Abstract
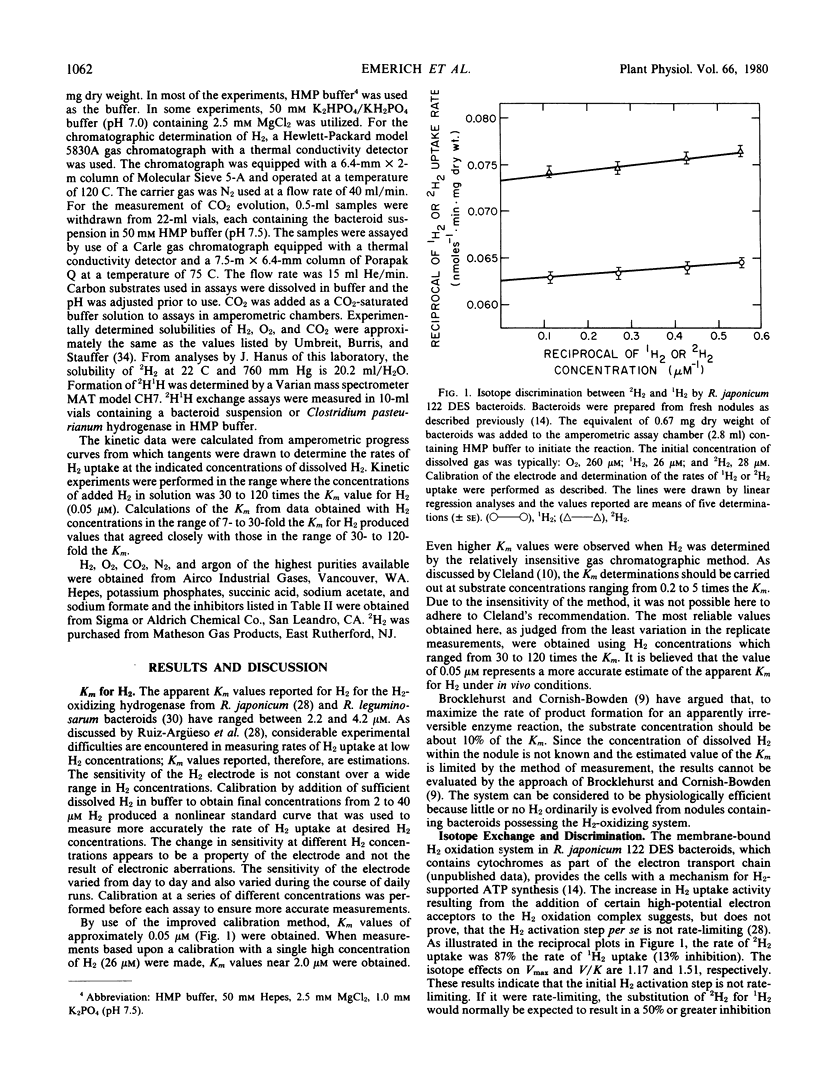
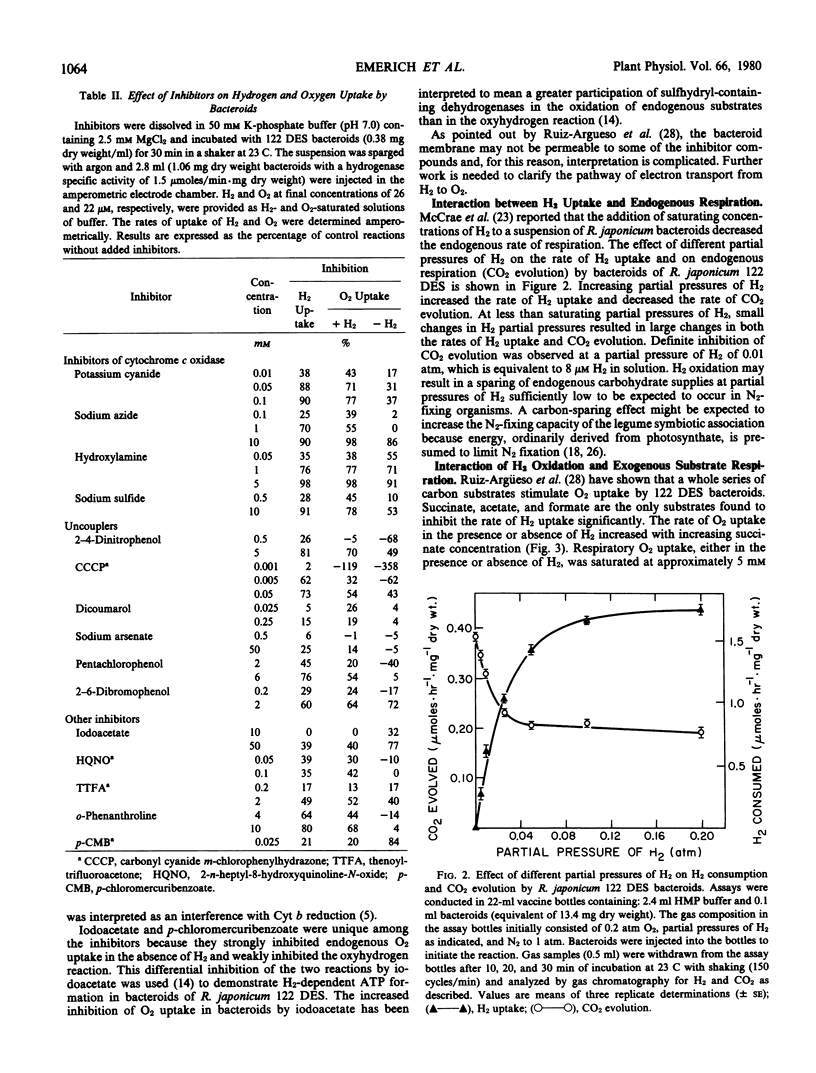
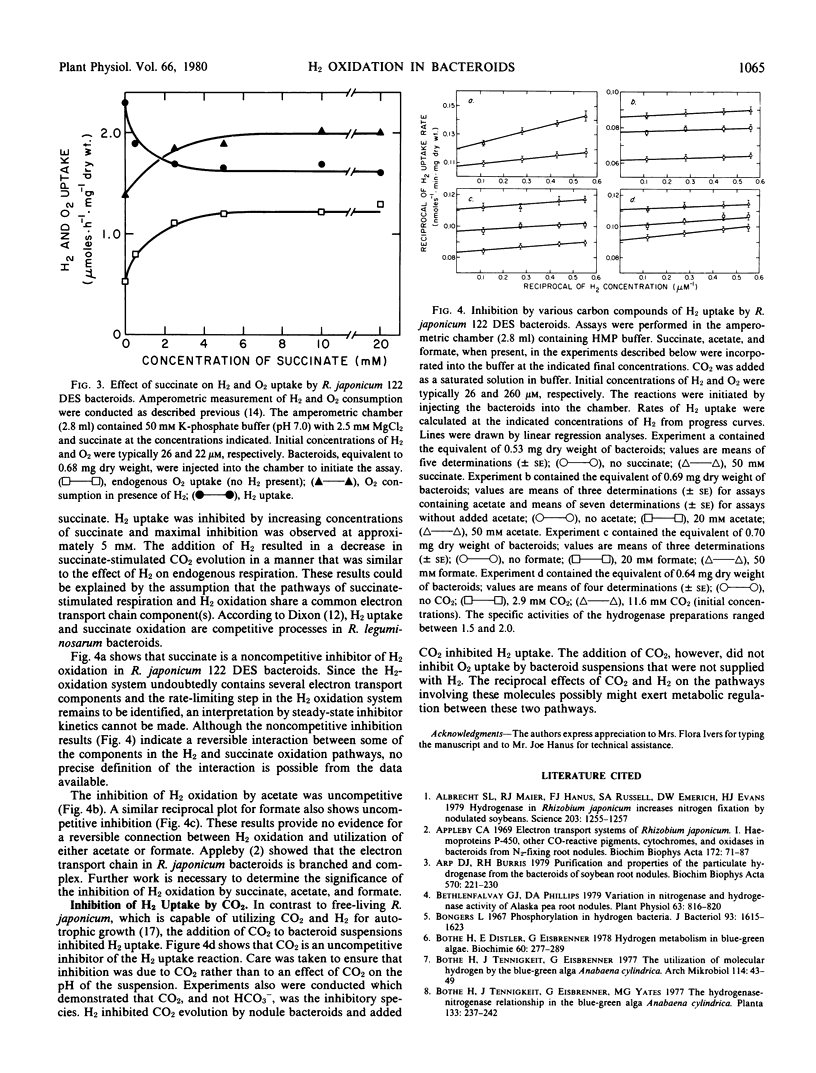
The H2-oxidizing complex in Rhizobium japonicum 122 DES bacteroids failed to catalyze, at a measurable rate, 2H1H exchange from a mixture of 2H2 and 1H2 in presence of 2H2O and 1H2O, providing no evidence for reversibility of the hydrogenase reaction in vivo. In the H2 oxidation reaction, there was no significant discrimination between 2H2 and 1H2, indicating that the initial H2-activation step in the over-all H2 oxidation reaction is not rate-limiting. By use of improved methods, an apparent Km for H2 of 0.05 micromolar was determined. The H2 oxidation reaction in bacteroids was strongly inhibited by cyanide (88% at 0.05 millimolar), theonyltrifluoroacetone, and other metal-complexing agents. Carbonyl cyanide m-chlorophenylhydrazone at 0.005 millimolar and 2,4-dinitrophenol at 0.5 millimolar inhibited H2 oxidation and stimulated O2 uptake. This and other evidence suggest the involvement of cytochromes and nonheme iron proteins in the pathway of electron transport from H2 to O2. Partial pressures of H2 at 0.03 atmosphere and below had a pronounced inhibitory effect on endogenous respiration by bacteroid suspensions. The inhibition of CO2 evolution by low partial pressures of H2 suggests that H2 utilization may result in conservation of oxidizable substrates and benefits the symbiosis under physiological conditions. Succinate, acetate, and formate at concentrations