsy conditions is similar in β-cells of mice (n = 5–9) and pigs (n = 4). *P < 0.005.
Discussion
Our data indicate that exposure of the pancreas to cold and warm ischemia, as occurs with autopsies and cadaveric pancreas collection, leads to reduced Ki67 protein staining in β-cells and acinar cells and PCNA staining in β-cells. Pathologists have long known that delays of fixation for even less than 2 h can lead to a marked reduction in the frequency of mitotic figures (13) and more recently to loss of Ki67 (14–16). These findings suggest that estimates of β-cell proliferation using pancreatic tissue from autopsies or cadaveric donors are unreliable.
If the measurements of Ki67 positivity in autopsied and cadaveric donor pancreases are unreliable, valid information should be obtained from freshly fixed pancreatic tissue. Indeed, data from surgical specimens, transplanted human islets, and cultured human islets support the concept that adult human β-cells have Ki67 positivity in the range of 0.1–0.7% (9,11,12,17).
Major questions for diabetes are: How much β-cell turnover occurs in the adult human pancreas and can this be harnessed to regenerate meaningful numbers of new β-cells? In addition to β-cell replication, there is growing evidence that new β-cells are produced from neogenesis. Some argue that the presence of β-cells in or adjacent to the duct epithelium supports this concept (18), but these cells could have been there since fetal life or early childhood. Others point out the increase in β-cells as singlets or in small clumps that can be seen with pregnancy (19) or insulin resistance (17), but it is possible that these resulted from replication. A stronger argument comes from the findings of increased numbers of cells costained for duct markers and islet hormones, in particular, the combination of insulin and cytokeratin, which suggests an active process (11,20,21).
What can we say about the rate of β-cell turnover in the adult human pancreas if replication and neogenesis are both taking place? We know there is some rate of β-cell death as seen in studies using markers such as TUNEL (22). This should be balanced by an equivalent rate of β-cell birth. However, TUNEL staining represents an event and not a rate. Ki67 staining might provide some insight, although positive staining does not necessarily represent the birth of a new cell. The appearance of Ki67 means that a cell has entered the cell cycle, yet division may not occur. Moreover, there can be death of replicating cells (23,24). Despite these caveats, because the length of the cell cycle may be ∼27 h (25), Ki67 staining might be seen for a slightly shorter time. Thus, if β-cells in an adult human had 0.4% Ki67 positivity and if there were no neogenesis or cell death, β-cell mass could double in less than a year.
In summary, the current study finds that the frequency of Ki67-stained β-cells falls markedly when mouse and neonatal pig pancreases are subjected to warm and cold ischemia. These findings suggests that pancreases obtained from autopsies or organ donors will have reduced Ki67 staining of β-cells, leading to the mistaken conclusion that adult human β-cells do not replicate. The rates of β-cell turnover in the human pancreas and the capacity for β-cell regeneration remain to be determined.
Article Information
Funding. This study was supported by grants from JDRF (G.C.W.), National Institutes of Health grants R01-DK-066056 and DK-093909 (S.B.-W.) and P30-DK-036836 (Joslin Diabetes Research Center and its Advanced Microscopy Core), and the Diabetes Research & Wellness Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.A.S. and J.H.-L. researched data. S.B.-W. and G.C.W. conceived the project. B.A.S. and G.C.W. wrote the manuscript. All authors reviewed and edited the manuscript. G.C.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
References
- 1.Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci 2013;1281:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012;97:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner JA. The role of aging upon β cell turnover. J Clin Invest 2013;123:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecil RL. On hypertrophy and regeneration of the islands of Langerhans. J Exp Med 1911;14:500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 2010;95:E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010;53:321–330 [DOI] [PubMed] [Google Scholar]
- 8.In’t Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes 2010;59:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brelje TC, Scharp DW, Lacy PE, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993;132:879–887 [DOI] [PubMed] [Google Scholar]
- 10.Takane KK, Kleinberger JW, Salim FG, Fiaschi-Taesch NM, Stewart AF. Regulated and reversible induction of adult human β-cell replication. Diabetes 2012;61:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caballero F, Siniakowicz K, Hollister-Lock J, et al. Birth and death of human β-cells in pancreases from cadaver donors, autopsies, surgical specimens, and islets transplanted into mice. Cell Transplant 2014;23:139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menge BA, Tannapfel A, Belyaev O, et al. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes 2008;57:142–149 [DOI] [PubMed] [Google Scholar]
- 13.Cross SS, Start RD, Smith JH. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol 1990;43:597–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yildiz-Aktas IZ, Dabbs DJ, Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol 2012;25:1098–1105 [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Tolles J, Cheng H, et al. Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Invest 2011;91:1253–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 2010;134:907–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneda S, Uno S, Iwahashi H, et al. Predominance of β-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab 2013;98:2053–2061 [DOI] [PubMed] [Google Scholar]
- 18.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010;53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez-Pinzon WL, Lakey JR, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant 2008;17:631–640 [DOI] [PubMed] [Google Scholar]
- 21.Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 2014;63:994–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 23.Rieck S, Zhang J, Li Z, et al. Overexpression of hepatocyte nuclear factor-4α initiates cell cycle entry, but is not sufficient to promote β-cell expansion in human islets. Mol Endocrinol 2012;26:1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev 1996;10:2105–2116 [DOI] [PubMed] [Google Scholar]
- 25.Hija A, Salpeter S, Klochendler A, et al. G0-G1 transition and the restriction point in pancreatic β-cells in vivo. Diabetes 2014;63:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]