of 50 millimolar inhibited rates of H2 uptake by 8, 29, and 25%, respectively. The inhibition by succinate was noncompetitive and that by acetate and formate was uncompetitive. A concentration of 11.6 millimolar CO2 (initial concentration) in solution inhibited H2 uptake by bacteroid suspensions by 18%. Further research is necessary to establish the significance of the inhibition of H2 uptake by succinate, acetate, formate, and CO2 in the metabolism of the H2-uptake-positive strains of Rhizobium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Variation in nitrogenase and hydrogenase activity of alaska pea root nodules. Plant Physiol. 1979 May;63(5):816–820. doi: 10.1104/pp.63.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H., Distler E., Eisbrenner G. Hydrogen metabolism in blue-green algae. Biochimie. 1978;60(3):277–289. doi: 10.1016/s0300-9084(78)80824-4. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Cornish-Bowden A. The pre-eminence of k(cat) in the manifestation of optimal enzymic activity delineated by using the Briggs-Haldane two-step irreversible kinetic model. Biochem J. 1976 Oct 1;159(1):165–166. doi: 10.1042/bj1590165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- HYNDMAN L. A., BURRIS R. H., WILSON P. W. Properties of hydrogenase from Azotobacter vinelandii. J Bacteriol. 1953 May;65(5):522–531. doi: 10.1128/jb.65.5.522-531.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Burris R. H. The hydrogenase of Clostridium pasteurianum. Kinetic studies and the role of molybdenum. Biochim Biophys Acta. 1970 Sep 16;212(3):417–427. doi: 10.1016/0005-2744(70)90247-0. [DOI] [PubMed] [Google Scholar]
- Klinman J. P. Kinetic isotope effects in enzymology. Adv Enzymol Relat Areas Mol Biol. 1978;46:415–494. doi: 10.1002/9780470122914.ch7. [DOI] [PubMed] [Google Scholar]
- Lim S. T. Determination of Hydrogenase in Free-living Cultures of Rhizobium japonicum and Energy Efficiency of Soybean Nodules. Plant Physiol. 1978 Oct;62(4):609–611. doi: 10.1104/pp.62.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- Quebedeaux B., Havelka U. D., Livak K. L., Hardy R. W. Effect of Altered pO(2) in the Aerial Part of Soybean on Symbiotic N(2) Fixation. Plant Physiol. 1975 Dec;56(6):761–764. doi: 10.1104/pp.56.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhinshtein M. V., Melik-Sarkisian S. S., Zaigraeva G. G., Kretovich V. L. Ingibitornyi analiz dykhaniia bakteroidov iz kluben'kov zheltogo liupina. Mikrobiologiia. 1976 Mar-Apr;45(2):210–216. [PubMed] [Google Scholar]
- Ruiz-Argüeso T., Emerich D. W., Evans H. J. Hydrogenase system in legume nodules: a mechanism of providing nitrogenase with energy and protection from oxygen damage. Biochem Biophys Res Commun. 1979 Jan 30;86(2):259–264. doi: 10.1016/0006-291x(79)90860-x. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. C., Yates M. G. The hydrogen cycle in nitrogen-fixing Azotobacter chroococcum. Biochimie. 1978;60(3):225–231. doi: 10.1016/s0300-9084(78)80818-9. [DOI] [PubMed] [Google Scholar]



